저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Protective immunity in mice immunized
with the Nfa1 protein for pathogenic
Naegleria fowleri infection
by
Yang Jin Lee
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Protective immunity in mice immunized
with the Nfa1 protein for pathogenic
Naegleria fowleri infection
by
Yang-Jin Lee
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Ph.D. in Medicine
Supervised by
Ho-Joon Shin, Ph.D.,
Sun Park, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Yang Jin Lee is approved.
SUPERVISORY COMMITTEE
Kyongmin Kim
Ho-Joon Shin
Sun Park
Myung-Hee Kwon
Tai-Soon Yong
The Graduate School, Ajou University
December, 22th, 2008
- ABSTRACT -
Protective Immunity in Mice Immunized with the Nfa1 Protein
for Pathogenic Naegleria fowleri Infection
Pathogenic Naegleria fowleri causes a fatal disease, called as primary amoebic menigoencephalitis, in humans and experimental animals. In the present study, to examine the protective immunity of the Nfa1 protein for N. fowleri infection in a mouse model, BALB/c mice were immunized with the Nfa1 protein either by intraperitoneal or intranasal route, and then infected intranasally with N. fowleri trophozoites. In regardless of the immunization routes, Nfa1-immunized mice showed the prolonged mean time to death. The specific immunoglobulin G (IgG), IgG subclass, IgA and IgE of mice induced against Nfa1 protein immunization was measured by ELISA. The levels of IgG and IgA were significantly increased in sera obtained from mice immunized with rNfa1 protein. Analysis of IgG subclass profiles revealed that IgG1 showed the greatest increasing followed by IgG2b, IgG2a and IgG3. In contrast, IgE level displayed low level of IgE similar to non-immunized mice. In addition, splenocytes of mice immunized with Nfa1 protein secreted significantly high levels of both gamma-interferon and interleukin-10 after stimulation with rNfa1 protein. These findings suggest that the rNfa1 protein may induce the humoral and cell-mediated immunity leading to the host defense in N. fowleri infection.
Key words: Naegleria fowleri, primary amoebic menigoencephalitis, Nfa1 protein, protective immunity
TABLE OF CONTENTS
ABSTRACT ···ⅰ TABLE OF CONTENTS ···ⅱ LIST OF FIGURES ···ⅴ LIST OF TABLES ···ⅶ ABBREVIATION ···ⅷ Ⅰ. INTRODUCTION ···1 A. Background ···1 1. Free-living amoebae ···1 2. Naegleri fowleri ···2(A) Life cycle, morphology, growth and culture ···3
(B) Pathogenicity ···4
(C) Primary amoebic meningoencephalitis ···5
(D) Symptoms ···7
(E) Diagnosis ···7
(F) Treatment ···8
(G) Immunity ···9
B. Introduction of Naegleria fowleri nfa1gene ··· 12
Ⅱ. MATERIALS AND METHODS ···14
A. Culture of Naegleria fowleri trophozoites ···14
B. Mice ···14
C. Expression and purification of the recombinant His-tag Nfa1 protein ···15
D. Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting ···16
E. Immunization ···17
1. Intraperitoneal immunization ···17
2. Intranasal immunization ···18
F. Infection of Naegleria fowleri trophozoites ···18
G. Histopathological examination of mice brain tissue infected with N. fowleri trophozoites ···19
H. Serum collection ···19
I. Antibody detection by Enzyme-linked immunosorbent Assay (ELISA) ···20
J. Spleen cell culture ···21
K. Cytokines assay ···22
L. Duration of the Nfa1- specific IgG levels in immune sera ···22
. RESULTS Ⅲ ···24
A. Expression and purification of the recombinant His-tag Nfa1 protein ···24
C. Histopathological examination of mice brain tissue infected with N. fowleri
trophozoites ···29
D. Serum antibody responses to recombinant Nfa1 protein ···31
E. Cytokine responses to recombinant Nfa1 protein ··· 38
F. Duration of the Nfa1-specific IgG levels in immunized mice ···41
. DISCUSS Ⅳ ION ···43 . CONCLUSION Ⅴ ···48 REFERENCES ···50 국문요약 ···60
LIST OF FIGURES
Fig. 1. SDS-PAGE and Western blotting of the purified Nfa1 protein ···25
Fig. 2. The survival rate in immune mice post infection of N. fowleri trophozoites ··· 27
Fig. 3. Histologic findings of mice brain tissues post infection of N. fowleri trophozoites ···30
Fig. 4. The Nfa1-specific IgG levels in immune mice sera ··· 33
Fig. 5. Levels of IgG subclass specific the Nfa1 protein in immune mice sera ···34
Fig. 6. Levels of IgG subclass specific the Nfa1 protein in immune mice sera ···35
Fig. 7. The Nfa1-specific IgA levels in immune mice sera ···36
Fig. 8. The Nfa1-specific IgE levels in immune mice sera ···37
Fig. 9. Production of IL-2 and IFN-γ from splenocytes of immune mice stimulated with the rNfa1 protein ··· 39
Fig. 10. Production of IL-4 and IL-10 from splenocytes of immune mice stimulated
with the rNfa1 protein ···40
LIST OF TABLE
Table 1. Mortality of mice infected with N. fowleri trophozoites after they were immunized intraperitoneally with the rNfa1 protein in adjuvant or
ABBREVIATION
AP: Alkaline Phosphatase
BCIP/NBT: 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium
BSA: Bovine Serum Albumin
CNS: Central Nervous System
ELISA: Enzyme-Linked Immunosorbent Assay
FBS: Fetal Bovine Serum
IFN-
γ: Interferon gamma
IL-2: Interleukin-2
IL-4: Interleukin-4
IL-10: Interleukin-10
I.N.: Intranasally
I.P.: Intraperitoneally
IPTG: Isopropylthiogalactoside
OD: Optical Density
PAGE: Polyacrylamide gel electrophoresis
PAME: Primary Amoebic Meningoencephalitis
PBS: Phosphate Buffered Saline
p-NPP: p-Nitrophenyl Phosphate
SDS: Sodium dodecyl sulfate
. INTRODUCTION
Ⅰ
Ⅰ
Ⅰ
Ⅰ
A. Background 1. Free-living amoebaeThe free-living amoebae include the genera Naegleria, Acanthamoeba, Balamuthia, and Sappinia, are preadapted for a pseudoparasitic and potentially pathogenic life style. These organisms have been called amphizoic amoebae in recognition of their ability to live endozoically, yet they are capable of free-living existence (Page, 1974).
It was due to the prescient observations of Culbertson that the pathogenic potential of free-living amoebae was first realized (Culbertson et al., 1958). Cytopathology produced in monkey kidney tissue cultures used for growing poliovirus was shown to be caused by Acanthamoeba and not by a simian virus as originally thought (Culbertson, 1971). Culbertson later confirmed that, when inoculated into mice or monkeys, the amoebae killed the animals. The first human case of primary amoebic meningoencephalitis was reported from Australia, but the etiologic agent, first thought to be Acanthamoeba, was later identified as Naegleria (Fowler and Carter, 1965). Not long after, however, the first human infection by Acanthamoeba was described (Jager and Stamm, 1972). Acanthamoeba infections were characterized as opportunistic infections by Martinez, who recognized their occurrence in debilitated or chronically ill patients, and who distinguished between the pathologies caused by Acanthamoeba and Naegleria (Martinez, 1980). Keratitis cases caused by Acanthamoeba
were diagnosed in the United Kingdom and in the United States, respectively (Naginton et al., 1974; Jones et al., 1975). The first recognized case of a Balamuthia infection was identified in a baboon that died in a zoological park, but the amoeba was detected in humans soon after (Visvesvara et al., 1990; Visvesvara et al., 1993).
2. Naegleri fowleri
Naegleria fowleri is the causal agent of primary amoebic meningoencephalitis (PAME). Naegleria spp. are amoeboflagellates found in water and soil. Although, some 30 species of Naegleria have been recognized based upon sequencing data (De Jonckheere, 2004). N. fowleri is the only one that has been isolated from cases of primary amoebic meningoencephalitis. Other species (Naegleria italica, Naegleria philippinensis, Naegleria australiensis) may be pathogenic in the mouse model of PAME, but have not been identified from any human cases. Because, it grows best at somewhat elevated temperatures, the amoeba has been isolated from warm-water bodies including ponds and lakes, hot springs, and thermally polluted streams and rivers. N. fowleri is thermotolerant, being able to survive temperatures up to 45℃, preadapting the species to mammalian body temperature. Indeed, incubation at 45℃ is routinely used to isolate the amoeba from water samples, while suppressing growth of other amoebae in the samples (De Jonckheere and van de Voorde, 1977b). But thermotolerance is not the sole factor determining pathogencity of Naegleria spp. Naegleria Lovaniensis is a thermotolerant species, but is non-pathogenic in the mouse model for PAME (De Jonckheere and van de Voorde, 1977a; Stevens et al., 1980).
PAME is a fulminating disease, developing within several days following exposure to the water source, and causing death within 1~2 weeks after hospitalization. The ability of N. fowleri to produce such a rapidly fatal infection has encouraged search for virulence properties of the amoeba that might account for its destructiveness. Among candidates that might serve as virulence factor are the release of the enzymes phospholipase (Cursons et al., 1978), or neuraminidase (Eisen and Franson, 1987), the creation of pores in target cell membranes that may have a lytic effect (Young and Lowery, 1989) and aggressive phagocytotic activity (Brown, 1979). The amoeboid stage forms food cups that are capable of pinching off bits of target cell cytoplasm (Brown, 1979; John et al., 1985).
(A) Life cycle, morphology, growth and culture
There are both trophozoite and cyst stages in the life cycle, with the stage primarily depending on environmental conditions. Trophozoites can be found in water or moist soil and can be maintained in tissue culture or other artificial media.
The trophozoites can occur in two forms, amoeboid and flagellate. Mortility can be observed in hanging-drop preparations from cultures of cerebrospinal fluid (CSF); the amoeboid form is elongate with a broad anterior end and tapered posterior end. The size ranges from 7 to 35 ㎛. The diameter of the rounded forms is usually 15 ㎛. There is a large, central karyosome and no peripheral nuclear chromatin. The cytoplasm is somewhat granular and contains vacuoles. The amoeboid form organisms change to the transient, pear-shaped flagellate form when they are transferred from culture or teased from tissue into water and
maintained a temperature of 27℃ to 37℃. The change may occur very quickly or may take as long as 20 h. The flagellate form has two flagella at the brad end. Mortility is typical, with either spinning or jerky movements. These flagellate forms do not divide, but when the flagella are lost, the amoeboid forms resume reproduction.
Cysts form nature and from agar cultures look the same and have a single nucleus almost identical to that seen in the trophozoite. They are generally round, measuring from 7 to 15 ㎛, and there is a thick double wall.
In nature and in the laboratory, the amoeba feeds actively on bacteria. Isolations of N. fowleri from environmental water and soil samples are accomplished by growth on non-nutrient agar plates coated with E. coli at 45℃ (Lares-Villa et al., 1993). Other bacteria, such as Enterobacter aerogenes or Klebsiella pneumoniae, may also be used. Once established in vitro, the amoebae can also grown in an axenic medium following elimination of bacteria by antibiotic treatment of the culture (Schuster, 2002).
(B) Pathogenicity
The pathogenesis of PAME is poorly understood. Both pathogenic and nonpathogenic species of Naegleria have been isolated from the environment, but the determinants of virulence and pathogenicity are unknown. As with many pathogenic organisms, prolonged growth of N. fowleri in axenic culture in vitro results in attenuation of virulence, while serial passage through mice restores and maintains virulence (Wong et al., 1977; Whiteman and Marciano-Cabral, 1987).
Adherence of pathogens to host cells is a critical initial step in the infection process. The ability of trophozoites to attach to the nasal mucosal, an increased rate of locomotion, and a chemotactic response to nerve cell components may be important in disease progression (Cline et al., 1986; Brinkley and Marciano-Cabral, 1992; Han et al., 2004). A variety of in vitro cell culture systems have been used to study the infection of N. fowleri with mammalian cells. N. fowleri trophozoites have been shown to destroy nerve cells, as well as other cell types, by trogocytosis using food-cup structure on their surface (Brown, 1979; Marciano-Cabral et al., 1982; Marciano-Cabral and Fulford, 1986) and by the release of cytolytic molecules (Lowery and McLaughlin, 1984, 1985; Marciano-Cabral and Fulford, 1986; Leippe and Herbst, 2004). The mode that is applied to destroy target cells in vitro, however, is dependent on the amoeba strain. For example, weakly pathogenic strains destroy nerve cells by ingestion using the food-cup structure, while highly pathogenic strains lyse nerve cells on contact and subsequently ingest the cell debris that is generated (Marciano-Cabral et al., 1982; Marciano-Cabral, 1988).
(C) Primary amoebic meningoencephalitis
Once entering into the nostrils of swimmers, others engaging in water sports, and the nasal cavity by inhalation or aspiration of dust, water, or aerosols containing the trophozoites or cysts. N. fowleri penetrates the mucosal epithelial layer and migrates along the olfactory nerve tracts, crossing the cribriform plate, to the brain. The cribriform plate in children is more porous than in adults, another possible reason for the higher incidence of PAME in
young persons. Because of their proximity to the point of entrance of amoebae into the central nervous system (CNS), the frontal and olfactory lobes of the brain are the initial targets of amoebic destruction. Other areas affected are the base of the brain, the brainstem, and the cerebellum (Martinez, 1985). Amoebae are found in large numbers in the perivascular regions in brain tissue. A purulent exudates containing trophic amoebae can be found in the subarachnoid space of the meninges. Involvement of the meninges is the basis for the distinction made between PAME and Acanthamoeba and Balamuthia enceephalitides, which are typically granulomatous amoebic encephalitides. PAME can resemble acute purulent bacterial meningitis, and these conditions may be difficult to differentiate, particularly in the early stages. The CSF may have a predominantly polymorphonuclear leukocytosis, increased protein concentrations, and decreased glucose concentration like those seen with bacterial meningitis. Unfortunately, if the CSF Gram stain is interpreted incorrectly, the resulting antibacterial therapy has no impact on the amoebae and patient will usually die within several days.
Extensive tissue damage occurs along the path of amoebic invasion; nasopharyngeal mucosa shows ulceration, and the olfactory nerves are inflamed and necrotic. Hemorrhagic necrosis is concentrated in the region of the olfactory bulbs and the base of the brain. Organisms can be found in the meninges, perivascular spaces, and sanguinopurulent exudates.
(D) Symptoms
Primary amoebic meningoncephalitis caused by N. fowleri is an acute, supperative infection of the brain and meninges. With extremely rare exceptions, the disease is rapidly fatal in humans. The period between contact with the organism and onset of clinical symptoms such as headache, fever, and rhinitis may vary from 2 to 3 days to as long as 7 to 15 days. Early symptoms include vague upper respiratory distress, lethargy, headache, and occasionally olfactory problems. The acute phase includes sore throat, stuffy blocked or discharging nose, and severe headache. Progressive symptoms include pyrexia, vomiting, and stiffness of the neck. Mental confusion and coma usually occur approximately 3 to 5 days prior to death. The cause of death is usually cardiorespiratory arrest and pulmonary edema.
(E) Diagnosis
Rapid diagnosis is by microscopic examination, preferably phase-contrast, of freshly drawn CSF, to visualize motile amoebae. Suspending amoebae in 1 ml of distilled water can further confirm the identity of the amoebae as Naegleria, by watching for development of actively swimming flagellates (Visvesvara, 1999). Further corroboration can be obtained by isolation of the amoeba from CNS or macerated brain tissue on a non-nutrient agar plate that has been spread with a lawn of E. coli, and incubated overnight at 37℃. The amoebae will grow out in large numbers, feeding on the bacteria. Because this is an acute disease, early
diagnosis is essential in order that appropriate antimicrobial therapy may be initiated before the amoebae do extensive damage. Diagnosis may be delayed when amoebae in CSF are mistaken for leukocyte being sluggish in motion, while N. fowleri move relatively swiftly with a distinctive ectoplasmic pseudopod.
(F) Treatment
Many antiparasitic and antimicrobial drugs have been screened for in vitro and in vivo activity against N. fowleri. Naegleria spp. are highly sensitive to the antifungal drug amphotericin B, and it has been used in virtually all cases as the core antimicrobial where recovery occurred. Minimum amoebacidal concentrations of amphotericin B were determined to be 0.02∼0.078 ㎍/ml for three different clinical isolates of N. fowleri tested in vitro (Duma et al., 1971). Ultrastructural examination of amoebae treated with amphotericin B revealed membrane distortions, including the nuclear envelope, rough and smooth endoplasmic reticula, and plasma membrane blebbing (Schuster and Rechthand, 1975).
Although N. fowleri is very sensitive to amphotericin B in vitro, only a few patients have recovered after receiving intravenous injections and intrathecal of this drug alone or in combination with miconazole (Schuster and Visvesvara, 2004).
Naegleria infections have also been treated successfully with amphotericin B, rifampin, and chloramphenicol; amphotericin B, oral rifampin, and oral ketoconazole; and amphotericin B alone (Abramowicz, 2004; Schuster and Visvesvara, 2004).
The variables in determining the survival of PAME cases are how early the diagnosis is made and treatment initiated, the infectious dose of amoebae, the virulence of the infecting strain and the health of the patient. It is of interest to note that the N. fowleri strain isolated from this patient appeared to be less virulent than other clinical isolates when tested for cytopathogenicity in monkey cell cultures (John and John, 1989).
The macrolide antibiotic azithromycin is effective against Naegleria in vitro and in the mouse model, but it is reported to penetrate poorly into the CSF (Schuster et al., 2001; Goswick and Brenner, 2003a). Other antimicrobials that have been tested, mostly in vitro, are clotrimazole, itraconazole, fluconazole, and ketoconazole, with varying degrees of efficacy. Differences in reported drug sensitivity are due to the use of different N. fowleri strains in different laboratories, which show variation to drugs. Amphotericin B, however, remains the drug of choice for PAME treatment.
(G) Immunity
N. fowleri are among the protozoa that humans are exposed to in the course of their lives, either through direct contact with water or soil, or by wind-blown cysts that might lodge in the nasal mucosa. In a study of selected groups in Czechoslovakia (students, psychiatric patients), referred to earlier, percentages of individuals giving positive antibody responses for N. fowleri ranged from 1 to 4% (Cerva, 1989). Much higher percentages of positive responses were found in serum samples of hosptitalised patients in the United States in which antibodies of the IgG and IgM classes were detected to N. fowleri and the
non-pathogenic N. lovaniensis. Titters ranged from 1:20 to 1:640. IgG, but not IgM antibodies were detected in sera from newborn infants, supporting evidence for cross-placental transfer (Dubray et al., 1987). Antibodies against Naegleria were found in a variety of wild mammals including raccoons, some rodents and opposums, mirroring the presence of Naegleria antibodies in human population (Kollars and Wilhelm, 1996).
Sera obtained from wild animals showed lytic activity attributable to complement, since heat treatment destroyed amoebacidal activity (John and Smith, 1997). Of various species tested, amoebacidal activity was strongest in sera from raccoon, muskrat and bullfrog, all species closely associated with aquatic environments. Given the close association of wild animals with water and soil, it is not surprising that protective immune agents should be encountered.
Immunofluorescence staining for Naegleria antibody in PAME patients is not very effective as a diagnostic tool. The reason, of course, is that there is insufficient time for a humoral antibody reaction to occur. Differences in surface antigens exist among Naegleria spp. N. fowleri and N. lovaniensis share similar antigens (Marciano-Cabral et al., 1987).
The humoral immune response to N. fowleri has been studied in experimental animals and humans. Serum samples from healthy individuals from the United States (Reill et al., 1983a; Marciano-Cabral et al., 1987), New Zealand (Cursons et al., 1977, 1980a), and the Czech Republic (Cerva, 1989) have been examined for antibodies to N. fowleri. Although the antibody titers recorded have differed from study to study, almost all human sera from healthy individuals have been found to be positive for N. fowleri, indicating that exposure to the amoeba is common. Naegleria spp. Antibodies to N. fowleri have also been detected in
101 of 115 randomly obtained serum samples from hospitalized patients in the United States (Dubray et al., 1987). Using immunoblot analysis, the examined serum samples from army recruits with acute respiratory disease for antibodies to free-living amoebae (Powell et al., 1994).
A number of studies have been conducted to determine the role of antibody by idiotype in host resistance to infection with Naegleria. It survey to detect IgA antibodies in the serum and saliva of individuals living in Mexico in areas where Naegleria is endemic as compared with those where it is nonendemic (Rivera et al., 2001). The serum obtained before death from a patient with PAME, which showed no increase in specific antibody titers by indirect immunofluorescent antibody assay, revealed very low levels of IgA upon radial-immunodiffusion testing for quantitation of serum IgG, IgA and IgM (Cursons et al., 1979). In contrast, the serum obtained before death from a patient with PAME and noted that the levels of serum immunoglobulins, including those of IgA, were within normal limits (Cain et al., 1979). Using immunofluorescence testing, it was reported an antibody titer of 1:4096 after 42 days of infection in a patient who survived PAME following treatment by intravenous and intraventricular administration of amphotericin B (Seidel et al., 1982). Circulating IgG antibodies in serum were demonstrated by ELISA from day 7 after infection in ICR mice infected with N. fowleri (Park et al., 1987b) and from day 14 after infection in ICR mice infected with N. jadini, but no anti-N.gruberi antibody was detected (Lee et al., 1985).
B. Introduction of Naegleria fowleri nfa1 gene
In an attempt to identify antigen- or pathogen-related molecules with a potential for use as diagnostic agents or immunogens in pathogenic N fowleri infections, we previously cloned an antigenic gene (nfa1) from an N. fowleri cDNA library by means of immunoscreening using infected and immune mouse sera. This gene had a 360 bp coding nucleotide sequence and expressed a recombinant Nfa1 protein (rNfa1) with a molecular weight of 13.1 kDa (Shin et al., 2001). For immunocytochemical analysis of the properties of this protein, an anti-Nfa1 monoclonal antibody using cell fusion techniques has been used to demonstrate pseudopodia- and food cup-specific localization of the Nfa1 protein (Lee et al., 2007). Also, anti-Nfa1 antibody treatment reduced the cytotoxicity of N. fowleri for Chinese hamster ovary (CHO) cells in a dose-dependent manner, supporting the notion that the nfa1 gene may be related to the pathogenicity of N fowleri (Jeong et al., 2004).
In spite of the widespread use amoebae to understand pathogenicity of N. fowleri, the role of afresh cloned Nfa1 protein has not been fully carried out regarding the presence of antigen- or pathogen-related protein and their functions in N. fowleri.
C. Purpose
To examine the protective immunity in mice immunized with the rNfa1 protein for pathogenic N. fowleri infection, in the present study, the immunogenicity of the rNfa1 protein and host protective efficacy were evaluated. Mice were intraperitoneally or
intranasally immunized with the rNfa1 protein, and survival rate and mean time to death of immunized mice were observed to evaluate the host protective immunity against lethal challenge of N. fowleri trophozoites in vivo. In addition, host immune response was evaluated by analyzing the Nfa1-specific IgG, IgG subclass, IgA and IgE antibody induction against the rNfa1 protein in mice bloods as well as by measuring the level of cytokines ( γ-IFN, IL-2, IL-10 and IL-4) in mice splenocytes.
Ⅱ
Ⅱ
Ⅱ
Ⅱ. MATERIALS AND METHODS
A. Culture of Naegleria fowleri trophozoites
The trophozoites of N. fowleri (Carter NF69 strain; American Type Culture Collection [ATCC] No. 30215) were cultured at 37℃ in an axenic Nelson's medium supplemented with 10% fetal bovine serum (FBS) (Willaert, 1971).
To assure consistent pathogenicity of N. fowleri trophozoites, amoebae were cultured after at least tow continuous passages in mice. The trophozoites of N. fowleri were harvested during the logarithmic phase of growth at 48h. After centrifugation at 1,500 × g for 3 min, trophozoites of N. fowleri were washed three times with sterile phosphate-buffered saline (PBS) and counted by using a hemocytometer.
B. Mice
Female specific pathogen-free BALB/c mice of 6 weeks of age, weighing 18 g, were purchased from Samtaco (O San, Korea). The animals were housed in groups of three per cage, and kept in conventional animal facilities, where they received water and food ad libitum. The animals were under a natural light and dark cycle of 12 h/12 h, relative humidity of 50~60% and ambient temperatures of 20 ± 1℃. The animals were acclimated for 1 weeks prior before the start of the experiment. Approval for animal experiments was obtained from
the institutional animal welfare committee.
C. Expression and purification of the recombinant His-tag Nfa1 protein
The rNfa1 protein was produced by inducing the expression of nfa1 and purifying the recombinant His-tag Nfa1 (rNfa1) protein according to the method of previous study (Lee et al., 2007). Purified DNA (5 µg/µl) obtained from a PCR-T7/NT TOPO expression vector (Invitrogen; Grohingen, Netherlands) and containing the nfa1 gene was subsequently transferred to the BL21(DE3)pLysS Escherichia coli strain using the heat-shock method. The clones were selected by growing the cultures at 37℃ on Luria-Bertani (LB) agar containing 100 µg/mL of ampicillin and 34 µg/mL of chloramphenicol (LAC). A transformed colony was selected and inoculated into 1 ℓ LB medium cotaining 100 µg/mL of ampicillin and 34
µg/mL of chloramphenicol (LAC) incubated at 37℃ with aeration. The transformed colony
were grown to an optical density at 600 nm (OD600) of 0.5~0.8 at 37℃, 250 rpm.
Isopropyl-β-D-thiogalactoside (IPTG) was added to LB medium to a final concentration of 1 mM, and
the cultures were grown for an additional 4 h at 37℃, 250 rpm. The E. coli cells were harvested by centrifugation at 6,000 × g for 15 min. The pellets were resuspended in the buffer consisting of 2 mM imidazole, 200 mM NaCl, and 50 mM Tris-Cl (pH 7.5). The E. coli cells were lysed by freezing-thawing method and sonication. Soluble and insoluble fractions were separated by centrifugation at 10,000 × g for 10 min. The rNfa1 protein lysates were analysed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and the presence of the expressed gene product an rNfa1 protein was confirmed by
Western blotting using immune serum.
The rNfa1 protein was purified by metal affinity chromatography using a Ni-NTA agarose column (Qiagen Inc, Hilden, Germany). Elutions were carried out using imidazole buffer (5 M urea, 20 mM Na2HPO4, 5 M NaCl, and 500 mM imidazole). The protein was
dialyzed in phosphate-buffered saline (PBS) for 24 h with buffer changes every 8 h. The rNfa1 protein purity was evaluated by SDS-PAGE.
D. Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot
SDS-PAGE was performed as described by Laemmli (Laemmli, 1970). The samples were mixed sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer containing 2-mercaptoethanol, boiled for 5 min, and loaded onto a 15% polyacrylamide gels. The rNfa1 protein lysates and purified were migrated in SDS-PAGE gels, and the protein bands were stained with Coomassie brilliant blue (Amersham Biosciences Co., Piscataway, NJ, USA) for 12 h. And then, it was destained with destaining solution (10% glacial acetic acid, 60% distilled water, 30% Methanol) for 4 h.
The rNfa1 protein lysates separated by SDS-PAGE were transferred to nitrocellulose membranes (Amersham Biosciences) at 250 mA for one and half an hour in transfer buffer (25 mM sodium phosphate, pH 7.5). The nitrocellulose membranes were blocked with Phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) at 4℃ overnight, and washed three times with Tris-buffered saline containing 0.05% Tween 20
(TBST; 0.5 M NaCl, 0.02 M Tris [pH 7.5], 0.05% Tween 20). The nitrocellulose membranes were reacted with mouse anti-recombinant His-tag Nfa1 protein serum for 2 h at room temperature. After reaction with the primary antibody, the nitrocellulose membranes were washed three times with TBST and incubated for 1 h at room temperature with alkaline phosphatase-conjugated goat anti mouse immunoglobulin G (IgG) (Sigma) as a secondary antibody. After three times wash with TBST, the proteins bound to the secondary antibody were developed following incubation at room temperature with 5-bromo-4-chloro-3-indoly-1-phoaphate and nitroblue tetrazolium chloride (BCIP/NBT) (Sigma).
E. Immunization
1. Intraperitoneal immunization
For intraperitoneally immunization, female BALB/c mice (6 weeks old, 18 g, purchased from Samtaco) were randomly divided into four experimental groups of each 60 animals. Four groups; Group I, Normal (PBS); Group II, Adjuvant+PBS; Group III, rNfa1+Adjuvant; Group IV, rNfa1 only. Group II mice were intraperitoneally immunized with Freund’s complete adjuvant (sigma) mixed with PBS in a total volume of 200 ㎕. The mice were boosted with same dose of Freund’s incomplete adjuvant mixed with PBS at 2 weeks after the primary immunization. A final booster of same adjuvant mixed with PBS was given at 2 weeks after the second immunization. Group III mice were intraperitoneally immunized with 50 ㎍ of rNfa1 protein mixed with Freund’s complete adjuvant (sigma) in a total volume of
200 ㎕. The mice were boosted with 25 ㎍ of rNfa1 protein mixed with Freund’s incomplete adjuvant at 2 weeks after the primary immunization. A final booster of 25 ㎍ of rNfa1 protein mixed with Freund’s incomplete adjuvant was given at 2 weeks after the second immunization. Group IV mice were immunized with rNfa1 protein only using the same dose and same route as Group III.
2. Intranasal immunization
For intranasally immunization, female BALB/c mice (6 weeks old, 18 g, purchased from Samtaco) were randomly divided into two experimental groups of each 60 animals. Two groups; Group Ⅴ, Normal (PBS); Group Ⅵ, rNfa1 only. Mice were anesthetized with a mixture of 5 ㎎/ml of ketamine, 0.5 ㎎/ml of rompun and saline. In group Ⅴ as a control, saline was slowly instilled into the nostril of each mouse in a 20 ㎕. Group Ⅵ mice were intranasally immunized with 50 ㎍ of rNfa1 protein mixed with PBS in a total volume of 20
㎕. The mice were boosted with 25 ㎍ of rNfa1 protein mixed with PBS at 2 weeks after
the primary immunization. A final booster of 25 ㎍ of rNfa1 protein mixed with PBS was given at 2 weeks after the second immunization.
F. Infection of Naegleria fowleri trophozoites
In each group, two weeks after the final immunization, fifty-one mice were used for infection studies. The trophozoites of N. fowleri were harvested by centrifugation at 1,500 ×
g for 3 min and washed three times with sterile PBS. The trophozoites of N. fowleri were then resuspended in sterile PBS and counted by using a hemocytometer. The suspensions were adjusted to concentrations of 1 × 104 trophozoites/20 ㎕. Mice were lightly anesthetized with a mixture of 5 ㎎/ml of ketamine, 0.5 ㎎/ml of rompun and saline. The inoculum was slowly instilled into the nostril of each mouse in a 20 ㎕. Mice were infected intranasally with 1 × 104 trophozoites suspended in 20 ㎕ sterile PBS. The dates of disease onset and death were recorded for each mouse. The mice were observed at least twice a day for everyday for survival rate and mean time to death.
G. Histopathological examination of mice brain tissue infected with N. fowleri trophozoites
Tissue samples from the brains of dead mice were fixed in 10% neutral-buffered formalin (4% formaldehyde in phosphate buffered saline). The brains were then carefully dissected and representative fragments of brain tissue were embedded in paraffin. Thick sections of 5 ㎛ were cut on albuminized glass slides. The sections were stained with haematoxylin and eosin for light microscopic analysis.
H. Serum collection
Bloods were collected from immunized mice at two weeks after the third immunization. Sera containing the anti-Nfa1 polyclonal antibody were collected from the mice bloods by
centrifugation at 2,500 × g for 30 min at 4℃. Immune sera were used for antibody titer analysis and Western blotting.
I. Antibody detection by Enzyme-linked immunosorbent Assay (ELISA)
In each group, nine mice were used for antibody detection. Sera were tested for the presence of the Nfa1-specific IgG, IgG subclass (IgG1, IgG2a, IgG2b and IgG3) ,IgA and Ig E by ELISA. Briefly, The experiments were performed using 96-well ELISA plates (Nunc, Roskilde, Denmark) in 100 ㎕/well reaction, with the rNfa1 protein at 2 ㎍/ml mixed with coating buffer (0.05 M carbonate-bicarbonate, pH 9.6). The plates were incubated at 4℃ for 12 h and washed with PBS containing 0.05% Tween 20 (PBST) three times, and blocked with 3% bovine serum albumin (Sigma) in PBS at 4℃ for 2 h. After the plates were washed three times with PBST, serum samples were diluted in PBS and added to each wells. After the incubation at 37℃ for 2 h, the wells were washed three times with PBST, and 100 ㎕ of alkaline phosphatase-conjugated goat anti-mouse IgE (Bethyl Laboratories, Montgomery, Tex) or 100 ㎕ of horse-radish peroxidase (HRP)–conjugated goat anti-mouse IgG or IgA or IgG subclass antibodies (Bethyl Laboratories, Montgomery, Tex) diluted 1:10,000 in PBS were added to the wells. The plates were incubated at 37℃ for 1 h and washed three times with PBST. Detection of antibody was performed at 37℃ with 100 ㎕ of 2,2' – azinobis (3-ethylbenzthiazoline-6-sulfonic acid) substrate solution (ABTS; Kirkegaard and Laboratories, Gaithersburg, MD), and the coloring reaction was terminated with 100 ㎕ of 1% SDS. The plates were read at 405 nm in a microplate reader (Bio-Rad).
J. Spleen cell culture
Spleens were removed under aseptically conditions from immunized mice two weeks after the third immunization. Spleens were cut into small pieces. Single cell suspensions were prepared by mashing the tissue through sterile metal screens. Cells were suspended in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco BRL, Life Technologies, Grand Island, N.Y.) supplemented with 2 mM L–glutamine, 100 U of penicillin/ml, and 100
㎍ of streptomycin/ml. Cell suspensions were prepared after lysis of the erythrocytes (red
blood cell lysing buffer; Sigma Chemical Co, St. Louis, MO, USA). After the suspended cells were washed three times with PBS by centrifugation at 1,500 × g for 30 min at 4℃ each, cells were resuspended in complete RPMI 1640 medium containing 10% fetal bovine serum, 25 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), 2 mM L– glutamine, 100 U of penicillin/ml, and 100 ㎍ of streptomycin/ml, 1 mM nonessential amino acids, 50 uM 2-mercaptoethanol and 1 mM sodium pyruvate. Cell viability was determined by trypan-blue dye exclusion. The splenocytes were counted and seeded out on 24-well tissue culture plates in a final volume 1 ml per well at a final concentration of 4 × 106 cells. For cytokine production, the splenocytes were stimulated in vitro alone or with the rNfa1 protein at 10 ㎍/ml. The culture were incubated at 37℃ in a humidified atmosphere with 5% CO2. After 72 h, the splenocytes culture supernatants were harvested for
K. Cytokines assay
Cytokine produced by activated splenocytes were measured by standard sandwich ELISA using purified anti-γ-IFN, IL-2, IL-10 and IL-4 as capture antibody and the corresponding biotinylated antibody as reporter antibody according to manufacturer's instructions (Biosource International, California). The concentrations of primary capture antibody and biotinylated reporter antibody were used according to protocol. Purified recombinant γ-IFN, IL-2, IL-10 and IL-4 were used as standards (Biosource International, California). Tetramethylbenzidine (TMB) microwell peroxidase was used as substrate and the reaction was terminated by 1 M H3PO4. Absorbance of wells was read at 450 nm in a
microplate reader (Bio-Rad).
L. Duration of the Nfa1- specific IgG levels in immune sera
For intraperitoneally immunization, female BALB/c mice (6 weeks old, 18 g, purchased from Samtaco) were randomly divided into tow experimental groups of each 3 animals. Two groups; Group Ⅶ, Normal (PBS); Group Ⅷ, rNfa1+Adjuvant. Group Ⅷ mice were intraperitoneally immunized with 50 ㎍ of the rNfa1 protein mixed with Freund’s complete adjuvant (sigma) in a total volume of 200 ㎕. The mice were boosted with 25 ㎍ of the rNfa1 protein mixed with Freund’s incomplete adjuvant at 2 weeks after the primary immunization. A final booster of 25 ㎍ of the rNfa1 protein mixed with Freund’s incomplete adjuvant was given at 2 weeks after the second immunization.
For intranasally immunization, female BALB/c mice (6 weeks old, 18 g, purchased from Samtaco) were randomly divided into two experimental groups of each 3 animals. Two groups; Group Ⅸ, Normal (PBS); Group Ⅹ, rNfa1 only. Mice were anesthetized with a mixture of 5 ㎎/ml of ketamine, 0.5 ㎎/ml of rompun and saline. In group Ⅸ as a control, saline was slowly instilled into the nostril of each mouse in a 20 ㎕. Group Ⅹ mice were intranasally immunized with 50 ㎍ of the rNfa1 protein mixed with PBS in a total volume of 20 ㎕. The mice were boosted with 25 ㎍ of the rNfa1 protein mixed with PBS at 2 weeks after the primary immunization. A final booster of 25 ㎍ of the rNfa1 protein mixed with PBS was given at 2 weeks after the second immunization.
The Nfa1-specific IgG responses were evaluated in each group at 2, 6, 12 weeks following immunization at two weeks after the third immunization.
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS
A. Expression and purification of the recombinant His-tag Nfa1 protein
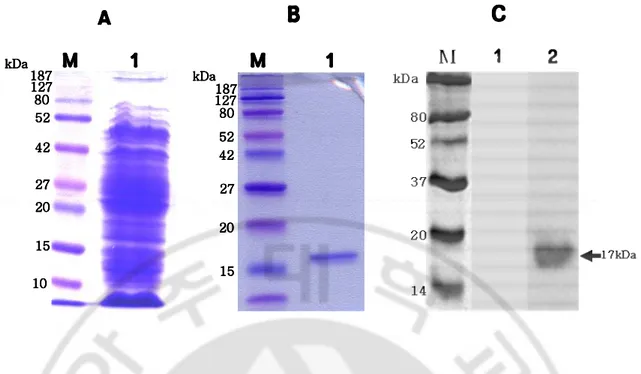
The recombinant His-tag Nfa1 protein was purified by metal affinity chromatography using a Ni-NTA agarose column. The lysates of transformed E. coli BL21 strain and the purified recombinant His-tag Nfa1 protein were migrated in the SDS-PAGE gel, and the staining results demonstrated the recombinant His-tag Nfa1 protein as a 17 kDa band (Fig. 1A, 1B). Additionally, the recombinant His-tag Nfa1 protein was confirmed in Western blotting using normal mouse serum or immune serum against the Nfa1 protein (Fig. 1C, 2C).
Fig. 1. SDS-PAGE and Western blotting of the purified Nfa1 protein. Lane 1 (A) shows a fractionated E. coli lysate by SDS-PAGE. Lane 1 (B) and lane 2 (C) show a purified recombinant His-tag fusion Nfa1 protein by SDS-PAGE and Western blotting, respectively. Lane 1(C) is reacted with normal serum of healthy mouse. Arrow indicates a 17 kDa molecular weight. M, prestained marker.
B
B
B
B
15 1515 15 20 20 20 20 27 2727 27 kDa kDa kDa kDa 42 42 42 42 52 52 52 52 80 80 80 80 127 127 127 127 187 187 187 1871
1
1
1
M
M
M
M
kDa kDa kDa kDa 10 10 10 10 15 15 15 15 20 20 20 20 27 27 27 27 42 42 42 42 52 52 52 52 80 80 80 80 127 127 127 127 187 187 187 187
A
A
A
A
M
M
M
M
1
1
1
1
C
C
C
C
B. Survival rate and mean time to death of mice
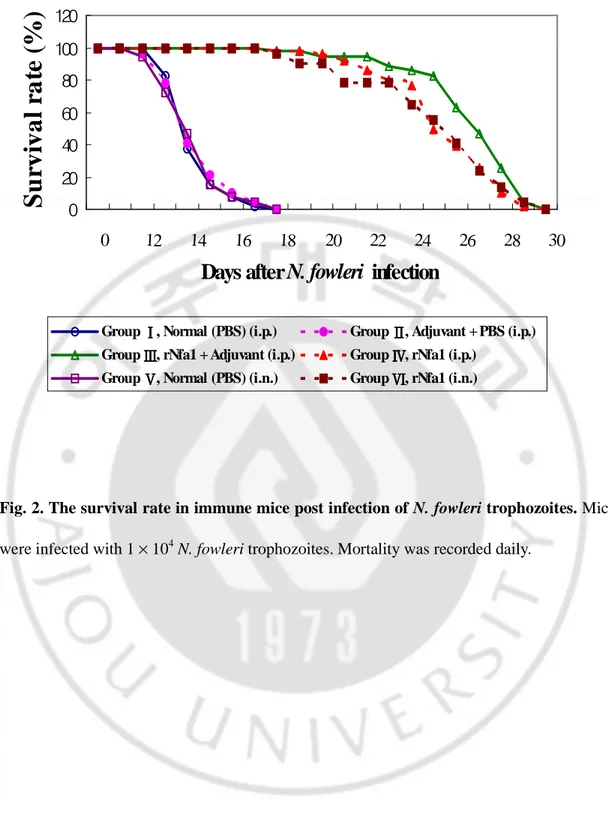
In order to determine whether the host protective immunity is induced by intraperitoneal or intranasal immunization with the rNfa1 protein, mice from each group were intranasally challenged with trophozoites of N. fowleri 1 × 104 cells/20 ㎕. The survival rate and the mean time to death of mice were monitored for all experimental periods.
For intraperitoneally immunization groups (Group I, II, III and IV), mice in group I (Normal: PBS) died at 13 day post N. fowleri trophozoites, and all mice died at 18 day. Their mean time to death was 15.5 days. In group II (Adjuvant+PBS), mice died at 12 day post infection, and all mice died at 18 day. Their mean time to death was 15.0 days. In contrast, mice in group III (rNfa1+Adjuvant) and group IV (rNfa1 only) died at 18 day post infection, and all mice died at 30 day, respectively (Fig. 2). Also, their mean time to death was 25.0 and 24.4 days, respectively (Table 1).
For intranasally immunization groups (Group Ⅴ and Ⅵ), mice in group Ⅴ (Normal:
PBS) died at 12 day post infection, and all mice died at 18 day. Their mean time to death was 15.0 days. As opposed to the mice in group Ⅴ, mice in group Ⅵ (rNfa1 only) died at 18 day post infection, and all mice died at 30 day (Fig. 2). Their mean time to death was 24.7 days (Table 1).
The mean time to death of mice immunized intraperitoneally with the rNfa1 protein mixed with Freund’s complete adjuvant or intranasally with the rNfa1 protein only showed similarly as 25 or 24.7 days after challenge, respectively.
Fig. 2. The survival rate in immune mice post infection of N. fowleri trophozoites. Mice were infected with 1 × 104 N. fowleri trophozoites. Mortality was recorded daily.
0 20 40 60 80 100 120 0 12 14 16 18 20 22 24 26 28 30
Days after N. fowleri infection
S
u
rv
iv
a
l
ra
te
(
%
)
Group ⅠⅠⅠⅠ, Normal (PBS) (i.p.) Group ⅡⅡⅡⅡ, Adjuvant + PBS (i.p.) Group ⅢⅢⅢⅢ, rNfa1 + Adjuvant (i.p.) Group ⅣⅣⅣⅣ, rNfa1 (i.p.)
24.4±3. 38 12 26 31 10 7 4 2 1 51 Ⅳ Ⅳ Ⅳ Ⅳ (i.p.) 38 27 19 9 7 6 3 1 51 Ⅲ Ⅲ Ⅲ Ⅲ (i.p.) 51 49 46 40 30 11 2 51 Ⅱ Ⅱ Ⅱ Ⅱ (i.p.) 50 47 43 32 9 51 Ⅰ Ⅰ Ⅰ Ⅰ (i.p.) 29 28 27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 12 Group 30 51 50 46 51 49 51 No. of Mice Exam 15±2 25±3.7 (i.n.) Ⅴ ⅤⅤ Ⅴ Ⅵ ⅥⅥ Ⅵ (i.n.) 51 51 3 14 27 43 47 49 51 2 5 11 18 23 30 39 44 49 51 15±2 24.7±4 Mean time to death (mean±S.D)
Cumulative number of death on days after infection
15.5±1.7 Table 1. Mortality of mice infected with N. fowleri trophozoites after they were immunized intraperitoneally with the rNfa1 protein in adjuvant or in the absence of adjuvant, or intranasally with the rNfa1 protein only.
Group ⅠⅠⅠⅠ: Normal (PBS) (i.p.) Group ⅡⅡⅡⅡ: Adjuvant + PBS (i.p.) Group ⅢⅢⅢⅢ: rNfa1 + Adjuvant (i.p.) Group ⅣⅣⅣⅣ: rNfa1
(i.p.)
Group ⅤⅤⅤ: Normal (PBS) (i.n.) Ⅴ Group ⅥⅥⅥ: rNfa1 (i.n.) Ⅵ
C. Histopathological examination of mice brain tissue infected with N. fowleri trophozoites
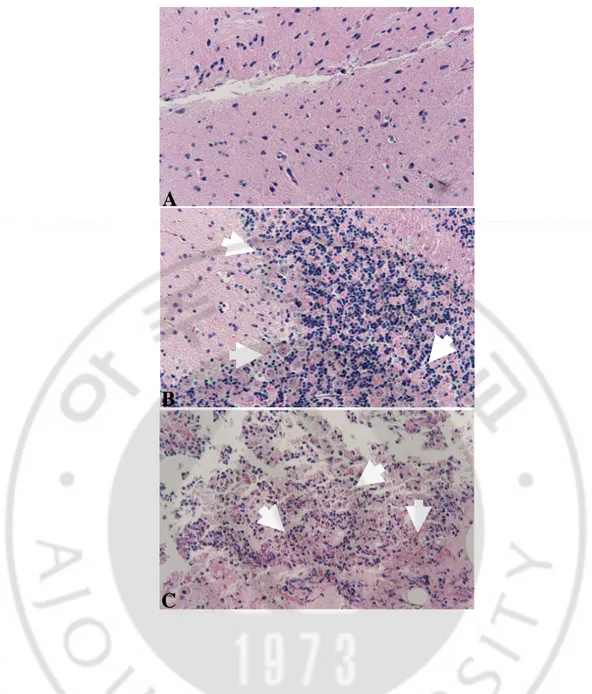
To confirm the cause of mice death, mouse brain tissue was sectioned and stained with hematoxylin and eosin, and other brain tissues were cultured at 37℃ in 75-cm2 culture flasks. Histopathological examination was observed by a light microscopy.
In results of experimental PAME, numerous N. fowleri trophozoites with inflammatory cells infiltration were observed in mice brain tissues immunized intraperitoneally or intranasally with rNfa1 protein (Fig. 3). Also, N. fowleri trophozoites existed in culture of mice brain tissue (data not shown).
Fig. 3. Histologic findings of mice brain tissues post infection of N. fowleri trophozoites. In results of experimental PAME, numerous N. fowleri trophozoites (arrows) with inflammatory cells infiltration were observed in mice brain tissue of all experimental groups (×400). Normal brain tissue was not infected with N. fowleri trophozoites. A, Normal mouse brain tissue; B, intraperitoneally immunized mouse brain tissue; C, intranasally immunized mouse brain tissue.
C
B
A
D. Serum antibody responses to recombinant Nfa1 protein
To elicit host protective response, the Nfa1-specific IgG, IgG subclass, IgA and IgE antibodies were measured in immunized mice sera by ELISA.
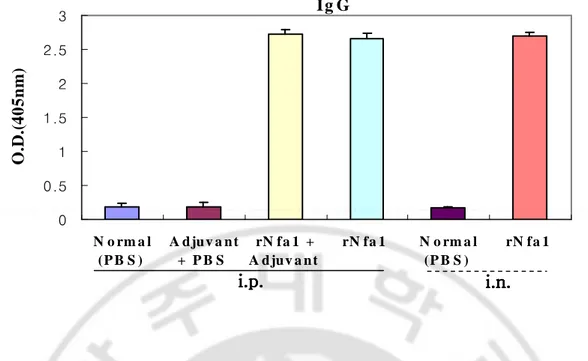
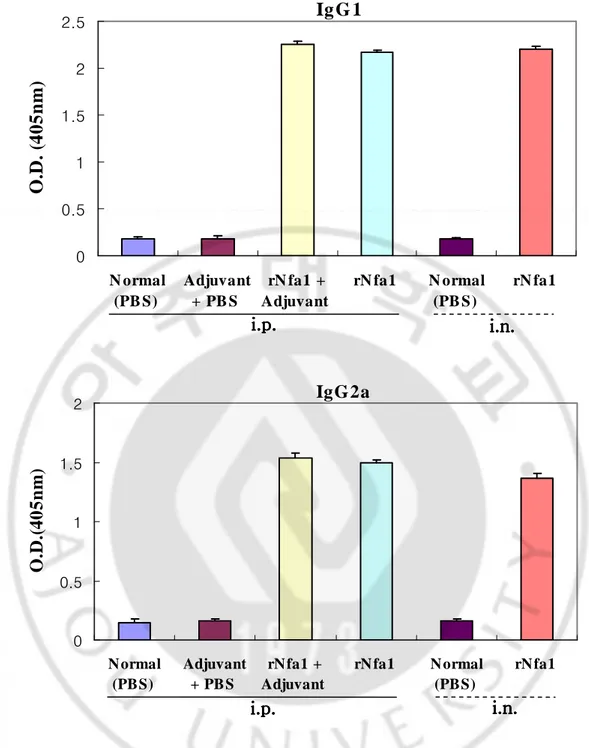
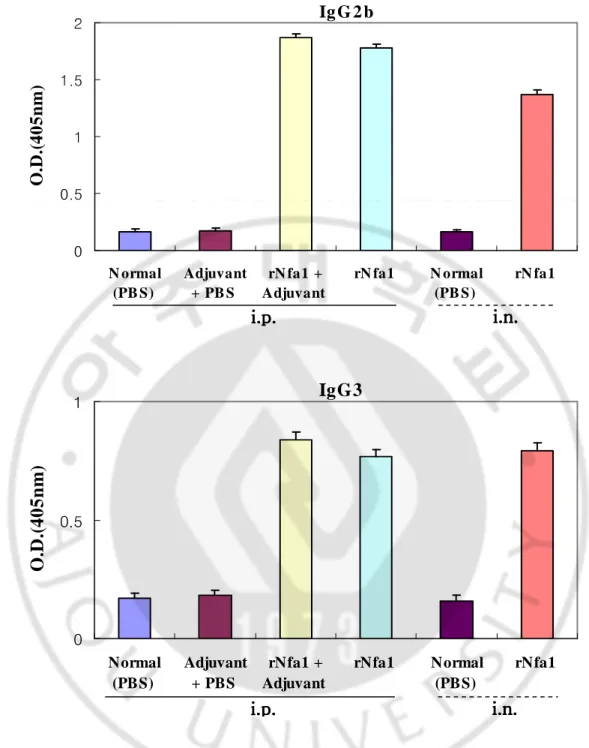
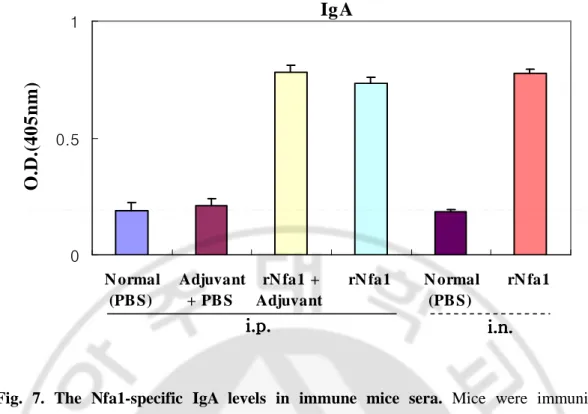
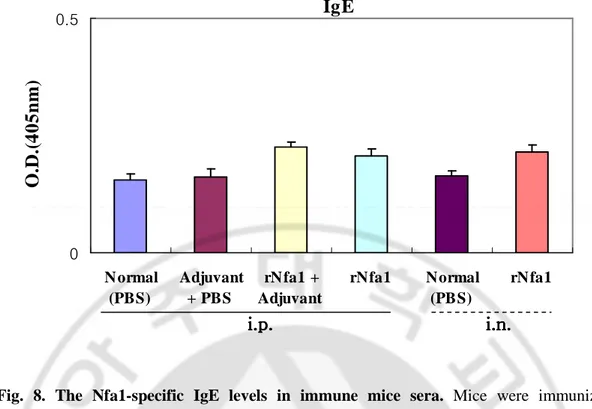
Mice immunized intraperitoneally with the rNfa1 protein mixed with Freund’s complete adjuvant (Group III) displayed a significantly high level of the Nfa1-specific serum IgG and IgA in comparison with either non-immunized mice (Group I) or mice immunized with Freund’s complete adjuvant mixed with PBS (Group II). In addition, mice immunized with the rNfa1 protein only (Group IV) displayed similar to Group III (rNfa1+Adjuvant) showed high level of the Nfa1-specific serum IgG (Fig. 4, 7). In addition, in results of measurement of the Nfa1-specific serum IgG subclass antibody levels, mice of Group III (rNfa1+Adjuvant) induced a mixed pattern of IgG1, IgG2b, IgG3 and IgG2a responses (Fig. 5, 6). In contrast, the Nfa1-specific serum IgE responses were not seen significantly in sera of these all mice groups (Fig. 8).
Mice immunized intranasally with the rNfa1 protein only (Group Ⅵ) displayed a significantly high level of the Nfa1-specific serum IgG and IgA in comparison with non-immunized mice (Group Ⅴ) (Fig. 4, 7). The similar pattern of the Nfa1-specific serum IgG subclass responses were seen in mice immunized with rNfa1 protein only as shown intraperitoneal immunized mice (Fig. 5, 6). In contrast, no significant levels of the Nfa1-specific serum IgE responses were seen in all mice sera (Fig. 8).
IgG, IgG subclass, IgA and IgE levels in immune sera of mice immunized intraperitoneally with the rNfa1 protein mixed with Freund’s complete adjuvant have
similarly high levels to the mice immunized intranasally with the rNfa1 protein only.
Fig. 4. The Nfa1-specific IgG levels in immune mice sera. Mice were immunized intraperitoneally (i.p.) with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally (i.v.) with the rNfa1 only. At two weeks post last immunization, mice were sacrificed. The values are expressed as mean±SD in nine mice of each group.
Ig G 0 0.5 1 1.5 2 2.5 3 N o rm a l (P B S ) A d ju v a n t + P B S rN fa 1 + A d ju v a n t rN fa 1 N o rm a l (P B S ) rN fa 1 O .D .( 4 0 5 n m )
i.p.
i.p.
i.p.
Fig. 5. Levels of IgG subclass specific the Nfa1 protein in immune mice sera. Mice were immunized intraperitoneally (i.p.) with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally (i.v.) with the rNfa1 only. At two weeks post last immunization, mice were sacrificed. The values are expressed as mean±SD in nine mice of each group.
IgG2a
0 0.5 1 1.5 2 N ormal (PBS) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PBS) rN fa1O
.D
.(
4
0
5
n
m
)
Ig G 1
0 0.5 1 1.5 2 2.5 N ormal (PB S) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PB S) rN fa1O
.D
.
(4
0
5
n
m
)
i.p.
i.p.
i.p.
i.p.
i.n.
i.n.
i.n.
i.n.
i.p.
i.p.
i.p.
Fig. 6. Levels of IgG subclass specific the Nfa1 protein in immune mice sera. Mice were immunized intraperitoneally (i.p.) with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally (i.v.) with the rNfa1 only. At two weeks post last immunization, mice were sacrificed. The values are expressed as mean±SD in nine mice of each group.
IgG3
0 0.5 1 Normal (PBS) Adjuvant + PBS rNfa1 + Adjuvant rNfa1 Normal (PBS) rNfa1O
.D
.(
4
0
5
n
m
)
Ig G 2 b 0 0.5 1 1.5 2 N ormal (PB S) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PB S) rN fa1 O .D .( 4 0 5 n m )i.p.
i.p.
i.p.
i.p.
i.n.
i.n.
i.n.
i.n.
i.p.
i.p.
i.p.
Fig. 7. The Nfa1-specific IgA levels in immune mice sera. Mice were immunized intraperitoneally (i.p.) with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally (i.v.) with the rNfa1 only. At two weeks post last immunization, mice were sacrificed. The values are expressed as mean±SD in nine mice of each group.
IgA
0 0.5 1 N ormal (PB S) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PB S) rN fa1O
.D
.(
4
0
5
n
m
)
i.p.
i.p.
i.p.
Fig. 8. The Nfa1-specific IgE levels in immune mice sera. Mice were immunized intraperitoneally (i.p.) with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally (i.v.) with the rNfa1 only. At two weeks post last immunization, mice were sacrificed. The values are expressed as mean±SD in nine mice of each group.
IgE
0 0.5 Normal (PBS) Adjuvant + PBS rNfa1 + Adjuvant rNfa1 Normal (PBS) rNfa1O
.D
.(
4
0
5
n
m
)
i.p.
i.p.
i.p.
E. Cytokine responses to recombinant Nfa1 protein
To determine the T cell response to rNfa1 protein in immunized mice, the cytokine levels were evaluated by ELISA in culture supernatants from rNfa1 protein-simulated splenocytes.
The in vitro stimulated splenocytes from rNfa1 protein mixed with Freund’s complete adjuvant-intraperitoneally immunized mice (Group III) exhibited a significantly high level of Th2-type cytokine, IL-10 (Fig. 9). Also, IL-2 and IL-4 levels were increasing in comparison with the non-immunized controls (Group I) or mice immunized with Freund’s complete adjuvant mixed with PBS (Group II) (Fig. 9, 10). Interestingly, stimulated splenocytes from mice of Group III (rNfa1+Adjuvant) also showed a significantly increased level of Th1-type
cytokine, γ-IFN, in comparison with Group I (Normal [PBS]) or Group II (Adjuvant+PBS)
(Fig. 9). Also, in mice of Group IV (rNfa1 only), γ-IFN, IL-2, IL-10 and IL-4 levels were similar to Group III (rNfa1+Adjuvant).
Mice immunized intranasally with rNfa1 protein only (Group Ⅵ) exhibited a significantly high level of Th1-type cytokine, γ-IFN, and Th2-type cytokine, IL-10, in comparison with the non-immunized controls (Group Ⅴ) (Fig. 9, 10). Also, IL-2 and IL-4 levels were increasing in comparison with Group Ⅴ (Normal [PBS]) (Fig. 9, 10).
The γ-IFN, IL-2, IL-10 and IL-4 levels in splenocytes from mice immunized intraperitoneally with rNfa1 protein mixed with Freund’s complete adjuvant were similar to the mice immunized intranasally with rNfa1 protein only.
39
Fig. 9. Production of IFN-γ and IL-2 from splenocytes of immune mice stimulated with the rNfa1 protein. At two weeks post last immunization, splenocytes were isolated from immunized mice. Results are expressed as the cytokine concentration in the supernatant of the cell cultures collected at 72 h after stimulation with 10 ㎍/ml rNfa1 protein, as detected by ELISA. The values are expressed as mean±SD in nine mice of each group.
IFN-r
0 100 200 300 400 500 600 700 800 Normal (PBS) Adjuvant + PBS rNfa1 + Adjuvant rNfa1 Normal (PBS) rNfa1p
g
/m
l
i.p.
i.p.
i.p.
i.p.
i.n.
i.n.
i.n.
i.n.
IL-2
0 50 100 150 200 N ormal (PB S) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PB S) rN fa1p
g
/m
l
i.p.
i.p.
i.p.
Fig. 10. Production of IL-10 and IL-4 from splenocytes of immune mice stimulated with the rNfa1 protein. At two weeks post last immunization, splenocytes were isolated from immunized mice. Results are expressed as the cytokine concentration in the supernatant of the cell cultures collected at 72 h after stimulation with 10 ㎍/ml rNfa1 protein, as detected by ELISA. The values are expressed as mean±SD in nine mice of each group.
IL-1 0 0 100 200 300 400 500 600 700 N ormal (PB S) Adjuvant + PB S rN fa1 + Adjuvant rN fa1 N ormal (PB S) rN fa1 p g /m l
i.p.
i.p.
i.p.
i.p.
i.n.
i.n.
i.n.
i.n.
IL -4 0 100 200 300 400 N o rm a l (P B S ) A djuv a nt + P B S rN fa 1 + A djuv a nt rN fa 1 N o rm a l (P B S ) rN fa 1 p g /m l
i.p.
i.p.
i.p.
F. Duration of the Nfa1-specific IgG levels in immunized mice
To evaluate the duration of the Nfa1-specific IgG antibody responses, the Nfa1-specific IgG antibody formation were evaluated by ELISA in each group at 2, 6 and 12 weeks following third immunization.
In intraperitoneally immunized mice, sera from rNfa1+adjuvant immunized mice demonstrated significantly high levels of the rNfa1-specific IgG at 2 weeks (Fig. 11). These high levels was continued and slightly decreased with 6 and 12 weeks post immunization. But, during 12 weeks, the value of O.D. of the rNfa1-specific IgG showed significantly high levels compared to normal mice.
In intranasally immunized mice, sera from rNfa1 only immunized mice demonstrated high significantly levels of the rNfa1-specific IgG which was similar to mouse immunized with Nfa1 protein intraperitoneally (Fig. 11).
The duration of the Nfa1-specific IgG levels of mice immunized intraperitoneally with the rNfa1 protein mixed with Freund’s complete adjuvant showed similar to the mice immunized intranasally with the rNfa1 protein only.
Fig. 11. Duration of the Nfa1-specific IgG levels in immune mice sera. The Nfa1-specific IgG responses were evaluated by ELISA in each group at 2, 6 and 12 weeks following immunization.
0
0.5
1
1.5
2
2.5
3
2
6
12
Weeks
O
.D
.(
4
0
5
n
m
)
Group Ⅶ
Ⅶ
Ⅶ, Normal (PBS) (i.p.)
Ⅶ
Group Ⅷ
Ⅷ
Ⅷ
Ⅷ, rNfa1+Adjuvant (i.p.)
Ⅳ
Ⅳ
Ⅳ
Ⅳ. DISCUSSION
N. fowleri is the causal agent of primary amoebic meningoencephalitis (PAME). Naegleria spp. are amoeboflagellates found in water and soil. Although, some 30 species of Naegleria have been recognized based upon sequencing data (De Jonckheere, 2004), N. fowleri is the only one that has been isolated from cases of primary amoebic meningoencephalitis. Other species (Naegleria italica, Naegleria philippinensis, Naegleria australiensis) may be pathogenic in the mouse model of PAME, but have not been identified from any human cases.
PAME is a fulminating disease, developing within several days following exposure to the water source, and causing death within 1~2 weeks after hospitalization. Adherence of pathogens to host cells is a critical initial step in the infection process. The ability of trophozoites to attach to the nasal mucosal, an increased rate of locomotion, and a chemotactic response to nerve cell components may be important in disease progression (Cline et al., 1986; Brinkley and Marciano-Cabral, 1992; Han et al., 2004).
A variety of in vitro cell culture systems have been used to study the infection of N. fowleri with mammalian cells. N. fowleri trophozoites have been shown to destroy nerve cells, as well as other cell types, by trogocytosis using food-cup structure on their surface (Brown, 1979; Marciano-Cabral et al., 1982; Marciano-Cabral and Fulford, 1986) and by the release of cytolytic molecules (Lowery and McLaughlin, 1984, 1985; Marciano-Cabral and Fulford, 1986; Leippe and Herbst, 2004).
Many antiparasitic and antimicrobial drugs have been screened for in vitro and in vivo activity against N. fowleri. Naegleria spp. are highly sensitive to the antifungal drug amphotericin B, and it has been used in virtually all cases as the core antimicrobial where recovery occurred. Minimum amoebacidal concentrations of amphotericin B were determined to be 0.02∼0.078 ㎍/ml for three different clinical isolates of N. fowleri tested in vitro (Duma et al., 1971).
Particularly in the early stages of PAME, Naegleria infections have also been treated successfully with amphotericin B, rifampin, and chloramphenicol; amphotericin B, oral rifampin, and oral ketoconazole; and amphotericin B alone (Abramowicz, 2004; Schuster and Visvesvara, 2004; Schuster and Visvesvara, 2004).
Circulating IgG antibodies in serum were demonstrated by ELISA from day 7 after infection in ICR mice infected with N. fowleri (Park et al., 1987b) and from day 14 after infection in ICR mice infected with N. jadini, but no anti-N.gruberi antibody was detected (Lee et al., 1985).
In the present study, the immunogenicity of the rNfa1 protein and protective efficacy for pathogenic N. fowleri infection were evaluated. The survival times of intraperitoneally or intranasally immunized mice with the rNfa1 protein prolonged about 10 or 9 days in comparison with the normal group mice. Mice immunized intraperitoneally with the Nfa1 protein in adjuvant or in the absence of adjuvant, or intranasally with the rNfa1 only elicited significantly high levels of the Nfa1-specific IgG antibody. Furthermore, analysis of IgG subclass profiles revealed that anti-Nfa1 IgG1 showed the greatest increase followed by IgG2b, IgG2a and IgG3, suggesting that rNfa1-adjuvant or only rNfa1 immunization resulted
in a Th1/Th2 mixed type immunity. Also, intraperitoneal or intranasal immunization of Nfa1 showed significantly high levels of the Nfa1-specific serum IgA antibody. It is generally known that mucosal IgA plays an important role in protection against several bacterial, viral and protozoan (Renegar et al., 1991; Michetti et al., 1992; Marcotte et al., 1998). The inhibition of bacterial adherence by mucosal IgA is considered to be one of the most important defense mechanisms against mucosal bacterial invasion, has been shown to limit the attachment of bacteria to epitherial cells (Svanborg-Eden et al., 1978; Kurono Y et al., 1989).
Also, adherence of N. fowleri to host cells is a critical initial step in the infection process. N. fowleri penetrates the mucosal epithelial layer and migrates along the olfactory nerve tracts, crossing the cribriform plate, to the brain. Mice immunized intranasally with antigen-adjuvant showed low levels of serum IgA and high levels of mucosal IgA (Masanori et al., 1998). In this study, mice immunized intraperitoneally with the rNfa1 in adjuvant or in the absence of adjuvant, or intranasally with the rNfa1 only showed significantly high levels of the Nfa1-specific serum IgA. Finally, the survival times were prolonged. It suggests that antibody may act to the resistance to N. fowleri penetration and invasion by immobilizing the N. fowleri and inhibiting the adherence of the N. fowleri to nasal mucosal. Interestingly, IgE responses were not detected in these mice, suggesting that immune responses to the rNfa1 are not mediated by hypersensitivity reactions.
Immunoglobulin isotype-specific responses seen in the rNfa1-adjuvant or only rNfa1 immunized mice are consistent with cytokine profiles in splenocyte culture significant levels of Th1 type cytokine, γ-IFN and IL-2 were demonstrated, together with Th2 type cytokine,