Clinical Pediatric Hematology-Oncology
Volume 20ㆍNumber 1ㆍApril 2013CASE REPORT
71
초중증재생불량빈혈 소아에서 진단된 유두형갑상샘암 1예
편종석ㆍ최혜윤ㆍ한승범ㆍ이재욱ㆍ정낙균ㆍ정대철ㆍ조 빈ㆍ강진한ㆍ김학기
가톨릭대학교 의과대학 소아과학교실
Papillary Thyroid Cancer in a Child with Very Severe Aplastic Anemia
Jong-Seok Pyeon, M.D., Hye-Yoon Choi, M.D., Seung Beom Han, M.D., Jae Wook Lee, M.D.,
Nack-Gyun Chung, M.D., Ph.D., Dae-Chul Jeong, M.D., Bin Cho, M.D., Jin Han Kang, M.D. and Hack-Ki Kim, M.D.
Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
The concurrent occurrence of both aplastic anemia and thyroid cancer in a child is a very rare event. Although cancer may occur in patients with aplastic anemia who pre- viously received immunosuppressive therapy or hematopoietic stem cell transplantation (HSCT), cancer in patients who are naive to such therapies is rare. We report a 12-year-old patient with idiopathic very severe aplastic anemia who was subsequently diagnosed with papillary thyroid cancer. This patient had a unique clinical presentation in that thyroid cancer was diagnosed prior to hematopoietic stem cell transplantation given to treat very severe aplastic anemia.
pISSN 2233-5250 / eISSN 2233-4580 Clin Pediatr Hematol Oncol 2013;20:71∼74
Received on February 7, 2013 Revised on March 23, 2013 Accepted on April 18, 2013
Corresponding author: Jae Wook Lee
Department of Pediatrics, College of Medicine, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul 137-701, Korea Tel: +82-2-2258-6192
Fax: +82-2-537-4544
E-mail: dashwood@catholic.ac.kr
Key Words: Thyroid cancer, Aplastic anemia, Child
Introduction
Hematologic diseases, such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), may develop in patients with inherited forms of aplastic anemia (AA) [1].
Fanconi anemia (FA), which is one of the inherited bone marrow failure syndromes, may result aplastic anemia dur- ing childhood. Various solid tumors, such as those of the head and neck, gastrointestinal tract, female genital tract, breast, lung, liver and brain tumors, as well as hematologic malignancies may develop in association with FA due to chromosomal instability [2]. In patients with idiopathic AA,
the relationship between cancer and AA is less clear, al-
though treatment for AA, such as immunosuppressive ther-
apy (IST) or hematopoietic stem cell transplantation (HSCT),
may lead to secondary cancer in some patients [3]. Thyroid
cancer may be caused by irradiation, as well as genetic mu-
tations that have been implicated in familial thyroid cancer
[4]. Although the incidence of thyroid cancer has been in-
creasing in adolescents and adults [5], thyroid cancer in pa-
tients with AA has rarely been reported. Here, we report
a 12-year-old boy with very severe aplastic anemia (VSAA)
who was diagnosed with papillary thyroid cancer (PTC)
soon after initial presentation with neutropenic fever.
Jong-Seok Pyeon, et al
72 Vol. 20, No. 1, April 2013
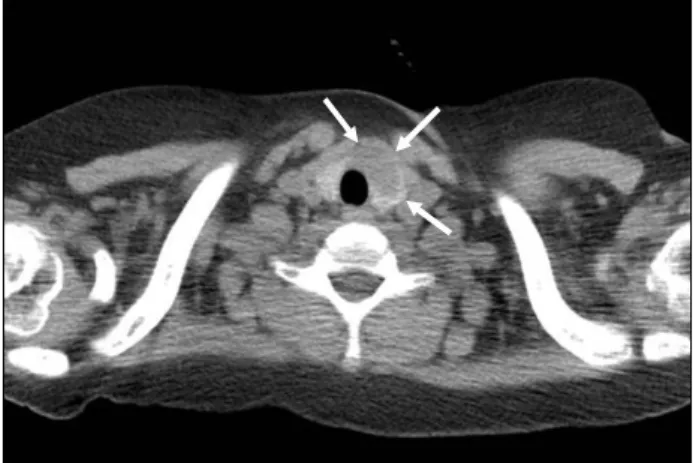
Fig. 1. Chest computed tomogram revealed an incidental thyroid
mass sized 2.5×1.3 cm (arrows) in the left lobe of the thyroid gland.Case Report
A 12-year-old boy with VSAA, who was scheduled to re- ceive HSCT, was admitted to our hospital because of fever and painful swelling of his right upper thigh. He had been diagnosed with VSAA 2 months prior to admission, and his complete blood count (CBC) at the time of diagnosis showed the following: white blood cell (WBC) count 2,220/μL (neutrophils 23%, lymphocytes 73%, monocytes 1%, eosinophils 3%), absolute neutrophil count (ANC) 510/μL, hemoglobin 10.7 g/dL, platelet count 5,000/μL, and corrected reticulocyte count 0.20%. Bone marrow study at the time revealed 10% hypocellularity with no features such as dyspoiesis or myelofibrosis. His bone marrow karyotype was 46, XY, and chromosomal breakage test was normal.
Flow cytometric analysis for paroxysmal nocturnal hemo- globinuria was negative. Follow-up CBCs showed a de- crease in ANC that failed to surpass 100/μL.
On admission, he had a fever of 38.2
oC, and his blood pressure was 100/60 mmHg, heart rate 98 beats/min, and respiratory rate 20 breaths/min. His right upper thigh was swollen with erythema and local heat, and a bulla was ob- served on his right thigh. The rest of the physical examina- tion was unremarkable. Laboratory findings showed a WBC count of 610/μL (neutrophils 0%, lymphocytes 100%), he- moglobin 6.7 g/dL, platelet count 28,000/μL, and C-reactive protein 19.96 mg/dL (normal range: <0.5 mg/dL). Magnetic resonance imaging (MRI) of his right thigh showed skin and soft tissue infection encircling the right upper thigh, a 3×2 cm sized abscess in the right inguinal area, and several en- larged inguinal lymph nodes.
He was treated with broad spectrum antibiotics for skin and soft tissue infection of his thigh considering his persis- tent neutropenia. After three weeks of antibiotic therapy, the patient no longer complained of pain in his right thigh and the size of the infected lesion decreased. However, his neutropenic fever continued. His chest X-ray showed no abnormality, but a further study with chest computed to- mography (CT) done for fever work-up revealed a 2.5×1.3 cm sized mass in the left lobe of the thyroid gland (Fig.
1). An ultrasound-guided fine needle aspiration of the thy-
roid mass was performed, and the pathologic examination revealed PTC. Thyroid function test was normal and thyroid autoantibodies were negative.
Priority was given to treatment of VSAA through HSCT before treatment of PTC. On hospital day 62, he received haploidentical peripheral blood stem cell transplantation from his father whilst continuing to show febrile neutropenia. After HSCT, full engraftment was achieved with complete donor chimerism. Debridement and closure using local flap was done for the necrotic skin lesion of his right thigh one month after HSCT. Total thyroidectomy and central lymph node dissection for PTC was done five months after HSCT.
The tumor size was 2.4×2.3 cm grossly, and pathologic ex- amination revealed PTC with extrathyroidal extension and lymph node metastasis to Level VI (Fig. 2).
Discussion
AA is characterized by pancytopenia in peripheral blood,
and hypocellularity in bone marrow. A key diagnostic ques-
tion in patients with bone marrow failure is differentiating
patients with an inherited disorder, such as patients with
FA, from those without a genetic etiology. In the majority
of patients, a positive chromosomal breakage test, sup-
ported by pertinent clinical findings, allow for the diagnosis
of FA. In rare situations, the breakage test may be negative
in patients with hematopoietic reversion, that is, the sponta-
neous correction of an FA allele [6]. In our patient, a clearly
Papillary Thyroid Cancer in a Very Severe Aplastic Anemia
Clin Pediatr Hematol Oncol
73
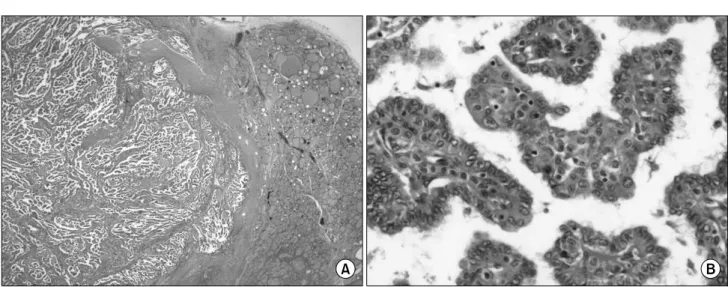
Fig. 2. Microscopic findings of the excised thyroid mass. (A) A huge mass occupied most of the left lobe of the thyroid gland.
(Hematoxylin and Eosin stain; original magnification 12.5×), (B) Papillary structures with enlarged epithelial cell proliferation were seen (Hematoxylin and Eosin stain; original magnification 400×).
negative breakage test and a normal phenotype allowed for the diagnosis of non-inherited VSAA.
Idiopathic AA is assumed to be caused by the destruction of immature hematopoietic stem cells by cytotoxic T cells and autoantibodies [7]. Idiopathic AA may be treated by IST or HSCT, and solid tumors, such as head and neck cancer, stomach cancer, hepatocellular carcinoma, and breast can- cer, as well as hematologic malignancies, such as acute leu- kemia and lymphoma, may occur after treatment [3].
Hematologic malignancies are assumed to develop as a re- sult of the lymphoproliferation triggered by ongoing anti- genic stimulation by an autoimmune mechanism, or by clo- nal evolution after IST, but how solid tumors develop in patients with idiopathic AA has not been well defined [1,7].
Relatively short telomeres are found in patients with dysker- atosis congenita (DC), which is one of the inherited bone marrow failure syndromes. Short telomeres are also found in some patients with idiopathic AA [8]. Telomeres shorten after each cell division, and eventually aged cells with short telomeres undergo apoptosis [8]. If cell proliferation con- tinues with a short telomere, chromosomal instability is trig- gered and malignancies may develop [8]. While mutations in genes coding telomerase, an enzyme that maintains telo- mere length, are found in patients with DC, patients with idiopathic AA may have short telomeres without such ge-
netic mutations [9]. Short telomeres are also found in pa- tients with various types of solid tumors including thyroid cancer, as well as in patients with AA, and thus, short telo- meres may have a role in oncogenesis [10,11]. However, the contribution of telomere dysfunction in the develop- ment of solid tumors in patients with AA requires further study.
Thyroid cancer is related to irradiation, and accordingly the incidence is higher in cancer patients who received ra- diation therapy [4]. Although genetic mutations of the RET proto-oncogene and BRAF gene may be found in familial thyroid cancer [4], the relationship between such genetic mutations and AA has not been reported. Improvements in diagnostic modalities have aided in the detection of thyroid cancer in recent decades [5]. Considering the increasing in- cidence of thyroid cancer, reports on thyroid cancers in pa- tients with AA are also expected to increase even though there is no defined causative relationship between AA and thyroid cancer.
The prognosis of thyroid cancer is dependent on patient age, gender, tumor size and histology, extrathyroidal ex- tension, lymph node metastasis, and distant metastasis [12].
In our patient, favorable prognostic factors included patho-
logically confirmed PTC, young age at diagnosis, a solitary
mass less than 4.5 cm, and having received total thyroi-
Jong-Seok Pyeon, et al
74 Vol. 20, No. 1, April 2013