Agric. Chem. Biotechnol. 46(3), 122-123 (2003)
Short Communication
Partial Purification and
Characterization of an Angiotensin- Converting Enzyme Inhibitor from Squid Ink
So-Youn Kim, Sun-Hye Kim and Kyung Bin Song*
Department of Food Science and Technology, College of Agriculture, Chungnam National University, Daejeon, 305-764, Korea Received June 13, 2003; Accepted August 4, 2003
Key words : ACE inhibitor, isolation, squid ink.
Among biologically-active molecules, the angiotensin- converting enzyme (ACE) inhibitory peptides have been extensively studied.1,2) ACE converts angiotensin I into angiotensin II by cleaving the C-terminal dipeptide (His-Leu) of angiotensin I and also inactivates bradykinin which depresses blood pressure. Thus, the ACE inhibitor acts on the inhibition of ACE, which results in a decrease in blood pressure. It was screened from various food sources.1) Since the discovery of an ACE inhibitory peptide in snake venom, many ACE inhibitory peptides have been identified from the enzymatic hydrolysates of various food sources. 3-6)
Squid ink is obtained as a waste product of squid product processing. A peptidoglycan that is isolated from squid ink has been reported to have anti-tumor activity7). Therefore, to further elucidate other biological functions of squid ink and to produce a high value-added product from the squid waste, its anti-hypertensive property was examined and an ACE inhibitor was isolated and purified from squid ink.
Fresh squid (Sepia esculenta) was purchased in Mookho, Korea. Crude squid ink (50 mL) was filtered through a cheese cloth and followed by centrifugation at 12,000× g for 30 min.
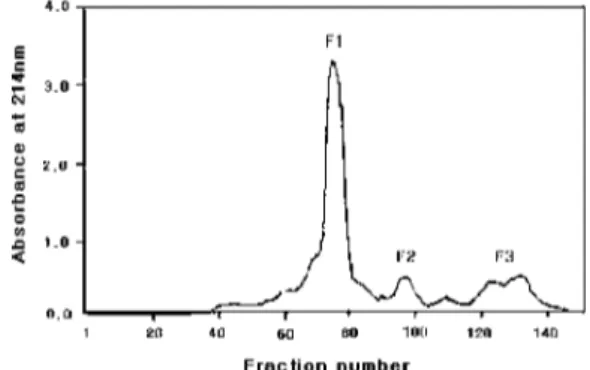
Squid ink was then filtered using a YM-3 (MW 3,000 cut-off) membrane. The membrane-filtered solution was loaded onto Sephadex G-15 (1.5× 100 cm) that had been equilibrated with a 10 mM phosphate buffer (pH 7.0). The eluate was monitored by measuring the absorbance at 214 nm. Three fractions were obtained from the column (Fig. 1). The ACE inhibitory activity of each fraction was measured by the method of Cushman and Cheung8) with modifications that were established in our laboratory. 9-11) The reaction mixture contained 150µl of 5 mM Hip-His-Leu as a substrate, 50 µl of rabbit lung ACE powder (5 munit) in a 50 mM sodium borate buffer (pH 8.3), and 50µl of the sample solution. The
reaction was carried out at 37oC for 30 min, and terminated by adding 250µl of 1 N HCl and 1 ml of ethylacetate. After centrifugation, the absorbance of the supernatants was measured at 228 nm. The IC50 value was defined as the amount of the ACE inhibitor concentration that was needed to inhibit 50% of the ACE activity.
The ACE assay result showed that the F1 fraction had the highest inhibitory activity (32%). Therefore, the fraction was pooled and loaded onto the normal phase HPLC (Thermo Separation Products Inc., FL, USA) with an amino column (4.6× 250 mm, Capcell pak NH2, Shiseido Co., Tokyo, Japan). Elution was performed on the A solvent (0.1%
trifluroacetic acid and 0.1% triethylamine in acetonirile: water (97 : 3, v/v) and B solvent (0.1% trifluroacetic acid and 0.1%
triethylamine in acetonirile: water (30 : 70, v/v) conditions, having a gradient of 0% of B to 55% at 0.5 ml · min−1. The F1 fraction was separated into three fractions by HPLC (Fig. 2).
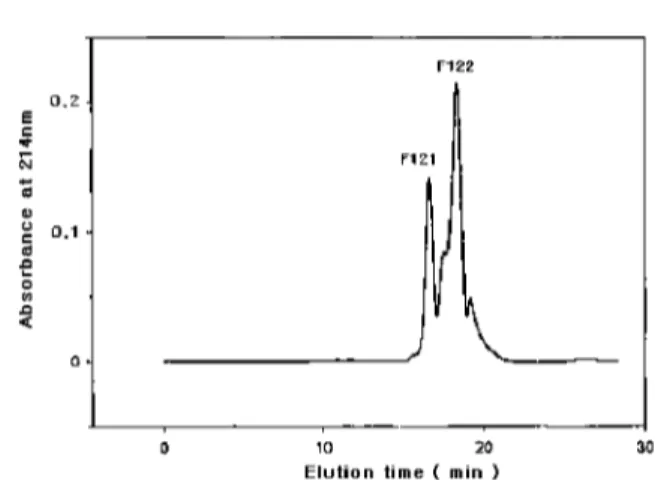
Among them, the F12 fraction had the highest inhibitory activity (36%). The F12 fraction was further purified using FPLC with a Superdex peptide HR 10/30 (10× 300 mm, Amersham Pharmacia Co., Uppsala, Sweden) that was
*Corresponding author
Phone: 82-42-821-6723; Fax: 82-42-825-2664 E-mail: kbsong@cnu.ac.kr
Fig. 1. Elution profile of crude extracts of squid ink on a GPC.
Fig. 2. Elution profile of fraction F1 on a normal phase HPLC.
ACE inhibitor from Squid Ink 123
equilibrated with 0.1% trifluroacetic acid (TFA) in acetonitrile: water (30 : 70, v/v). There were two fractions (Fig. 3). The F122 fraction had the highest inhibitory activity (65%). Since this fraction was homogeneous, based on re- chromatography, its molecular mass was determined using a mass spectrometer (JMS HX-110A, Jeol, Tokyo, Japan). The F122 fraction was identified as having a molecular mass of 294 (Fig. 4) and had 4.9µg · ml−1 as the IC50 value. The ACE inhibitor appears to be a peptide derivative since Edman degradation was unsuccessful in determining the amino acid sequence of the inhibitor. An additional NMR study is needed to identify the inhibitor.
This is the first report regarding the isolation of an ACE
inhibitor from squid ink. Although the chemical nature of the inhibitor should be further characterized, this small molecular- weight inhibitor is quite promising in terms of manufacturing a functional food product using squid ink. Further characterization of the inhibitor, and the development of the processing of a functional product, is currently being studied.
References
1. Ariyoshi, Y. (1993) Angiotensin-converting enzyme inhibi- tors derived from food proteins. Trends Food Sci. & Tech- nol., 4, 139-145.
2. Yamamoto, N. (1997) Antihypertensive peptides derived from food proteins. Biopoly., 43, 129-134.
3. Oshima, G., Shimabukuro, H. and Nagasawa, K. (1979) Peptide inhibitors of angiotensin I-converting enzyme in digests of gelatin by bacterial collagenase. Biochem. Bio- phys. Acta, 566, 128-137.
4. Matsumura, N., Fujii, M., Takeda, Y. and Shimizu, T.
(1993) Angiotensin I-converting enzyme inhibitory peptides derived from bonito bowels autolysate. Biosci. Biotech. Bio- chem., 57, 695-697.
5. Abubakar, A., Saito, T., Kitazawa, H., Kawai, Y. and Itoh, T. (1998) Structural analysis of new antihypertensive pep- tides derived from cheese whey protein by proteinase K digestion. J. Dairy Sci., 81, 3131-3138.
6. Wu, J. and Ding, X. (2001) Hypotensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. J. Agric. Food Chem., 49, 501-506.
7. Sasaki, J., Ishita, K., Takaya, Y., Uchisawa, H. and Matsue, H. (1997) Anti-tumor activity of squid ink. J. Nutr. Sci.
Vitaminol., 43, 455-61.
8. Cushman, D. W. and Cheung, H. S. (1971) Spectrophoto- metric assay and properties of the ACE of rabbit lung. Bio- chem. Pharmacol., 20, 1637-1638.
9. Park, E., Cho, Y. and Song K. B. (1998) Isolation of angio- tensin converting enzyme inhibitory peptide from beef bone extract hydrolysate. Agric. Chem. Biotech., 41, 270-272.
10. Noh, H. and Song, K. B. (2001) Isolation of an angio- tensin converting enzyme inhibitor from Oenanthe javanica.
Agric. Chem. Biotechnol., 44, 98-99.
11. Kim, J., Jung, H. and Song, K. B. (2002) Isolation of angiotensin converting enzyme inhibitor from Compositae plants. Nutraceut. Food, 7, 157-161.
Fig. 3. Elution profile of fraction F12 on a FPLC.
Fig. 4. Mass spectrum of fraction F122 isolated from squid ink.