Corresponding Author: Yu Na Kang, M.D., Department of Pathology, Keimyung University School of Medicine 56 Dalseong-ro, Jung-gu, Daegu 700-712, Korea
Tel: +82-53-250-7290, +82-53-580-3814
E-mail: yunakang@dsmc.or.kr, swmpath@gmail.com
전체 글
Corresponding Author: Yu Na Kang, M.D., Department of Pathology, Keimyung University School of Medicine 56 Dalseong-ro, Jung-gu, Daegu 700-712, Korea
Tel: +82-53-250-7290, +82-53-580-3814
E-mail: yunakang@dsmc.or.kr, swmpath@gmail.com
수치
관련 문서
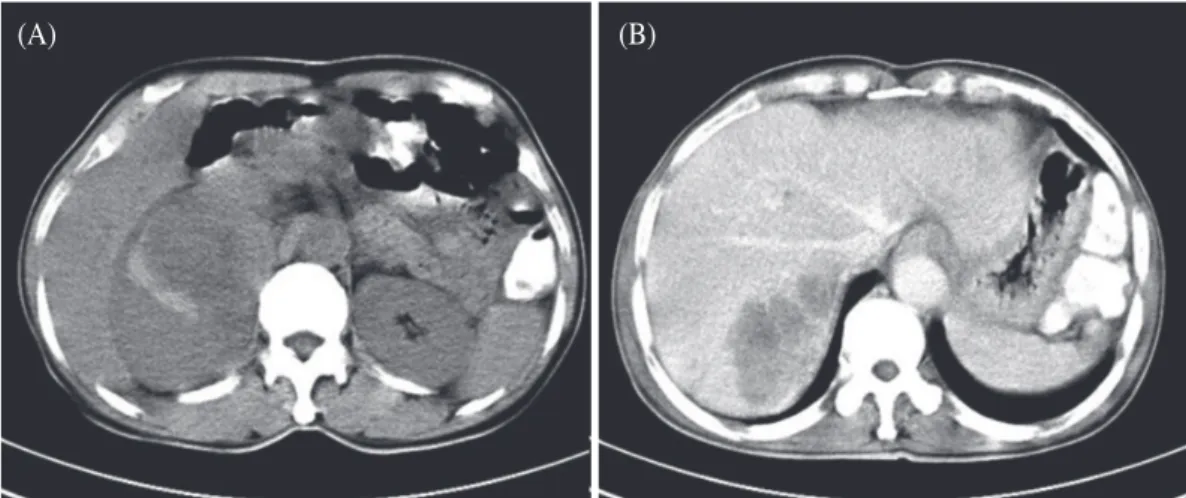
A retrospective analysis of invasive tumor percentages of GSS tumor samples showed that samples with high tumor content correlated well with FNAB samples
Effects of Tetrapanax papyriferum Extracts on the KB Human Oral
Especially, in the case of DPRK(Democratic People's Republic of Korea) of which energy consumption have been decreased by economic depression, decomposition
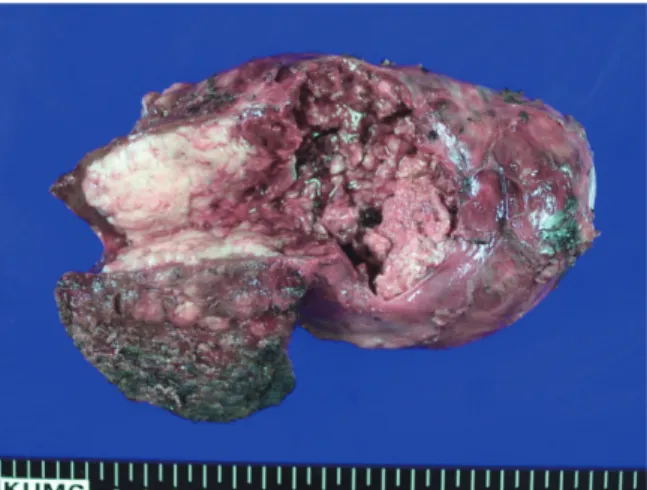
Polypoid lesions of the gallbladder; Report of 111 cases with surgical reference to operative indication.. Polypoid lesion of GB: Indication of carcinoma
1 John Owen, Justification by Faith Alone, in The Works of John Owen, ed. John Bolt, trans. Scott Clark, "Do This and Live: Christ's Active Obedience as the
Concentration-Dependent inhibition of cell viability by adenosine in human oral fibroblast and FaDu human head and neck squamous cell carcinoma · · ·
Differential Expression of Desmoglein1, Desmoglein3, Epithelial Membrane Antigen, Ber-EP4 and CD10 in Basal Cell Carcinoma and Squamous Cell Carcinoma
Objective: The purpose of this study was to analyze recent trend in incidence of basal cell carcinoma and squamous cell carcinoma in patients from the Gwangju City