Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
전체 글
수치
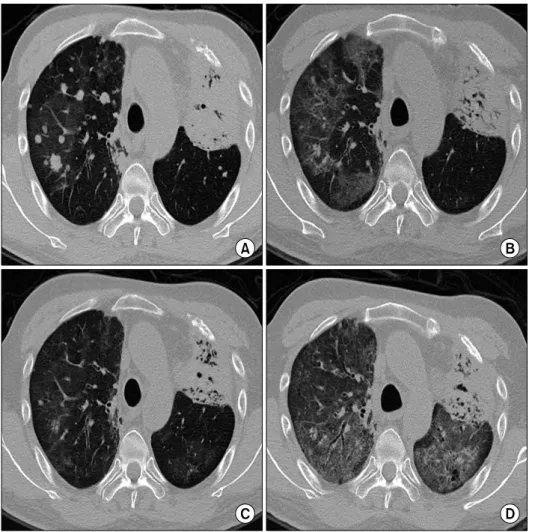
관련 문서
Patients with breast cancer; ovarian cancer; renal cell carcinoma; pancreatic neuroendocrine cancer; colorectal cancer; head and neck cancer; non-small cell lung
According to the result of this study, to do effective nursing that improve health promoting behaviors of early-stage lung cancer patients, nurses should
This paper is a case study on how Venture and Small-Business Executives managers can take advantage of their intuitions in situations where the
Beichel,“Automated 3-D segmentation of lungs with lung cancer in CT data using a novel robust active shape model approach,” IEEE Trans.. 저작물의
In the present study we investigated the proliferation effect of human oral cancer KB cell treated with pulsatilla koreana extract.. We analyzed the effects of this
In the present study, consistent to that report, up-regulation of VEGF mRNA was observed in the colon cancer cell with the acquired resistance to 5-FU and this
Anti‑cyclic citrullinated peptide antibody testing is used to diagnose rheumatoid arthritis and associated with interstitial lung disease in RA.. Herein, we
Background: Incidence of complications related extracorporeal membrane oxygenation (ECMO) support as a bridge to lung transplantation (BTT) and its association with the