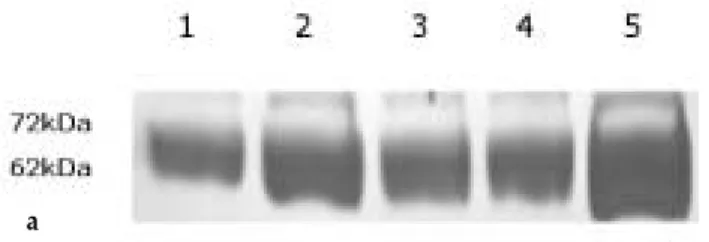
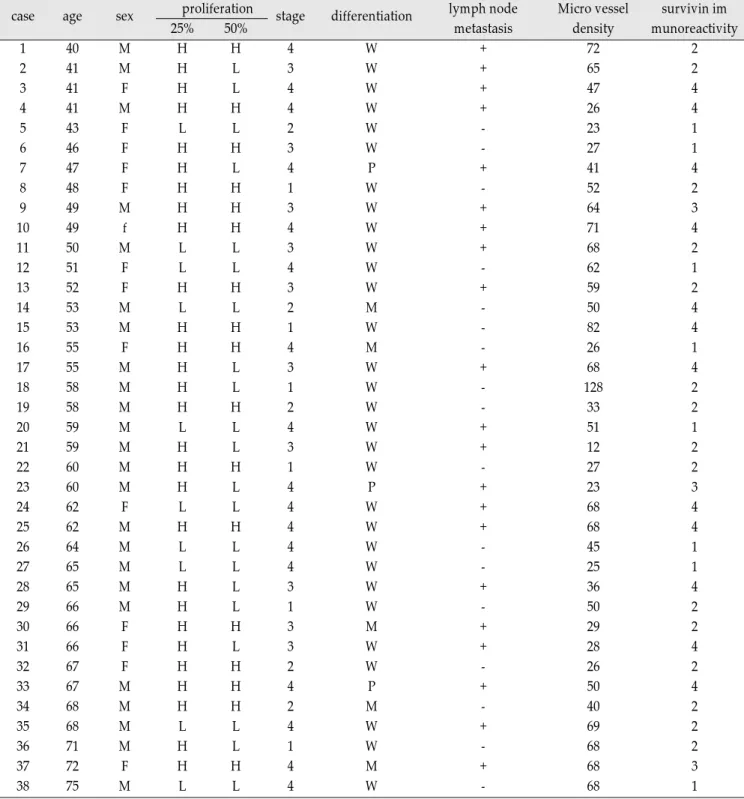
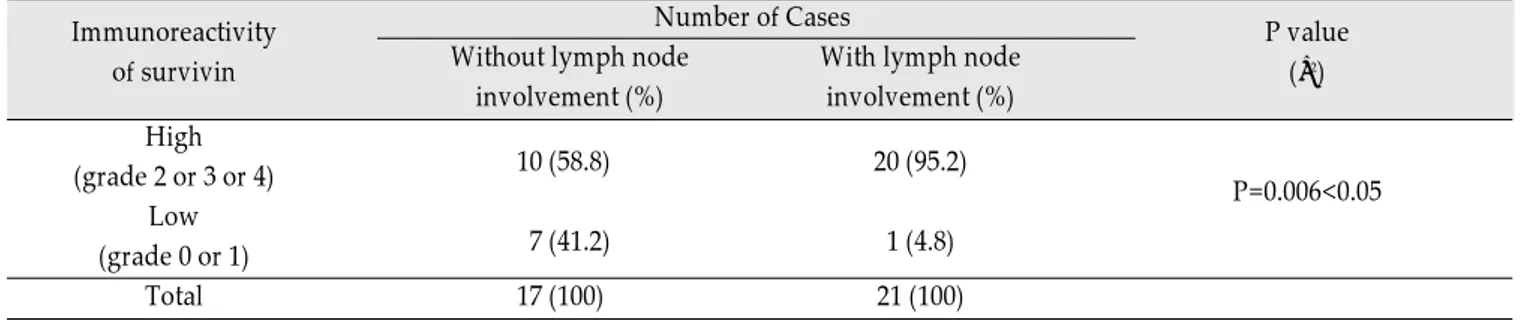
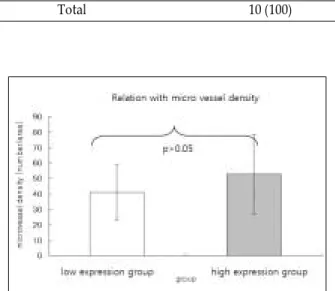
Survivin, Possible Marker and Prognostic Factor in Oral Squamous Cell Carcinomas
전체 글
수치
관련 문서
Examination of such materials led to the conclusion that Pak Chi-wŏn originally intended to have the five texts including “ Yangmaesihwa” in Yŏrha ilgi
The proposal of the cell theory as the birth of contemporary cell biology Microscopic studies of plant tissues by Schleiden and of animal tissues by Microscopic studies of
The index is calculated with the latest 5-year auction data of 400 selected Classic, Modern, and Contemporary Chinese painting artists from major auction houses..
In the present paper, L-shaped curves are general- ized and it is shown that every knot in the minor subfamilies, called sporadic examples of Berge’s lens space surgery, is
Concentration-Dependent inhibition of cell viability by adenosine in human oral fibroblast and FaDu human head and neck squamous cell carcinoma · · ·
The most important factor to level-differentiated instruction was to give attraction to student and let them have motivation themselves, and after finishing
Taylor Series
Objective: The purpose of this study was to analyze recent trend in incidence of basal cell carcinoma and squamous cell carcinoma in patients from the Gwangju City