dioxide pressure (pCO
2), and partial oxygen pressure (pO
2). More- over, the simultaneous measurement of electrolytes such as so- dium (Na
+), potassium (K
+), chloride (Cl
-), and ionized calcium (Ca
2+) is available with hematocrit (Hct). Integration and immedi- ate therapeutic response using these results allows for prompt treatment and improvement of prognosis. Therefore, blood gas analyzers are being demanded for both their rapid turn-around of test results and sophisticated result accuracy. While most of the blood gas analyzers are used on-site, such as in emergency de- partments and ICUs for point of care (POC) testing, even bench- top models are currently in use at most clinical laboratories.
In the use of POC analyzers, quality control is a big issue. The users of POC analyzers are often unfamiliar with laboratory in- struments and unacquainted with the maintenance and quality control of the instrument. Therefore, cartridge-type instruments are welcomed for most POC instruments, not only blood gas ana- lyzers, but also molecular diagnostic analyzers [3]. If, however,
INTRODUCTION
Blood gas analysis is a critical tool in assessing a clinical diag- nosis and in therapeutic monitoring of severely ill patients in the emergency department and intensive care unit (ICU) [1, 2]. Most blood gas analyzers measure the whole blood pH, partial carbon
카트리지 형태의 현장 검사 혈액 가스 장비 i-Smart 300의 성능 평가
Performance Evaluation of Cartridge-Type Blood Gas Analyzer: i-Smart 300
이아람·김한아·문희원·허미나·윤여민
Ahram Yi, M.D., Hanah Kim, M.D., Hee-Won Moon, M.D., Mina Hur, M.D., Yeo-Min Yun, M.D.
건국대학교 의학전문대학원 진단검사의학교실
Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea https://doi.org/10.3343/lmo.2017.7.1.20
Corresponding author: Yeo-Min Yun
Department of Laboratory Medicine, Konkuk University School of Medicine Konkuk University Hospital, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 05030, Korea
Tel: +82-2-2030-5582, Fax: +82-2-2030-5587, E-mail: ymyun@kuh.ac.kr Received: December 23, 2015
Revision received: April 26, 2016 Accepted: May 3, 2016
This article is available from http://www.labmedonline.org 2017, Laboratory Medicine Online
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Blood gas analysis plays a crucial role in critical care settings, and immediate and precise analysis improves clinical outcomes through prompt treatment. We evaluated the performance of a cartridge-type blood gas analyzer, i-Smart 300 (i-SENS, Korea), according to the Clinical and Laboratory Standard Institute (CLSI) guidelines and compared it to a conventional blood gas analyzer.
Methods: The precision was evaluated according to CLSI EP5-A3. The i-Smart 300 was compared to the Stat Profile Critical Care Xpress (STP CCX) (Nova CCX; Nova Biomedical, USA) according to CLSI EP9-A3 using the following eight parameters: pH, partial carbon dioxide pressure, partial oxygen pressure, sodium, potassium, chloride, ionized calcium, and hematocrit. Linearity was determined using five levels of control materials ac- cording to CLSI EP6-A.
Results: Within-run precision and total precision, demonstrated as coefficients of variation, ranged from 0.02 to 2.50% and from 0.05 to 3.46%, respectively. Correlation analysis yielded a correlation coefficient from 0.966 to 0.996 between the i-Smart 300 and the conventional analyzer (Nova CCX). The i-Smart 300 showed excellent linearity at eight parameters with acceptable percent recovery.
Conclusions: The i-Smart 300, a portable cartridge-type blood gas analyzer, showed high precision and good correlation with a traditional bench- top blood gas analyzer. It could be useful in critical care settings.
Key Words: i-Smart, Blood gas analyzer, Point-of-care systems, Performance
이아람 외: Cartridge-Type Blood Gas Analyzer
https://doi.org/10.3343/lmo.2017.7.1.20 www.labmedonline.org 21
there are discrepant results between a POC analyzer and central bench-top analyzer, this can confuse physicians and makes it more difficult to treat patients properly. Therefore, the performance eval- uation of blood gas analyzers and determination of analyzer-spe- cific differences in bias and precision are important to reduce the conflicting results between different instruments [4].
Recently, a brand new blood gas analyzer, i-Smart 300 (i-SENS, Seoul, Korea) was launched and is a cartridge-type blood gas an- alyzer, which is preferred in the clinical field. This study aims to evaluate the analytical performance of this new cartridge-type blood gas analyzer in comparison with the Stat Profile Critical Care Xpress (Nova CCX; Nova Biomedical, Waltham, MA, USA) as a reference method according to the Clinical and Laboratory Stan- dard Institute (CLSI) guidelines.
MATERIALS AND METHODS
1. Analyzer
The i-Smart 300 blood gas analyzer consists of an operating in- strument and a disposable cartridge containing all sensors, a re- agents waste bag, tubing, and sample probe for analysis. The stand- alone disposable cartridge is a self-contained miniature analyzer, a functioning analyzing unit that contains all necessary parts: re- agents, calibrators, reference solution, sample probe, valves, tubing, and sensors. Therefore, equipment maintenance is very conve- nient involving only replacing a cartridge. The i-Smart uses three levels of aqueous quality control (QC) material, RNA QC 623 Blood Gas-Electrolyte Control (RNA Medical, Devens, MA, USA), and two levels of RNA QC 900 Hematocrit Control (RNA Medical, De- vens, MA, USA). This analyzer measures the whole blood pH, pCO
2, pO
2, Na
+, K
+, Cl
-, Ca
2+, and Hct using 80 μL heparinized whole blood.
2. Specimens
In March 2015, a total of 40 heparinized whole blood specimens were prospectively and consecutively collected. The specimens were anaerobically drawn from arterial vessels using heparinized blood sample kits, Becton Dickinson’s Preset (BD Becton Dickin- son Co., Plymouth, UK), according to the CLSI guideline [5]. The remaining specimens were used in anonymous way, and this study was approved by the Institutional Review Board of the Konkuk University Medical Center (KUH1200047).
To determine the imprecision of i-Smart for eight analytes, aque- ous QC materials were used. For seven analytes, except Hct, three levels of aqueous QC material, RNA QC 623 Blood Gas-Electrolyte Control (RNA Medical, Devens, MA, USA) were used, and two levels of RNA QC 900 Hematocrit Control (RNA Medical, Devens, MA, USA) were used for Hct. For carry-over analysis, two levels of QC materials were tested. Five calibration verification materials [RNA CVC 123 Calibration Verification Controls and RNA CVC 9005 Hematocrit Calibration Verification Controls (RNA Medical, De- vens, MA, USA)] were used for linearity assessment.
3. Study Design
The total run imprecision was determined using within-labora- tory precision with the CLSI EP5-A3. It uses a design with 20 test- ing days, two runs per testing day, and two replicate measurements per run (20
×2
×2) for each samples using a single reagent and single calibrator lot. Carry-over was estimated with replicate mea- surements of the high-low sequences and was calculated by the equation: % carry-over
={L1-(L3+L4)/2}
×100/{(H2+H3)/2-(L3+L4)/2}.
An acceptability criterion was % carry-over of less than 1% [6, 7].
Linearity analysis was performed with five levels of commercially available linearity material with two repetitions of each level ac- cording to the CLSI EP6-A. Acceptability criteria were a slope be- tween 0.9 and 1.1 and a recovery of between 90% and 110% [8].
Method comparison and difference estimation were assessed using a Nova CCX in the core laboratory. Forty heparinized whole blood specimens were analyzed on the i-Smart and the Nova CCX according to the CLSI EP9-A3 [9].
4. Statistical Analysis
Before statistical analysis, all data were processed to detect and reject outliers according to the CLSI EP9-A3 [9]. To compare the results of the i-Smart and Nova CCX, Pearson correlation coeffi- cient and Passing-Bablok regression analysis were used. Each es- timated slope and intercept, as well as 95% confidence intervals (CI), was calculated. The Nova CCX is the laboratory’s current method and not a recognized reference method, so the trueness of the test methods could not be determined. The differences (ab- solute and relative) by the Bland-Altman difference plot were cal- culated. We used percent difference between the two methods, so bias from difference of scale or units could be excluded.
Statistical analysis was performed using Analyse-it Software
(version 3.90.5; Analyse-it Software, Ltd., Leeds, UK) and MedCalc Software (version 14.12.0; MedCalc Software, Mariakerke, Belgium).
P values of less than 0.05 were considered statistically significant.
RESULTS
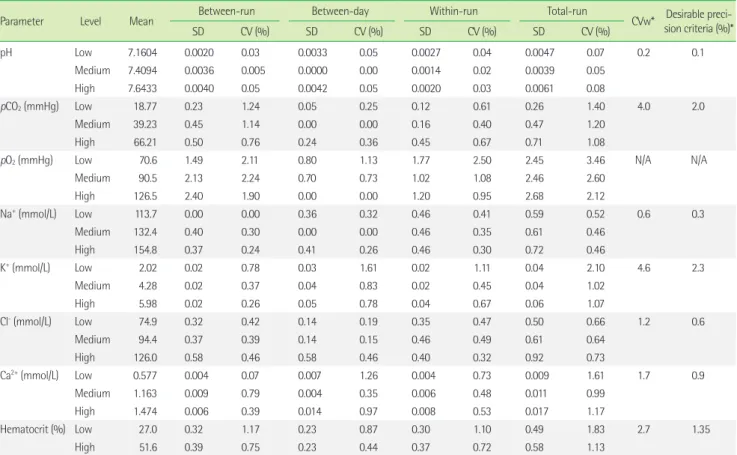
1. Precision and Carry-over
Total precision (percent coefficients of variation, CV) of eight parameters was determined by analyzing three levels of QC ma- terial on 20 consecutive days according to the CLSI guidelines (Table 1). Within-run CV ranged from 0.02 to 2.50%, and the total- run CV ranged from 0.05 to 3.46%. The %CV of Na
+at low level, Ca
2+at low level, Cl
-at high level, and Hct at low level were: 0.52, 1.61, 0.73, and 1.83%, respectively, and narrowly escaped the de- sirable precision criteria [10]. However, the other measured values
were within the allowable total error [11]. Carry-over ranged from -0.04 to 0.05% and was acceptable within 5% [7].
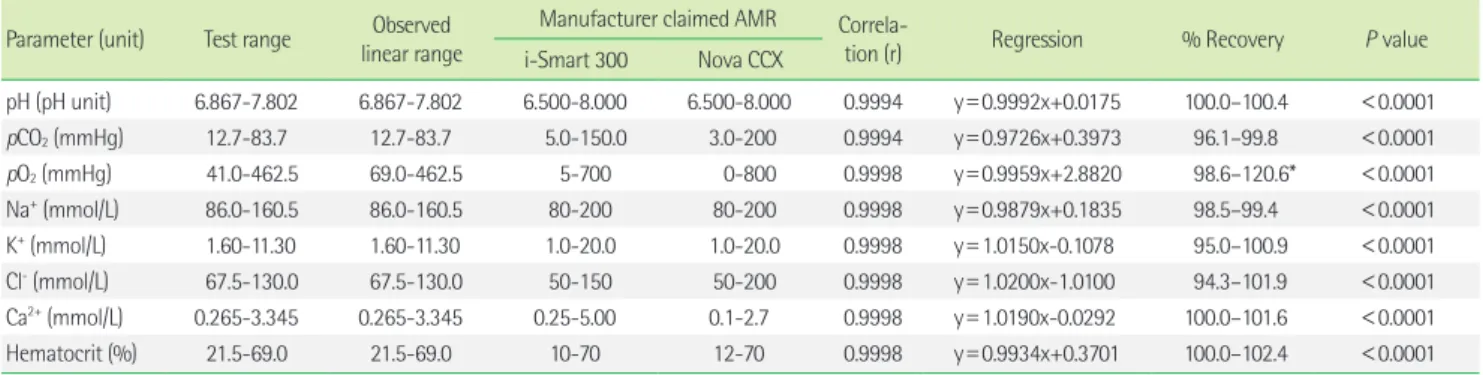
2. Linearity
Linearity was demonstrated according to the CLSI guidelines for all eight measurable parameters. Overall correlation coefficients (r) ranged from 0.9994 to 0.9998. The range of recovery was 94.3 – 120.6%. The slope and intercept of the regression equation are shown in Table 2. The recovery of pO
2at low levels was 120.6%, and narrowly escaped the acceptable criteria, while the other val- ues lay within the criteria.
3. Method Comparison
The results of Passing-Bablok regression analysis and mean dif- ference estimations are summarized in Table 3. Pearson correla-
Table 1. Evaluation of total precision for blood gas and electrolytes using three levels of control materials on the i-Smart 300 blood gas analyzer,
with allowable precision, difference, and total errorParameter Level Mean Between-run Between-day Within-run Total-run
CVw* Desirable preci- sion criteria (%)*
SD CV (%) SD CV (%) SD CV (%) SD CV (%)
pH Low 7.1604 0.0020 0.03 0.0033 0.05 0.0027 0.04 0.0047 0.07 0.2 0.1
Medium 7.4094 0.0036 0.005 0.0000 0.00 0.0014 0.02 0.0039 0.05
High 7.6433 0.0040 0.05 0.0042 0.05 0.0020 0.03 0.0061 0.08
pCO
2(mmHg) Low 18.77 0.23 1.24 0.05 0.25 0.12 0.61 0.26 1.40 4.0 2.0
Medium 39.23 0.45 1.14 0.00 0.00 0.16 0.40 0.47 1.20
High 66.21 0.50 0.76 0.24 0.36 0.45 0.67 0.71 1.08
pO
2(mmHg) Low 70.6 1.49 2.11 0.80 1.13 1.77 2.50 2.45 3.46 N/A N/A
Medium 90.5 2.13 2.24 0.70 0.73 1.02 1.08 2.46 2.60
High 126.5 2.40 1.90 0.00 0.00 1.20 0.95 2.68 2.12
Na
+(mmol/L) Low 113.7 0.00 0.00 0.36 0.32 0.46 0.41 0.59 0.52 0.6 0.3
Medium 132.4 0.40 0.30 0.00 0.00 0.46 0.35 0.61 0.46
High 154.8 0.37 0.24 0.41 0.26 0.46 0.30 0.72 0.46
K
+(mmol/L) Low 2.02 0.02 0.78 0.03 1.61 0.02 1.11 0.04 2.10 4.6 2.3
Medium 4.28 0.02 0.37 0.04 0.83 0.02 0.45 0.04 1.02
High 5.98 0.02 0.26 0.05 0.78 0.04 0.67 0.06 1.07
Cl
-(mmol/L) Low 74.9 0.32 0.42 0.14 0.19 0.35 0.47 0.50 0.66 1.2 0.6
Medium 94.4 0.37 0.39 0.14 0.15 0.46 0.49 0.61 0.64
High 126.0 0.58 0.46 0.58 0.46 0.40 0.32 0.92 0.73
Ca
2+(mmol/L) Low 0.577 0.004 0.07 0.007 1.26 0.004 0.73 0.009 1.61 1.7 0.9
Medium 1.163 0.009 0.79 0.004 0.35 0.006 0.48 0.011 0.99
High 1.474 0.006 0.39 0.014 0.97 0.008 0.53 0.017 1.17
Hematocrit (%) Low 27.0 0.32 1.17 0.23 0.87 0.30 1.10 0.49 1.83 2.7 1.35
High 51.6 0.39 0.75 0.23 0.44 0.37 0.72 0.58 1.13
It uses a design with 20 testing days, two runs per testing day, and two replicate measurements per run (n = 20×2×2 = 80) for each sample using a single reagent and single calibrator lot, according to the CLSI EP5-A3. Three levels of aqueous quality control (QC) material were used, RNA QC 623 Blood Gas-Electrolyte Control (RNA Medical, De- vens, MA, USA), and two levels of RNA QC 900 Hematocrit Control (RNA Medical, Devens, MA, USA) were used.
*Within-subject variation and desirable analytical precision criteria are referenced from Ricos et al. [10], and the biological variation database specification on Westgard’s web- site (https://www.westgard.com/biodatabase1.htm) [11].
Abbreviations: CV, coefficient variation; CVw, within-subject biological variation; NA, not available.
이아람 외: Cartridge-Type Blood Gas Analyzer
https://doi.org/10.3343/lmo.2017.7.1.20 www.labmedonline.org 23
tion analysis yielded generally very high correlation coefficients ranging from 0.966 to 0.996 for all parameters being evaluated.
The slopes of the regression equations ranged from 0.88 to 1.29 for all analytes.
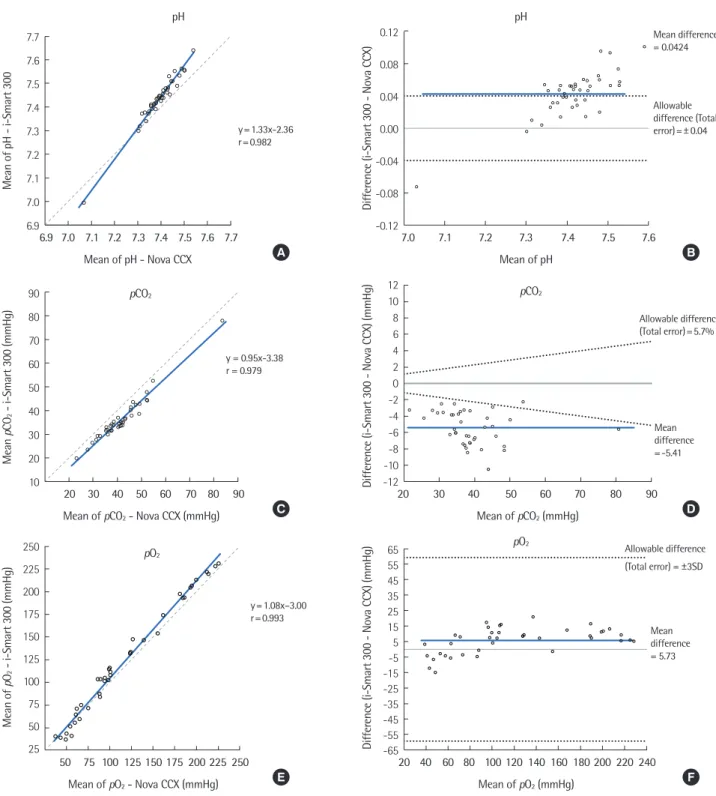
Bland-Altman plots showed the means of the paired difference in the concentrations of eight analytes obtained using the i-Smart 300 and Nova CCX, which are shown in Fig. 1(B, D, F, H, J, L, N, P). Comparison of the two blood gas analyzers showed accept- able comparability for all analytes. According to the Bland-Altman plots, most paired data lay within
±1.96 standard deviation (SD).
DISCUSSION
It is well known that results of blood gas and electrolyte analy- sis are crucial in managing the ICU and emergency department [12]. Furthermore, emergency conditions in the operating room, ICU, and emergency department require immediate and precise analysis to treat patients promptly. The i-Smart 300, a portable cartridge-type blood gas analyzer, can eliminate the time needed to deliver samples to central laboratory and help physicians to as-
sess disease and treat patients promptly. Unlike a conventional blood gas analyzer, the i-Smart 300 can be stored with the cartri- dges and reagents at room temperature, without occupying space in a refrigerator. Disposable cartridges include internal quality control materials and reagents, so it is easy to operate and use in- ternal quality controls and, thus, the results could be reliable even when users are inexperienced.
In this study, we evaluated the i-Smart 300 by comparing preci- sion, carry-over, linearity, and correlation with a conventional blood gas analyzer. For precision evaluation, various judgment parame- ters were applicable. Generally, the desirable analytical precision criteria are used according to intra-individual biologic variation [13]. Typically, if precision is within 50% of the intra-individual bi- ologic variation, it is regarded as suitable clinical test. Also, in a specific patient, when followed by serial tests, it means the ana- lytical errors allow for distinguishing between normal biologic variation and clinically significant changes [10, 14, 15]. For the pre- cision performance analysis of the i-Smart 300 using quality con- trol materials, each parameter’s within-run precision and total pre- cision were demonstrated as coefficients of variation and ranged Table 2. Evaluation of linearity from eight parameters using five levels of control materials with the i-Smart 300 blood gas analyzer
Parameter (unit) Test range Observed linear range
Manufacturer claimed AMR Correla-
tion (r) Regression % Recovery P value
i-Smart 300 Nova CCX
pH (pH unit) 6.867-7.802 6.867-7.802 6.500-8.000 6.500-8.000 0.9994 y = 0.9992x+0.0175 100.0–100.4 < 0.0001 pCO
2(mmHg) 12.7-83.7 12.7-83.7 5.0-150.0 3.0-200 0.9994 y = 0.9726x+0.3973 96.1–99.8 < 0.0001 pO
2(mmHg) 41.0-462.5 69.0-462.5 5-700 0-800 0.9998 y = 0.9959x+2.8820 98.6–120.6* < 0.0001 Na
+(mmol/L) 86.0-160.5 86.0-160.5 80-200 80-200 0.9998 y = 0.9879x+0.1835 98.5–99.4 < 0.0001 K
+(mmol/L) 1.60-11.30 1.60-11.30 1.0-20.0 1.0-20.0 0.9998 y = 1.0150x-0.1078 95.0–100.9 < 0.0001 Cl
-(mmol/L) 67.5-130.0 67.5-130.0 50-150 50-200 0.9998 y = 1.0200x-1.0100 94.3–101.9 < 0.0001 Ca
2+(mmol/L) 0.265-3.345 0.265-3.345 0.25-5.00 0.1-2.7 0.9998 y = 1.0190x-0.0292 100.0–101.6 < 0.0001 Hematocrit (%) 21.5-69.0 21.5-69.0 10-70 12-70 0.9998 y = 0.9934x+0.3701 100.0–102.4 < 0.0001
*The % recovery of pO
2ranged from 98.6% to 120.6%. Except for the coded concentration 34, the % recovery of pO
2ranged from 98.6% to 101.5%.
Abbreviation: AMR, analytical measurement range.
Table 3. Comparison of results obtained using the i-Smart 300 and Nova CCX (n=40)
Parameter Test range Correlation (r) (95% CI) Slope* (95% CI) Intercept* (95% CI) Mean difference
†(95% CI) pH (pH unit) 7.067-7.540 0.982 (0.967-0.991) 1.33 (1.25 to 1.40) -2.36 (-2.94 to -1.79) 0.0424 (0.0330 to 0.0518) pCO
2(mmHg) 19.9-77.9 0.979 (0.958-0.988) 0.95 (0.85 to 1.05) -3.38 (-7.44 to 0.68) -5.41 (-6.053 to -4.762) pO
2(mmHg) 37.2-225.8 0.993 (0.987-0.996) 1.08 (1.03 to 1.12) -3.00 (-8.97 to 2.97) 5.73 (3.10 to 8.36) Na
+(mmol/L) 124.5-144.8 0.966 (0.936-0.982) 1.02 (0.93 to 1.10) -3.09 (-14.24 to 8.06) -1.01 (-1.36 to 0.66) K
+(mmol/L) 2.67-6.59 0.996 (0.993-0.998) 0.88 (0.86 to 0.91) 0.34 (0.23 to 0.45) -0.128 (-0.161 to -0.095) Cl
-(mmol/L) 88.8-116.7 0.970 (0.943-0.984) 1.05 (0.95 to 1.14) -4.17 (-14.22 to 5.88) 0.86 (0.49 to 1.23) Ca
2+(mmol/L) 0.78-1.51 0.975 (0.952-0.987) 1.05 (0.95 to 1.15) -0.15 (-0.27 to -0.03) -0.091 (-0.100 to -0.082) Hematocrit (%) 24-53 0.974 (0.951-0.986) 1.07 (0.99 to 1.15) -5.08 (-8.08 to -2.08) -2.6 (-3.0 to -2.1)
*Determined using Deming fit;
†Mean difference is defined as measured values of the i-Smart 300 minus Nova CCX.
Abbreviation: CI, confidence interval.
이아람 외: Cartridge-Type Blood Gas Analyzer
https://doi.org/10.3343/lmo.2017.7.1.20 24 www.labmedonline.org
A
22 6.9
7 7.1 7.2 7.3 7.4 7.5 7.6 7.7
6.9 7 7.1 7.2 7.3 7.4 7.5 7.6 7.7
Mean of pH -i-Smart 300
Mean of pH - STP CCX pH
pH 17
18 19
20 21 22 23 24 25 26 27
28
‐0.120
‐0.080
‐0.040 0.000 0.040 0.080 0.120
7 7.1 7.2 7.3 7.4 7.5 7.6
Difference (i-Smart 300 -STP CCX)
Mean of pH
Allowable difference (Total error) = ± 0.04 (A)
(B)
y = 1.33x - 2.36 r = 0.982
Mean difference
= 0.0424
7.7 7.6 7.5 7.4 7.3 7.2 7.1 7.0 6.9
Mean of pH - i-Smart 300
Mean of pH - Nova CCX 6.9 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7
y=1.33x-2.36 r=0.982
Differ ence (i-Smart 300 - Nova CCX)
B
22 6.9
7 7.1 7.2 7.3 7.4 7.5
6.9 7 7.1 7.2 7.3 7.4 7.5 7.6 7.7
Mean of pH -i-Smart 300
Mean of pH - STP CCX
pH
20 21 22 23 24 25 26 27
28
‐0.120
‐0.080
‐0.040 0.000 0.040 0.080 0.120
7 7.1 7.2 7.3 7.4 7.5 7.6
Difference (i-Smart 300 -STP CCX)
Mean of pH
Allowable difference (Total error) = ± 0.04 (B)
y = 1.33x - 2.36 r = 0.982
Mean difference
= 0.0424
0.12 0.08 0.04 0.00 -0.04 -0.08 -0.12
Mean of pH
7.0 7.1 7.2 7.3 7.4 7.5 7.6
Mean difference
= 0.0424
Allowable difference (Total error)=±0.04 pH
C
24 10
20 30 40 50 60 70 80 90
10 20 30 40 50 60 70 80 90
Mean pCO2-i-Smart 300 (mmHg)
Mean of pCO2- STP CCX (mmHg) pCO2 35
36 37 38 39 40 41 42 43 44 45 46
47
Fig. 1. (continued) 48
‐12.0
‐10.0
‐8.0
‐6.0
‐4.0
‐2.0 0.0 2.0 4.0 6.0 8.0 10.0 12.0
20 30 40 50 60 70 80 90
Difference (i-Smart 300 -STP CCX)(mmHg)
Mean of pCO2(mmHg)
Allowable difference (Total error) = 5.7%
(A)
y = 0.95x -3.38 r = 0.979
Mean difference
= - 5.41 (B)
pCO2
90 80 70 60 50 40 30 20 10 Mean pCO
2- i-Smart 300 (mmHg)
Mean of pCO
2- Nova CCX (mmHg) 20 30 40 50 60 70 80 90
y = 0.95x-3.38 r = 0.979 pCO
2D
24 10
20 30 40 50 60 70 80 90
10 20 30 40 50 60 70 80 90
Mean pCO2-i-Smart 300 (mmHg)
Mean of pCO2- STP CCX (mmHg) pCO2 35
36 37 38 39 40 41 42 43 44 45 46
47
Fig. 1. (continued) 48
‐12.0
‐10.0
‐8.0
‐6.0
‐4.0
‐2.0 0.0 2.0 4.0 6.0 8.0 10.0 12.0
20 30 40 50 60 70 80 90
Difference (i-Smart 300 -STP CCX)(mmHg)
Mean of pCO2(mmHg)
Allowable difference (Total error) = 5.7%
(A)
y = 0.95x -3.38 r = 0.979
Mean difference
= - 5.41 (B)
pCO2
12 10 8 6 4 2 0 -2 -4 -6 -8 -10 Differ ence (i-Smart 300 - Nova CCX) (mmHg) -12
Mean of pCO
2(mmHg)
20 30 40 50 60 70 80 90
Allowable difference (Total error)=5.7%
Mean difference
=-5.41 pCO
225 50 75 100 125 150 175 200 225 250
25 50 75 100 125 150 175 200 225 250
Mean of pO2-i-Smart 300 (mmHg)
Mean of pO2- STP CCX (mmHg) pO2 49
50 51 52 53 54 55 56 57 58
59
Fig. 1. (continued) 60
‐65.0
‐55.0
‐45.0
‐35.0
‐25.0
‐15.0
‐5.0 5.0 15.0 25.0 35.0 45.0 55.0 65.0
20 40 60 80 100 120 140 160 180 200 220 240
Difference (i-Smart 300 -STP CCX) (mmHg)
Mean of pO2(mmHg)
y = 1.08x – 3.00 r = 0.993
Mean difference
= 5.73 (A)
(B) pO2 Allowable difference
(Total error) = ±3SD
E 250
225 200 175 150 125 100 75 50 25 Mean of pO
2- i-Smart 300 (mmHg)
Mean of pO
2- Nova CCX (mmHg) 50 75 100 125 150 175 200 225 250
y=1.08x–3.00 r=0.993 pO
2F
Differ ence (i-Smart 300 - Nova CCX) (mmHg)
25 25
50 75 100 125 150 175 200 225 250
25 50 75 100 125 150 175 200 225 250
Mean of pO2-i-Smart 300 (mmHg)
Mean of pO2- STP CCX (mmHg) pO2
49 50 51 52 53 54 55 56 57 58
59
Fig. 1. (continued) 60
‐65.0
‐55.0
‐45.0
‐35.0
‐25.0
‐15.0
‐5.0 5.0 15.0 25.0 35.0 45.0 55.0 65.0
20 40 60 80 100 120 140 160 180 200 220 240
Difference (i-Smart 300 -STP CCX) (mmHg)
Mean of pO2(mmHg)
y = 1.08x – 3.00 r = 0.993
Mean difference
= 5.73 (A)
(B) pO2 Allowable difference
(Total error) = ±3SD
65 55 45 35 25 15 5 -5 -15 -25 -35 -45 -55 -65
Mean of pO
2(mmHg)
20 40 60 80 100 120 140 160 180 200 220 240
Allowable difference (Total error) = ±3SD
Mean difference
= 5.73 pO
2Fig. 1. Comparison of pH, pCO
2, pO2, Na+, K+, Cl-, Ca2+, and Hematocrit results tested by the i-Smart 300 and STP CCX for 40 samples. (A, C, E, G, I, K, M, O) Correlation between the i-Smart 300 and Nova CCX. Solid line, Passing-Bablok regression; dashed line, identity line. (B, D, F, H, J, L, N, P) Dif- ference between the i-Smart 300 and Nova CCX. Bold line, mean difference between values; dotted lines, desirable specification for allowable to-tal error. (continued to the next page)
from 0.02 to 2.50% and from 0.05 to 3.46%, respectively. All pa- rameters except Na
+, Cl
-, Ca
2+, and Hct fit the total desirable preci- sion criteria. Therefore, pH, pCO
2, pO
2, and K
+can be used for as a patient follow-up purposes [11]. Cl
-and Hct were among the four
parameters that did not meet the precision criteria, however, they met the minimum precision criteria, which is 75% of biological variation [16].
The i-Smart 300 showed excellent carry-over, all of the parame-
pH
이아람 외: Cartridge-Type Blood Gas Analyzer
https://doi.org/10.3343/lmo.2017.7.1.20 www.labmedonline.org 25
ters’ carry-over were within 1.0%, and values were similar to a conventional blood gas analyzer. The specific carry-over ranged between -0.04% and 0.05%. Typically, the goal is within 1%, there- fore, the carry-over of the i-Smart 300 is excellent [17]. All eight analytes showed excellent linearity within the acceptable recov- ery range. Moreover, correlation statistics between the i-Smart 300 and Nova CCX using Pearson’s correlation coefficients all were grea-
ter than 0.96, meaning high correlation.
According to the Bland-Altman plots, most analytes of the i-Smart 300 such as pCO
2, Na
+, K
+, Ca
2+, and Hct showed lower values com- pared to those measured by the Nova CCX.
Some of the limitations of our study are that the results of the Nova CCX, which we use as the standard, have not been verified as the true value.
I
27
‐0.50
‐0.40
‐0.30
‐0.20
‐0.10 0.00 0.10 0.20 0.30 0.40 0.50
2.5 3 3.5 4 4.5 5 5.5 6 6.5
Difference (i-Smart 300 -STP CCX)(mmol/L)
Mean of K +(mmol/L)
K+ Allowable
difference (Total error) = 5.61%
2.5 3 3.5 4 4.5 5 5.5 6 6.5 7
2.5 3 3.5 4 4.5 5 5.5 6 6.5 7
Mean of K+-i-Smart 300 (mmol/L)
Mean of K+- STP CCX (mmol/L) K+ 76
77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99
Fig. 1. (continued) 100
y = 0.88x +0.34 r = 0.996
Mean difference
= -0.128 (A)
(B)
7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 Mean of K
+- i-Smart 300 (mmol/L)
Mean of K
+- Nova CCX (mmol/L) 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0
y = 0.88x+0.34 r = 0.996 K
+J
27
‐0.50
‐0.40
‐0.30
‐0.20
‐0.10 0.00 0.10 0.20 0.30 0.40 0.50
2.5 3 3.5 4 4.5 5 5.5 6 6.5
Difference (i-Smart 300 -STP CCX)(mmol/L)
Mean of K +(mmol/L)
K+ Allowable
difference (Total error) = 5.61%
2.5 3 3.5 4 4.5 5 5.5 6 6.5 7
2.5 3 3.5 4 4.5 5 5.5 6 6.5 7
Mean of K+-i-Smart 300 (mmol/L)
Mean of K+- STP CCX (mmol/L) K+ 76
77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98
99
Fig. 1. (continued) 100
y = 0.88x +0.34 r = 0.996
Mean difference
= -0.128 (A)
(B)
0.5 0.4 0.3 0.2 0.1 0.0 -0.1 -0.2 -0.3 -0.4 Differ ence (i-Smart 300 - Nova CCX) (mmol/L) -0.5
Mean of K
+(mmol/L)
2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 Allowable difference (Total error)
= 5.61%
Mean difference
= -0.128 K
+Fig. 1. Continued.
2885 90 95 100 105 110 115 120
85 90 95 100 105 110 115 120
Mean of Cl--i-Smart 300 (mmol/L)
Mean of Cl-- STP CCX (mmol/L) Cl- 101
102 103 104 105 106 107 108 109 110 111 112 113 114
115
Fig. 1. (continued) 116
‐4.0
‐3.0
‐2.0
‐1.0 0.0 1.0 2.0 3.0 4.0
85 90 95 100 105 110 115 120
Difference (i-Smart 300 -STP CCX)(mmol/L)
Mean of Cl -(mmol/L)
Allowable difference (Total error)= 1.5%
y = 1.05x -4.17 r = 0.970
Mean difference
= 0.86 (A)
(B)
Cl-
28 85
90 95 100 105 110 115 120
85 90 95 100 105 110 115 120
Mean of Cl--i-Smart 300 (mmol/L)
Mean of Cl-- STP CCX (mmol/L) Cl- 101
102 103 104 105 106 107 108 109 110 111 112 113 114
115
Fig. 1. (continued) 116
‐4.0
‐3.0
‐2.0
‐1.0 0.0 1.0 2.0 3.0 4.0
85 90 95 100 105 110 115 120
Difference (i-Smart 300 -STP CCX)(mmol/L)
Mean of Cl -(mmol/L)
Allowable difference (Total error)= 1.5%
y = 1.05x -4.17 r = 0.970
Mean difference
= 0.86 (A)
(B)
Cl-
K 120
115 110 105 100 95 90 85
Mean of Cl- - i-Smart 300 (mmol/L)
Mean of Cl
-- Nova CCX (mmol/L) 85 90 95 100 105 110 115 120
y = 1.05x -4.17 r = 0.970 Cl
-L
Differ ence (i-Smart 300 - Nova CCX) (mmol/L)
4 3 2 1 0 -1 -2 -3 -4
Mean of Cl
-(mmol/L)
85 90 95 100 105 110 115 120 Allowable difference (Total error)
= 1.5%
Mean difference
= 0.86 Cl
-26 120
122.5 125 127.5 130 132.5 135 137.5 140 142.5 145
120 122.5 125 127.5 130 132.5 135 137.5 140 142.5 145 Mean of Na+-i-Smart 300 (mmol/L)
Mean of Na+- STP CCX (mmol/L) Na+ 61
62 63 64 65 66 67 68 69 70 71 72 73
74
Fig. 1. (continued) 75
‐4.0
‐3.0
‐2.0
‐1.0 0.0 1.0 2.0 3.0 4.0
122.5 125 127.5 130 132.5 135 137.5 140 142.5 145
Difference ( i-Smart 300 -STP CCX)(mmol/L)
Mean of Na +(mmol/L) Na+
Allowable difference (Total error)= 0.73%
y = 1.02x - 3.09 r = 0.966
Mean difference
= -1.01 (A)
(B)
G
26 120
122.5 125 127.5 130 132.5 135 137.5 140 142.5 145
120 122.5 125 127.5 130 132.5 135 137.5 140 142.5 145 Mean of Na+-i-Smart 300 (mmol/L)
Mean of Na+- STP CCX (mmol/L) Na+ 61
62 63 64 65 66 67 68 69 70 71 72 73
74
Fig. 1. (continued) 75
‐4.0
‐3.0
‐2.0
‐1.0 0.0 1.0 2.0 3.0 4.0
122.5 125 127.5 130 132.5 135 137.5 140 142.5 145
Difference ( i-Smart 300 -STP CCX)(mmol/L)
Mean of Na +(mmol/L) Na+
Allowable difference (Total error)= 0.73%
y = 1.02x - 3.09 r = 0.966
Mean difference
= -1.01 (A)
(B) 145.0 142.5 140.0 137.5 135.0 132.5 130.0 127.5 125.0 122.5 120
Mean of Na
+- i-Smart 300 (mmol/L)
Mean of Na
+- Nova CCX (mmol/L)
122.5 125.0 127.5 130.0 132.5 135.0 137.5 140.0 142.5 145
y = 1.02x - 3.09 r = 0.966 Na
+Differ ence (i-Smart 300 - Nova CCX) (mmol/L)
H 4
3 2 1 0 -1 -2 -3 -4
Mean of Na
+(mmol/L)
122.5 125 127.5 130 132.5 135 137.5 140 142.5 145 Mean difference
= -1.01 Allowable difference (Total error)
= 0.73%
Na
+이아람 외: Cartridge-Type Blood Gas Analyzer
29 0.6
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4
0.6 0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5 1.6
Mean of Ca2+-i-Smart 300 (mmol/L)
Mean of Ca2+- STP CCX (mmol/L) 120
121 122 123 124 125 126 127 128
129
Fig. 1. (continued) 130
131
‐0.18
‐0.14
‐0.10
‐0.06
‐0.02 0.02 0.06 0.10 0.14 0.18
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5
Mean of Cl -(mmol/L)
Allowable difference (Total error) = 2.0%
Ca2+
Difference (i-Smart 300 -STP CCX)(mmol/L)
y = 1.05x – 0.15 r = 0.975
Mean difference
= -0.091 (B)
M
29 0.6
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5 1.6
0.6 0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5 1.6
Mean of Ca2+-i-Smart 300 (mmol/L)
Mean of Ca2+- STP CCX (mmol/L) Ca2+
117 118 119 120 121 122 123 124 125 126 127 128
129
Fig. 1. (continued) 130
131
‐0.18
‐0.14
‐0.10
‐0.06
‐0.02 0.02 0.06 0.10 0.14 0.18
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5
Mean of Cl -(mmol/L)
Allowable difference (Total error) = 2.0%
Ca2+
Difference (i-Smart 300 -STP CCX)(mmol/L)
y = 1.05x – 0.15 r = 0.975
Mean difference
= -0.091 (A)
(B)
1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 Mean of Ca
2+- i-Smart 300 (mmol/L)
Mean of Ca
2+- Nova CCX (mmol/L) 0.6 0.7 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5 1.6
y = 1.05x – 0.15 r = 0.975 Ca
2+Differ ence (i-Smart 300 - Nova CCX) (mmol/L)
N 0.18
0.14 0.10 0.06 0.02 -0.02 -0.06 -0.10 -0.14 -0.18
Mean of Cl
-(mmol/L)
0.7 0.8 0.9 1.0 1.1 1.2 1.3 1.4 1.5 Allowable difference (Total error)
= 2.0%
Mean difference
= -0.091
O
30
‐7
‐6
‐5
‐4
‐3
‐2
‐1 0 1 2 3 4 5 6 7
20 25 30 35 40 45 50 55
Difference (i-Smart 300 -STP CCX) (%)
Mean of Hematocrit (%)
Allowable difference (Total error)=3.97%
20 25 30 35 40 45 50 55
20 25 30 35 40 45 50 55
Mean of Hematocrit -i-Smart 300 (%)
Mean of Hematocrit - STP CCX (%) Hematocrit 132
133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154
Fig. 1. (continued) 155
y = 1.07x - 5.08 r = 0.974
Mean difference
= -2.5 (B)
(A)
Hematocrit
55 50 45 40 35 30 25 20
Mean of Hematocrit - i-Smart 300 (%)
Mean of Hematocrit - Nova CCX (%) 20 25 30 35 40 45 50 55
y = 1.07x - 5.08 r = 0.974 Hematocrit
P
30
‐7
‐6
‐5
‐4
‐3
‐2
‐1 0 1 2 3 4 5 6 7
20 25 30 35 40 45 50 55
Difference (i-Smart 300 -STP CCX) (%)
Mean of Hematocrit (%)
Allowable difference (Total error)=3.97%
20 25 30 35 40 45 50 55
20 25 30 35 40 45 50 55
Mean of Hematocrit -i-Smart 300 (%)
Mean of Hematocrit - STP CCX (%) Hematocrit 132
133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154
Fig. 1. (continued) 155
y = 1.07x - 5.08 r = 0.974
Mean difference
= -2.5 (B)
(A)
Hematocrit
7 6 5 4 3 2 1 0 -1 -2 -3 -4 -5 -6 Differ ence (i-Smart 300 - Nova CCX) (%) -7
Mean of Hematocrit (%)
20 25 30 35 40 45 50 55 Allowable difference (Total error)=3.97%
Mean difference
= -2.5 Hematocrit
Fig. 1. Continued.
We also used samplers, which contain lyophilized heparin, so that we could avoid the dilution effect.
In conclusion, this study is the first evaluation of the newly laun- ched i-Smart 300 according to the CLSI guidelines. The i-Smart 300 showed excellent precision, linearity, carry-over, and inter- analyzer correlation, even though it is a portable, cartridge-type blood gas analyzer. There are advantages in terms of the conve- nience to users and the stability of the analyzer, so the i-Smart 300 can be utilized in various clinical settings, such as ICU, operating room, and emergency department as a blood gas analyzer.
요 약
배경: 혈액가스 분석은 의료 분야에 있어서 중요한 역할을 하고 있 고, 즉각적이고 정확한 분석은 즉각적인 치료를 통해 임상적 결과를 향상시킨다. 우리는 카트리지 형태의 혈액가스 분석 장비인 i-Smart 300의 분석능을 기존 혈액가스 분석 장비와 비교하여, Clinical and
Laboratory Standard Institute (CLSI) 지침에 따라 평가하였다.
방법: 정밀도는 CLSI EP5-A3에 따라 검증하였다. i-Smart 300은 다 음과 같은 8가지 지표에 대하여 Stat Profile Critical Care Xpress (STP CCX) (Nova CCX; Nova Biomedical, USA)와 비교 평가를 시 행하였다. : pH, partial carbon dioxide pressure (pCO
2), partial ox- ygen pressure (pO
2), sodium (Na
+), potassium (K
+), chloride (Cl
-), ionized calcium (Ca
2+), and hematocrit (Hct). 직선성은 CLSI EP6-A 에 근거하여 5가지 농도의 control 물질을 이용하여 판정하였다.
결과: 검사차례내 정밀도는 0.02–2.50%, 총 정밀도는 0.05–3.46%
로 낮은 변이계수를 보였다. 장비 간 비교에서 상관계수는 0.966–
0.996의 값을 보였다. 8가지 측정항목에서 우수한 직선성과 수용 할 수 있는 범위내의 기대값 대비 회수율을 보였다.
결론: 본 연구는 i-Smart 300의 CLSI 가이드라인에 근거한 최초의 분석능 평가라는 의의를 갖는다. i-Smart 300은 이동식 카트리지 형태의 혈액가스 분석 장비로서, 높은 정밀도와 기존 장비와의 좋 은 상관관계를 보였다. 그러므로 의료 환경 내에서 유용하게 쓰일 수 있을 것으로 생각된다.
Ca
2+이아람 외: Cartridge-Type Blood Gas Analyzer
https://doi.org/10.3343/lmo.2017.7.1.20 www.labmedonline.org 27
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTERESTS
No potential conflicts of interest relevant to this article were re- ported.
ACKNOWLEDGEMENTS
This study was supported by i-SENS.
REFERENCES
1. Uyanik M, Sertoglu E, Kayadibi H, Tapan S, Serdar MA, Bilgi C, et al.
Comparison of blood gas, electrolyte and metabolite results measured with two different blood gas analyzers and a core laboratory analyzer.
Scand J Clin Lab Invest 2015;75:97-105.
2. De Koninck AS, De Decker K, Van Bocxlaer J, Meeus P, Van Hoovels L. Analytical performance evaluation of four cartridge-type blood gas analyzers. Clin Chem Lab Med 2012;50:1083-91.
3. Einstein MH, Smith KM, Davis TE, Schmeler KM, Ferris DG, Savage AH, et al. Clinical evaluation of the cartridge-based GeneXpert human papillomavirus assay in women referred for colposcopy. J Clin Micro- biol 2014;52:2089-95.
4. Leino A and Kurvinen K. Interchangeability of blood gas, electrolyte and metabolite results measured with point-of-care, blood gas and core laboratory analyzers. Clin Chem Lab Med 2011;49:1187-91.
5. CLSI. Blood Gas and pH Analysis and Related Measurements; Approved Guideline—Second Edition. CLSI document C46-A2. Wayne, PA: Clin- ical and Laboratory Standards Institute, 2009.
6. CLSI. Evaluation of Precision of Quantitative Measurement Procedures;
Approved Guideline—Third Edition. CLSI document EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute, 2014.
7. CLSI. Preliminary evaluation of quantitative clinical laboratory mea- surement procedures; Approved Guideline—Third Edition. CLSI doc- ument EP10-A3. Wayne, PA: Clinical and Laboratory Standards Insti-
tute, 2006.
8. CLSI. Evaluation of the Linearity of Quantitative Measurement Proce- dures: A Statistical Approach; Approved Guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute, 2003.
9. CLSI. Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline—Third Edition. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute, 2013.
10. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and prog- ress. Scand J Clin Lab Invest 1999;59:491-500.
11. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Desirable specification for total error, imprecision, and bias, de- rived from intra-and inter-individual biologic variation. http://www.
westgard.com/biodatabase1.htm (updated for 2014).
12. Nichols JH, Christenson RH, Clarke W, Gronowski A, Hammett-Stabler CA, Jacobs E, et al. Executive summary. The National Academy of Clin- ical Biochemistry Laboratory Medicine Practice Guideline: evidence- based practice for point-of-care testing. Clin Chim Acta 2007;379:14- 28.
13. Fraser CG and Harris EK. Generation and application of data on bio- logical variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409- 37.
14. Cotlove E, Harris EK, Williams GZ. Biological and analytic components of variation in long-term studies of serum constituents in normal sub- jects. 3. Physiological and medical implications. Clin Chem 1970;16:
1028-32.
15. Harris EK. Statistical principles underlying analytic goal-setting in clin- ical chemistry. Am J Clin Pathol 1979;72(2 Suppl):374-82.
16. Fraser CG. General strategies to set quality specifications for reliabil- ity performance characteristics. Scand J Clin Lab Invest 1999;59:487- 90.
17. Seok YM, Lee WH, Yoon SY, Won YC, Kwon OH. Evaluation of the i- Smart 30 Point-of-care analyzer for use in clinical laboratory settings. J Lab Med Qual Assur 2011:33;25-30.