이학 석사학위 논문
Studies on the production of
IL-1β, TNF-α, iNOS, IL-10, and ROS
in the APP/PS1 mouse model of
Alzheimer's disease
아 주 대 학 교 대 학 원
의 학 과
Studies on the production of IL-1β, TNF-α,
iNOS, IL-10, and ROS in the APP/PS1 mouse
model of Alzheimer's disease
By
Hwan Goo Lee
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
MASTER OF MEDICAL SCIENCES
Supervised by
Yong Beom Lee, Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
August, 2008
이환구의 이학 석사학위 논문을 인준함.
심사위원장 조 은 혜 인
심 사 위 원 김 병 곤 인
심 사 위 원 이 용 범 인
아 주 대 학 교 대 학 원
2008년 6월 23일
ACKNOWLEDGEMENTS
가장먼저 제가 공부할 수 있도록 뒤에서 묵묵히 도와주시고, 따뜻하게 감싸 주신 부모님과 사랑하는 가족에게 깊은 감사의 마음을 전합니다. 그리고 부족 한 저를 지도해주시고 때로는 부모님의 마음으로, 때로는 인생의 선배로써 이 끌어주신 이용범 지도교수님께 감사 드립니다. 격려와 충고로써 지켜봐 주신 진병관 교수님, 이명애 교수님, 조은혜 교수님, 김병곤 교수님 진심으로 감사 의 마음을 전합니다. 실험실을 떠나서도 늘 걱정 반 격려 반으로 아껴주었던 수윤누나, 옆에서 서로의 버팀목이 되었던 재석이, 대학원 동기로써 서로 힘 이 되어주었던 종현이와 래희, 정파에게 감사합니다. 우리 방의 세훈, 혜란, 옆방의 수연, 동철, 정용이 형, 명선누나, 안정희 박사님과 함께해서 즐거웠습 니다. 2년 동안 늘 옆에서 어려울 때마다 도와주고 충고를 아끼지 않은 나의 정신적 지주 은숙 누나, 근우 형, 영철이 형, 수윤 누나, 은수 형을 비롯한 여 러 형 누나들의 도움에 진심으로 감사 드립니다. 또 사랑하는 나의 친구들과 선배님들; 경진 누나, 혜경 누나, 범수 형, 인수 형, 동훈이 형, 혁민이 형, 광 수 형, 신애 누나, 순미 누나, 수현이, 혜선이, 여초, 수희, 유진, 영미, 수령, 달님이, 재호 형, 화정 누나등 많은 선배님들과 친구들의 격려가 있었기에 힘 낼 수 있었습니다. 이 모든 분들께 감사와 사랑의 마음 전합니다. 끝으로 부 족하지만 지금까지 믿고 잘 기다려준 짝꿍에게 고맙다고 말하고 싶습니다. i- ABSTRACT -
Studies on the Production of IL-1β, TNF-α, iNOS, IL-10,
and ROS in the APP/PS1 Mouse Model of Alzheimer's Disease
It is well known that excessive brain inflammation cause both acute and chronic neurodegeneration. Our previous study demonstrated the neuroprotective effects of IL-10 in LPS-injected rat cerebral cortex, which was mediated by inhibition of NADPH oxidase activation and pro-inflammatory mediators expression. Here, we observed the expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of transgenic mouse model of AD (APP/PS1). In transgenic mice, microglia and astrocytes were activated synchronously with Aβ deposits and were abundant and closely associated with senile plaques. At 14-17 months, microglia and astrocytes were morphologically damaged and excessively activated. The mRNA and protein levels of IL-1β, TNF-α and iNOS were detected from 3 months, and they were increased with age. The mRNA and protein levels of IL-10 were detectable in 6 month-old transgenic mouse, which is 3 months later than the expression of pro-inflammatory mediators. Double immunoflorescence staining showed that IL-1β, TNF-α and IL-10 expression was localized mainly in Iba1-immunopositive microglia. Reactive oxygen species (ROS) production was detected from 6 months, and there most significantly increased between the ages of 14 months and 17 months. A major subunit of NADPH oxidase, gp91phox protein was localized to
Iba1-immunopositive microglia in the APP/PS1 transgenic mice. And also, cerebral cortex of
14, 17 months showed significant decrease in NeuN-positive neurons and MAP2-immunoreactice dendrites. These results suggest that activated microglia surrounding plaques induce excessive production of pro-inflammatory mediators and ROS, leading to neuronal damage.
Key words: Alzheimer’s disease (AD), Interleukin 10 (IL-10), microglia, IL-1β, TNF-α, iNOS, reactive oxygen species (ROS)
TABLE OF CONTENTS
ACKNOWLEDGEMENTS ………... ⅰ ABSTRACT ………..……….………..…….... ⅱ TABLE OF CONTENTS ……….………..…... ⅳ LIST OF FIGURES ……….………..……... ⅵ LIST OF ABBREVIATION ……….………… ⅶ . I Ⅰ NTRODUCTION ……… 1 A. Brain inflammation ………...……...……….…….………... 1B. Brain inflammation in Alzheimer’s disease ...………..…………..….... 2
C. Aims of study ………. 3
Ⅱ. MATERIALS AND METHODS ……….. 4
A. Transgenic mice and genotyping …………...………...……... 4
B. Immunohistochemistry ……….………...………... 4
C. Double-immunofluorescence staining ..………. 5
D. Thioflavin S staining …..……...……….………...……… 6
E. Reverse transcription-polymerase chain reaction (RT-PCR) ………...…….. 6
F. In situ detection of O2- and O2--derived oxidants ………... 7
G. Western blot analysis …………...………..… 8
H. Statistical analysis …..………... 9
. R Ⅲ ESULTS ……..……….……… 10
A. Age-related Aβ deposition and Aβ-associated gliosis in the cerebral cortex of APP/PS1 mice …...……...………...………….…………. 10
B. Expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of
APP/PS1 mice …...……….………...……….. 13
C. IL-1β, TNF-α, IL-10 expression in microglia ... 15
D. ROS production and neuronal damage in the cerebral cortex of APP/PS1 mice ... 19 . D Ⅳ ISCUSSION ..………..… 22 Ⅴ. CONCLUSION ……..……… 25 REFERENCES ..…………..………. 26 국문요약 ... 37 v
LIST
OF FIGURES
Fig. 1. Age-related Aβ deposition and Aβ-associated gliosis in the cerebral cortex of APP/PS1 mice ……..…... 11
Fig. 2. Expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of APP/PS1 mice ……...…... 14
Fig. 3. IL-1β, TNF-α, and IL-10 expression in microglia ... 16
Fig. 4. ROS productionandneuronaldamagein thecerebralcortexofAPP/PS1
mice ... 20
vii
LIST OF ABBREVIATION
AD, Alzheimer’s disease
APP, amyloid precursor protein
CNS, central nervous system
fAβ, fibrillar β-amyloid
GFAP, glial fibrillary acidic protein
Iba1, ionized calcium-binding adapter molecule 1
IFN-γ, interferon- γ
IL-10, interleukin-10
IL-1β, interleukin-1β
iNOS, inducible nitric oxide synthase
MAP2, microtubule associated protein 2
NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase
NeuN, neuronal nuclei
PS1,presenilin1
ROS, reactive oxygen species
TNF-α, tumor necrosis factor-α
.
Ⅰ
INTRODUCTION
A. Brain inflammation
Inflammation in the central nervous system (CNS) has usually been considered to be detrimental (Perry et al. 1998; Gebicke-Haerter, 2001; Nguyen et al. 2002). In particular, activated microglia exert cytotoxic effects by releasing inflammatory mediators, such as reactive oxygen species (ROS), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, nitric oxide, arachidonic acid metabolites (Boje et al., 1992; Meda et al., 1995). In addition, several cytokines and inflammatory mediators produced by activated glia have the potential to initiate or exacerbate the progression of neuropathology (Chao et al., 1995; Koshinaga et al., 2000). On the other hand, the anti-inflammatory cytokines IL-10 and TGF-β1 have also been reported to be produced by activated microglia (Tsunawaki et al., 1988; Fiorentino et al., 1991). Microglia-derived IL-10 could down-regulate CNS inflammation by acting both in an autocrine and paracrine fashion (Mizuno et al., 1994). Increasing evidence indicates that IL-10 has the ability to improve neurological outcome after CNS injury (Grilli et al., 2000; Kremlev and Palmer, 2005). For example, IL-10 has neuroprotective properties against glutamate-induced (Bachis et al., 2001) or hypoxic-ischemic (Dietrich et al., 1999) neuronal death and against LPS- or interferon-induced oligodendrocyte cell death (Molina-Holgado et al., 2001). Well-balanced microglial activation should be reversible and does not cause secondary neuronal degeneration. Recent evidence indicates that inflammatory cells can be beneficial, and may even exert neuroprotective effects (Martino, 2004; Glezer et al., 2007).
B. Brain inflammation in Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative dementia of the elderly that is characterized by neurofibrillary tangles and extracellular senile plaques (Selkoe and Schenk, 2003). β-amyloid peptide (Aβ), a major component of senile plaque, is postulated to play a pivotal role in inducing the inflammatory process in the AD brain; Aβ can activate glial cells (microglia and astrocytes) in culture to induce or potentiate the production of a variety of inflammatory products, including cytokines (Gonzalez-Scarano, 1999; Akiyama et al., 2000), nitric oxide (Goodwin et al., 1995; Meda et al., 1995; Ii et
al., 1996), glutamate (Piani et al., 1991; Klegeris and McGeer, 1997), ROS (Klegeris et al., 1994; Van Muiswinkel et al., 1996). Furthermore, these molecules cause harmful
secondary reactions that may lead to the injury or death of neurons (Vila et al., 2001). In AD brain, evidence for locally induced inflammation includes: the presence of activated complement components (Rogers et al., 1992); alteration of cytokine levels, in particular of TNF-α (Dickson et al., 1993), IL-1β (Mrak et al., 2000), IL-6 (Strauss et al., 1992) and TGF-β (Van der Wal et al., 1993); and the presence of chemokines (Xia et al., 1999), and altered enzyme activities, including cyclooxygenase (COX)-2 isoform (Fiala et al., 2002), and inducible nitric oxide synthase (iNOS) (Haas et al., 2002). Recently, ongoing controversy exists regarding whether microglia are neuroprotective or neurotoxic when activated. Microglia and macrophages could play a neuroprotective role (Mallat et al., 1994) either by aiding the removal of amyloid deposits (Schenk et al., 1999), or by secreting neurotrophic factors and anti-inflammatory factors (Polazzi et al., 2001; Liao et
al., 2004; Morgan et al., 2004). However, at present little is known about the balance
between pro- and anti-inflammatory events occurring in Alzheimer’s disease. Moreover, it remains to be determined whether microglial activation plays a role in the initiation stage of disease progression or occurs merely as a response to neuronal death. The availability of a transgenic mouse (APP/PS1) that develops β-amyloid plaques and glial activation in the aged brain may represent a useful tool to study such regulatory events (Alpar et al., 2006).
C. Aims of study
The specific aims of this study
1. To investigate the temporal Aβ deposition and Aβ-associated gliosis in the cerebral cortex of APP/PS1 mice.
2. To investigate the expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of APP/PS1 mice.
. Materials and Methods
Ⅱ
A. Transgenic mice and genotyping
A pathogen-free transgenic line of Alzheimer’s disease mouse model, APP/PS1 mice (Jankowsky et al., 2004), was obtained from Jackson Laboratory (Bar Harbor, ME) and maintained by crossing transgenic males with B6C3F1 females that were purchased from Jackson Lab, also. APP/PS1 mice carry mouse APP with the double mutations (K670N and M671L) and human PS1 with a deletion of exon 9 found in familial AD patients. The genotyping for the APP/PS1 transgene was performed by the PCR-based method provided by the Jackson Lab. APP/PS1 mice used were 3–17 months old. For each experimental group, 3–4 mice were used. For PCR analysis, genomic DNA was prepared from tails of adult mice and specific primers were used. The following primer pairs were used: APP forward GAC TGA CCA CTC GAC CAG GTT CTG-3´; APP reverse 5´-CTT GTA AGT TGG ATT CTC ATA TCC G-3´; PS1 forward 5´-AAT AGA GAA CGG CAG GAG CA-3´; PS1 reverse 5´-GCC ATG AGG GCA CTA ATC AT-3´.
B. Immunohistochemistry
Animals were transcardially perfused with a saline solution containing 0.5% sodium nitrate and heparin (10 U/ml) followed by 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (PB). Brains were removed from the cranium and postfixed for 1 h, washed in 0.1 M PB and then immersed in 30% sucrose solution until they sank. Tissues were sectioned on a sliding microtome at a thickness of 35 µm, and every sixth serial
section was selected and processed for immunostaining as described previously (Ryu et
al., 2002). In brief, brain sections were incubated in 0.2% Triton X-100 for 30min, rinsed
two times in PBS-0.5% bovine serum albumin (BSA) and then incubated overnight at room temperature with the appropriate primary antibodies. The primary antibodies used were against rabbit Iba1 (1:400; Wako) for microglia, against mouse glial fibrillary acidic protein (GFAP, 1:400; Sigma) for astrocytes, and against mouse NeuN (1:400; Chemicon International, Temecula, CA) for neurons. The following day, brain sections were rinsed with PBS-0.5% BSA, incubated with appropriate biotinylated secondary antibody followed by avidin-biotin complex (Elite Kit purchased from Vector Laboratories, Burlingame, CA). The bound antiserum was visualized by incubating with 0.05% diaminobenzidine-HCl (DAB) and 0.003% hydrogen peroxide in 0.1 M PB. The DAB reaction was stopped by rinsing tissues in 0.1 M PB. Immunostained cells were analyzed under brightfield microscopy.
C. Double-immunofluorescence staining
For immunofluorescence staining, the brain sections were incubated with a combination of a rabbit Iba1 (1:250; Wako) and goat anti-IL-1β (1:50; R&D Systems); rabbit Iba1 (1:250) and goat anti-TNF-α (1:50; R&D Systems); rabbit Iba1 (1:250) and goat anti-IL-10 (1:150; R&D Systems); rabbit Iba1 (1:250) and mouse anti-gp91phox
(1:200; BD bioscience); mouse anti-GFAP (1:400; Sigma) and goat anti-IL-1β; mouse anti-GFAP (1:400) and goat anti-TNF-α; mouse anti-GFAP (1:400) and goat anti-IL-10; mouse anti- GFAP (1:400) and mouse anti-gp91phox. For cortex neuron (dendrite) staining,
sections were reacted with mouse anti-MAP2 (1:300) overnight at 4℃. After washing, the sectionswere subsequently incubated with FITC-labeled anti-rabbit IgG or FITC-labeled anti-mouse IgG (1:200; Kirkegaard & Perry Laboratories, Gaithersburg, MD) and Texas red-labeled anti-rabbit IgG (1:200; Vector) or Texas Red-labeled anti-goat IgG (1:200; Vector) or Red fluorescent Cy3-donkey-anti-goat IgG (1:600). Tissues were mounted with Vectashield mounting medium (Biomed Corp., Foster City, CA) and viewed using an Olympus IX71 confocal laser scanning microscope (Olympus; Tokyo, Japan).
D. Thioflavin S staining
For thioflavin S staining, brain sections were mounted on gelatine/chrome alum-coated slides. After drying, slides were rinsed with water and incubated with thioflavin S (1% w/v, sigma) for 5 min. Subsequently slides were rinsed in 70% ethanol for 5 min and briefly rinsed in water. For combination with Iba1 and GFAP immunocytochemistry, sections were first fluorescence labeled as described above and subsequently stained with thioflavin S.
E. Reverse transcription-polymerase chain reaction (RT-PCR)
Brains from the ipsilateral cortex after injection were used for RNA isolation using Trizol (Life Technology, MD). The reverse transcribed cDNA (4 μg of RNA) was subjected to polymerase chain reaction (PCR) amplification with following primer sets. For mouse IL-10, 5'-ATG CAG GAC TTT AAG GGT-3' (sense) and 5'-ATT TCG GAG AGA GGT ACA-3' (antisense) and for mouse IL-1β, 5'-GCA ACT GTT CCT GAA
3' (sense) and 5'-CTC GGA GCC TGT AGT GCA-3' (antisense) and for mouse TNF-α, 5'-ATG AGC ACA GAA AGC ATG ATC-3' (sense) and 5'-TAC AGG CTT GTC ACT CGA ATT-3' (antisense) and for mouse iNOS, 5'-TCA CTG GGA CAG CAC AGA AT-3' (sense) and 5'-TGT GTC TGC AGA TGT GCT GA-3' (antisense). The PCR cycles consisted of denaturation at 94°C for 30 sec, annealing at 43°C for 30 sec (IL-10) or 50°C for 30 sec (TNF-α, IL-1β) or 63.7°C for 30 sec (iNOS), and extension at 72°C for 90 sec for 33 cycles. Mouse actin was also amplified as an internal PCR control using the following primers, 5'-CAT GTT TGA GAC CTT CAA CAC CCC-3' (sense) and 5'-GCC ATC TCC TGC TCG AAG TCT AG-3' (antisense). PCR products were separated by electrophoresis, stained with ethidium bromide, and then detected using ultraviolet (UV) light.
F. In situ detection of O2- and O2--derived oxidants
Hydroethidine histochemistry was performed for in situ visualization of O2- and O2-
-derived oxidants (Wu et al., 2003). Mice wereinjected intraperitoneally with 200 µL of hydroethidine (1 mg/ml in PBS containing 1% dimethylsulfoxide; Molecular Probes, Eugene, OR). After 30 min, the animals were transcardially perfused with a saline solution containing 0.5% sodium nitrate and heparin (10 U/ml) and then fixed with 4% paraformaldehyde in 0.1M PB. After fixaton, the brains were cut into 35 µm slices using a sliding microtome. The brain sections were incubated with Hoechst 33258 (1:1000; Molecular Probes) in PBS for 20 min in a darkchamber, rinsed with distilled water and coverslipped with Vectashieldmounting medium (Biomed Corp., Foster City, CA). The
brain sections with oxidized hydroethidine product, ethidium, were examined by confocal microscopy (Olympus).
G. Western blot analysis
Cerebral cortex tissues were isolated from transgenic mice and age-matched nontransgenic littermates at each time point and snap frozen. Tissues were homogenized with ice-cold lysis buffer containing 50 mM Tris-HCl pH 8, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, supplemented with a protease inhibitors cocktail (Sigma). The homogenate was then centrifuged at 13,000 ×g for 10 min and the supernatants collected. Protein concentration was determined using the DC protein assay kit (Bio-Rad, Hercules, CA). Total protein (20-40 μg) from each sample was separated on a 10-14% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After transfer, membranes were incubated in blocking buffer (TBS containing 0.5% skin milk) for 1 h at room temperature (RT) and then incubated overnight at 4 °C with following primary antibodies: goat IL-1β (1:5000, R&D Systems), goat anti-TNF-α (1:5000, R&D Systems), rabbit anti-iNOS (1:5000, Millipore), goat anti-IL-10 (1:5000, R&D Systems) and monoclonal anti-β-actin (1:5000, Sigma). After several washes in 0.1% Tween-20 (Sigma) in tris-buffered saline (TBS), membranes were incubated with the corresponding HRP conjugated secondary antibodies (Amersham Biosciences, UK) for 1 h at RT. After washing, the bands were visualized with ECL detection (Amersham Biosciences), exposing to X-ray film (AGFA, Belgium). The
average intensity value of the pixels in a background selected region was calculated and was subtracted from each pixel in the samples using IamgeQuant software (Image Guage 4.0; Fuji Film). The densitometry values obtained were normalized with respect to the values obtained with anti-β-actin to ascertain the same amount of protein.
H. Statistical analysis
All values are represented as mean ± SEM. Statistical significance (P < 0.01 for all analyses) was assessed by analysis of variance (ANOVA) using Instat 3.05 (GraphPad Software, San Diego, CA), followed by Student-Newman-Keuls analyses.
. R
Ⅲ
ESULTS
A. Age-related Aβ deposition and Aβ-associated gliosis in the cerebral cortex of APP/PS1 mice
In transgenic mice carrying AD-linked APP and PS1 mutations, we observed a rapid accumulation of fibrillar Aβ from 6 months of age up to the oldest age studied, 17 months (Fig. 1 A). By immunohistochemical study using Iba1 antibody, we investigated microglial activation in the cerebral cortex of APP/PS1 transgenic mouse at the different time points (3, 6, 10, 14 and 17 months). An aggregate of activated microglia were increased synchronously with Aβ deposition. GFAP-immunopositive reactive astrocytes were also observed in parallel with Aβ deposition. At 14-17 months, microglia and astrocytes were morphologically damaged (Fig. 1 B). Iba1-positive microglia and GFAP-positive astrocyte were abundant and closely associated with fibrillar (thioflavin S-positive) Aβ deposits throughout the cortex (Fig. 1 C).
Fig. 1. Age-related Aβ deposition and Aβ-associated gliosis in the cerebral cortex of APP/PS1 mice. Tissue sections were obtained from cerebral cortex of APP/PS1
transgenic mouse at the different time points (3, 6, 10, 14 and 17 months). (A) Representative images of thioflavine S staining in the cerebral cortex. Scale bar: 200 μm. (B) Immunohistochemistry staining with antibodies to Iba1 for microglia and GFAP for astrocyte at the indicated time point. Scale bar: 20 μm. (C) Iba1-positive microglia and GFAP-positive astrocyte immunoreactivity (red) showed in the area surrounding the plaque (thioflavine S, green) at 10 months in the APP/PS1 mice. Scale bar: 20 μm.
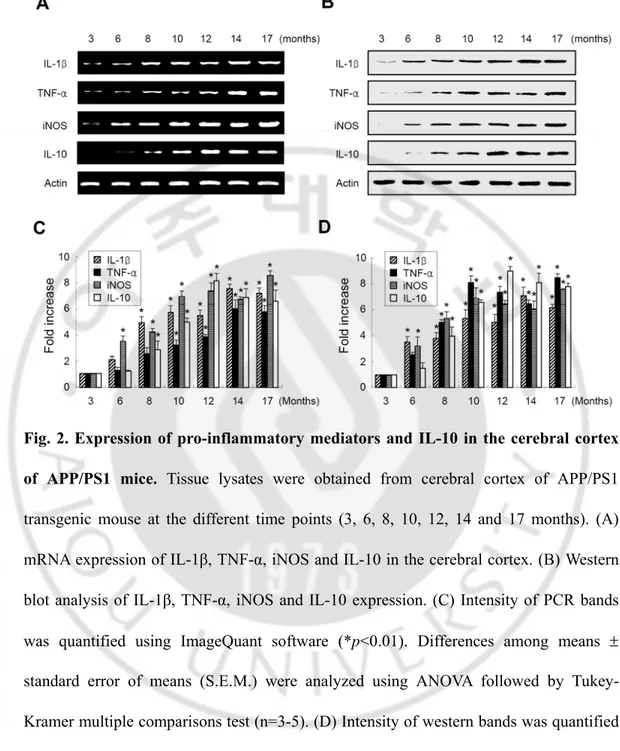
B. Expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of APP/PS1 mice
To elucidate the mechanisms involved in Aβ-mediated inflammation, we investigated the expression of IL-1β, TNF-α, iNOS and IL-10 in the APP/PS1 transgenic mouse. The level of mRNA and protein expression was detected in brain sections from cortex regions at various postnatal ages ranging from 3 to 17 months. The mRNA levels of IL-1β, TNF-α and iNOS were detected from 3 months, and there was increased with the increasing age. The mRNA of IL-10 was detectable in 6 month-old transgenic mouse, which is 3 months later than the expression of pro-inflammatory mediators (Fig. 2 A, C). The protein levels of IL-1β, TNF-α, iNOS and IL-10 were also similarly reflected at the mRNA expression.
The protein levels of IL-1β, TNF-α and iNOS were detected from 3 months, and there was increased with age. The protein levels of IL-10 were detectable in 6 month-old transgenic mouse, which is 3 months later than the expression of pro-inflammatory mediators (Fig. 2 B, D).
Fig. 2. Expression of pro-inflammatory mediators and IL-10 in the cerebral cortex of APP/PS1 mice. Tissue lysates were obtained from cerebral cortex of APP/PS1
transgenic mouse at the different time points (3, 6, 8, 10, 12, 14 and 17 months). (A) mRNA expression of IL-1β, TNF-α, iNOS and IL-10 in the cerebral cortex. (B) Western blot analysis of IL-1β, TNF-α, iNOS and IL-10 expression. (C) Intensity of PCR bands was quantified using ImageQuant software (*p<0.01). Differences among means ± standard error of means (S.E.M.) were analyzed using ANOVA followed by Tukey-Kramer multiple comparisons test (n=3-5). (D) Intensity of western bands was quantified using ImageQuant software (*p<0.01). Differences among means ± standard error of means (S.E.M.) were analyzed using ANOVA followed by Tukey-Kramer multiple comparisons test (n=3-5).
C. IL-1β, TNF-α and IL-10 expression in microglia
To identify the specific cell types expressing TNF-α, IL-1β and IL-10 in the cerebral cortex, double immunofluorescence staining with Iba1 for microglia and TNF-α, IL-1β, IL-10 (Fig. 3 A) or GFAP for astrocyte and IL-1β, TNF-α, IL-10 (Fig. 3 B) was performed at 3, 6, 10, 14 and 17 months. Fluorescence images from each channel of the double-labeled sections were merged. The results showed that IL-1β, TNF-α and IL-10 expression was localized mainly in Iba1-immunopositive microglia.
Fig. 3. IL-1β, TNF-α and IL-10 expression in microglia. Double-immunofluorescence
staining with antibodies to (A) Iba1 for microglia and IL-1β, TNF-α and IL-10, (B) GFAP for astrocyte and IL-1β, TNF-α and IL-10 at the different time points (3, 6, 10, 14 and 17 months). Images from the double-labeled tissue were merged. Scale bar: 10 μm.
D. ROS production and neuronal damage in the cerebral cortex of APP/PS1 mice
Aβ1-42 was found to generate ROS through NADPH oxidase, leading to
neurodegeneration in rat brain in vivo (Rosales-Corral et al., 2004) and in vitro (Abramov
et al., 2007). Thus, we investigated whether fibrillar Aβ mediates the production of ROS
in the cerebral cortex of transgenic mouse model of AD (APP/PS1). To examine this possibility, we performed in situ analysis of ROS production by hydroethidine histochemistry (Wu et al., 2003). The fluorescent products of oxidized hydroethidine were detected from 6 months, and there were significantly increased between the ages of 14 months and 17 months (Fig. 4 A). Recent studies have demonstrated that NADPH oxidase is a significant source of ROS in Alzhimer’s disease (Wilkinson et al., 2006). Double-immunofluorescence staining was performed with a combination of antibodies against Iba1 and gp91phox or GFAP and gp91phox. gp91phox protein was localized to
Iba1-immunopositive microglia in the cerebral cortex of 14 month-old APP/PS1 mice (Fig. 4 B). Next, we have investigated the neuronal damage in the APP/PS1 transgenic mouse model of AD. Cerebral cortex of 14, 17-month-old APP/PS1 transgenic mice showed significant decreases in NeuN-positive neurons and MAP2-immunoreactive dendrites (Fig. 4 C).
Fig. 4. ROS production and neuronal damage in the cerebral cortex of APP/PS1 mice. (A) In situ visualization of O2- and O2--derived oxidant production. Animals were
injected with hydroethidine at 3, 6, 8, 10, 12, 14 and 17 months. Confocal micrographs show ethidium fluorescence (red). Nuclei were counterstained with Hocheset33258 (blue). Scale bar: 100 μm. (B) Double-immunofluorescence staining with antibodies to Iba1 for microglia and gp91phox at 14 months. Images from the double-labled tissue were merged.
Scale bar: 10 μm. (C) Immunohistochemistry staining with antibodies to NeuN (neuronal nuclei) and MAP2 (microtubule associated protein 2) for neuron at the different time points (3, 6, 8, 10, 12, 14 and 17 months). Scale bar: 50 μm., 100 μm.
. D
Ⅳ
ISCUSSION
Microglia and astrocyte are a key component of the inflammatory response inthe brain and are associated with Aβ plaques in Alzheimer'sdisease (AD). Although there is evidence that glial activationis important for the pathogenesis of AD, the role of gliain cerebral amyloidosis remains obscure. The present study wasundertaken to investigate the temporal relationship between Aβ deposition and glial activation in the cerebral cortex of APP/PS1 transgenic mice. In transgenic mice, we showed accumulation of fibrillar Aβ from 6 months of age up to the oldest age studied, 17 months. Activated microglia and astrocytes increased synchronously with Aβ deposits and were abundant and closely associated with senile plaques. At 14-17 months, microglia and astrocytes were morphologically damaged and excessively activated (Fig. 1). Recent studies showed that despite the capability of microglia to degrade amyloid deposits, microglial cells are unable to effectively reduce the Aβ deposition in the brains of APP23 transgenic mice and human AD patients (Bornemann et al., 2001; Fiala et al., 2005).
Previous studies indicate the potentially toxic effects of activated microglia, due to their production of neurotoxic substances, such as glutamate (Piani et al., 1991; Piani et
al., 1992), nitric oxide (Chao et al., 1992; Dawson et al., 1994), superoxide anion (Thery et al., 1991), and cytokines with neurotoxic actions (Banati et al., 1993).
Pro-inflammatory cytokines such as IL-1β and TNF-α are over-expressed in activated microglia surrounding plaques in AD (McGeer et al., 2001). They can then also stimulate the production of Aβ peptides (Blasko et al., 1999). The balance between pro- and
inflammatory cytokines determines the magnitude of the inflammatory response. IL-10 is known as an inhibitor of the synthesis of pro-inflammatory cytokine, including TNF-α and IL-1β (Bogdan et al., 1992; Wang et al., 1994; Bethea et al., 1999; Sawada et al., 1999). There is evidence that IL-10 has an anti-inflammatory role in the brain (Strle et al., 2001; Lee et al., 2007) and therefore might be important in down-regulating inflammatory processes associated whit AD pathology (Remarque et al., 2001). Of relevance to AD, IL-10 has been shown in vitro to inhibit Aβ-induced cytokine production in differentiated THP-1 monocytes and primary murine microglia (Szczepanik
et al., 2001). We examined the endogenous expression of IL-10 in the cerebral cortex of
APP/PS1 mice, but did not find of an association between IL-10 expression and down-regulation of brain inflammation. A notable feature is that the expression of IL-10 was detectable in 6 month-old transgenic mouse, which is 3 months later than expression of pro-inflammatory mediators (Fig. 2). Double immunoflorescence staining showed that IL-1β, TNF-α and IL-10 expression was localized mainly in Iba1-immunopositive microglia (Fig. 3). These results suggest that IL-10 production act as a negative feedback regulation in pro-inflammatory mediators expression.
In the present study, reactive oxygen species (ROS) production was detected from 6 months, and there significantly increased between the ages of 14 months and 17 months. A major subunit of NADPH oxidase, gp91phox protein was localized to
Iba1-immunopositive microglia in the APP/PS1 transgenic mice. Additionally, cerebral cortex of 14, 17 months showed significant decrease in NeuN-positive neurons and MAP2-immunoreactice dendrites (Fig 4). These results suggest that activated microglia
surrounding plaques induce excessive expression of pro-inflammatory mediators and ROS production, leading to neuronal damage.
Ⅴ
. CONCLUSION
This study showed that the activated microglia surrounding plaques induced excessive expression of pro-inflammatory mediators such as IL-1β, TNF-α, iNOS and ROS, leading to neuronal damage in the cerebral cortex of APP/PS1 mice. Our previous study demonstrated that LPS-induced endogenous expression of IL-10 in microglia down-regulated brain inflammation and neuronal damage in the rat cerebral cortex. However, in the present study, we did not find of an association between IL-10 expression and down-regulation of brain inflammation. Therefore, further understanding the mechanisms of IL-10 expression in chronic brain inflammation may be an important point to comprehend the pathogenesis of Alzheimer’s disease.
REFERENCES
1. Abramov AY, Scorziello A, Duchen MR: Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation.
J Neurosci 27: 1129-1138, 2007
2. Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T: Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383-421, 2000
3. Alpar A, Ueberham U, Bruckner MK, Seeger G, Arendt T, Gartner U: Different dendrite and dendritic spine alterations in basal and apical arbors in mutant human amyloid precursor protein transgenic mice. Brain Res 1099: 189-198, 2006
4. Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I: Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci 21: 3104-3112, 2001
5. Banati RB, Gehrmann J, Schubert P, Kreutzgerg GW: Cytotoxicity of microglia. Glia 7: 111-118, 1993
6. Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD: Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma 16: 851-863, 1999
7. Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B: TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. Faseb J 13: 63-68, 1999
8. Bogdan C, Paik J, Vodovotz Y, Nathan C: Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J
Biol Chem 267: 23301-23308, 1992
9. Boje KM, Arora PK: Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587: 250-256, 1992
10. Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M: Aβ-Induced Inflammatory Processes in Microglia Cells of APP23 Transgenic Mice. Am J Pathol 158: 63-73, 2001
11. Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK: Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149: 2736-2741, 1992
12. Chao CC, Hu S, Peterson PK: Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol 9: 189-205, 1995
13. Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM: Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology 33: 1425-1430, 1994
14. Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C: Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia 7: 75-83, 1993
15. Dietrich WD, Busto R, Bethea JR: Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol 158: 444-450, 1999
16. Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV: Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest 32: 360-371, 2002
17. Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G: Ineffective phagocytosis of amyloid-β by macrophages of Alzheimer's disease patients. J
Alzheimers Dis 7(3): 221-232; discussion 255-262, 2005
18. Fiorentino DF, Zlontnik A, Mossman TR, Howard M O’Garra A: IL-10 inhibits cytokine production by activated macrophages. J Immunol 147: 3815–3822, 1991
19. Gebicke-Haerter PJ, Spleiss O, Ren LQ, Li H, Dichmann S, Norgauer J, Boddeke HW: Microglial chemokines and chemokine receptors. Prog Brain Res 132: 525-532, 2001
20. Glezer I, Simard AR, Rivest S: Neuroprotective role of the innate immune system by microglia. Neuroscience 147: 867-883, 2007
21. Gonzalez-Scarano F, Baltuch G: Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22: 219-240, 1999
22. Goodwin JL, Uemura E, Cunnick JE: Microglial release of nitric oxide by the synergistic action of beta-amyloid and IFN-gamma. Brain Res 692: 207-214, 1995
23. Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E:
10modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci 12: 2265-2272, 2000
24. Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B: Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer's dementia and following stimulation with beta-amyloid 1-42 in vitro.
Neurosci Lett 322: 121-5, 2002
25. Ii M, Sunamoto M, Ohnishi K, Ichimori Y: beta-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res 720: 93-100, 1996
26. Jankowsky JL, Slun HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR: APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1.
Neurobiol Aging 25: 885-892, 2004
27. Klegeris A, Walker DG, McGeer PL: Activation of macrophages by Alzheimer beta amyloid peptide. Biochem Biophys Res Commun 199: 984-991, 1994
28. Klegeris A, McGeer PL: beta-amyloid protein enhances macrophage production of oxygen free radicals and glutamate. J Neurosci Res 49: 229-235, 1997
29. Koshinaga M, Katayama Y, Fukushima M, Oshima H, Suma T, Takahata T: Rapid and widespread microglial activation induced by traumatic brain injury in rat brain slices.
J Neurotrauma 17: 185-192, 2000
30. Kremlev SG, Palmer C: Interleukin-10 inhibits endotoxin-induced proinflammatory cytokines in microglial cell cultures. J Neuroimmunol 162: 71-80, 2005
31. Lee HG, Park KW, Jin BK, Lee YB: Interleukin-10 endogenously expressed in microglia prevents lipopolysaccharide-induced neurodegeneration in the rat cerebral cortex in vivo. Exp Mol Med 39: 812-819, 2007
32. Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS: Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J
Biol Chem 279: 49523-49532, 2004
33. Mallat M, Chamak B: Brain macrophages: neurotoxic or neurotrophic effector cells?
J Leukoc Biol 56: 416-422, 1994
34. Martino G: How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS disorders. Lancet Neurol 3: 372-378, 2004
35. McGeer PL, McGeer EG: Inflammation, autotoxicity and Alzheimer disease.
Neurobiol Aging 22: 799-809, 2001
36. Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F: Activation of microglial cells by beta-amyloid protein and interferon-gamma.
Nature 374: 647-650, 1995
37. Mizuno T, Sawada M, Marunouchi T, Suzumura A: Production of IL-10 by mouse glial cells in culture. Biochem Biophys Res Comm 205: 1907-1915, 1994
38. Molina-Holgado E, Vela JM, Arevalo-Martin A, Guaza C: LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci 13: 493-502, 2001
39. Morgan JH, Gamblin TC, Adkins JR, Groves JR, Dalton ML, Ashley DW: Norepinephrine is a more potent inhibitor of tumor necrosis factor over a range of doses than dopamine. Am Surg 70: 526-528, 2004
40. Mrak RE, Griffin WS: Interleukin-1 and the immunogenetics of Alzheimer disease. J
Neuropathol Exp Neurol 59: 471-476, 2000
41. Nguyen MD, Julien JP, Rivest S: Innate immunity: the missing link in neuroprotection
and neurodegeneration? Nat Rev Neurosci 3: 216-227, 2002
42. Perry VH, Bolton SJ, Anthony DC, Betmouni S: The contribution of inflammation to acute and chronic neurodegeneration. Res Immunol 149: 721-725, 1998
43. Piani D, Frei K, Do KQ, Cuenod M, Fontana A: Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett 133: 159-162, 1991
44. Piani D, Spranger M, Frei K, Schaffner A, Fontana A: Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol 22: 2429-2436, 1992
45. Polazzi E, Gianni T, Contestabile A: Microglial cells protect cerebellar granule neurons from apoptosis: evidence for reciprocal signaling. Glia 36: 271-280, 2001
46. Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG: atients with Alzheimer's disease display a pro-inflammatory phenotype. Exp Gerontol 36: 171-176, 2001
47. Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH,
Brachova L, Bradt B, Ward P: Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 89: 10016-10020, 1992
48. Rosales-Corral S, Tan DX, Reiter RJ, Valdivia-Velazquez M, Acosta-Martinez JP, Ortiz GG: Kinetics of the neuroinflammation-oxidative stress correlation in rat brain following the injection of fibrillar amyloid-beta onto the hippocampus in vivo. J
Neuroimmunol 150: 20-28, 2004
49. Ryu JK, Kim J, Choi SH, Oh YJ, Lee YB, Kim SU, Jin BK: ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport 13: 1611-1615, 2002
50. Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T: Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J
Neurochem 72: 1466-1471, 1999
51. Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P: Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173-177, 1999
52. Selkoe DJ, Schenk D: Alzheimer's disease: molecular understanding predicts
amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43: 545-584, 2003
53. Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B: Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer's disease patients. Lab Invest 66: 223-230, 1992
54. Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW: Interleukin-10 in the brain. Crit Rev Immunol 21: 427-449, 2001
55. Szczepanik AM, Funes S, Petko W, Ringheim GE: IL-4, IL-10 and IL-13 modulate A beta (1-42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol 113: 49-62, 2001
56. Thery C, Chamak B, Mallat M: Cytotoxic Effect of Brain Macrophages on Developing. Eur J Neurosci 3: 1155-1164, 1991
57. Tsunawaki S, Sporn M, Ding A, Nathan CF: Deactivation of macrophages by transforming growth factor β. Nature 334: 260–262, 1988
58. Van der Wal EA, Gomez-Pinilla F, Cotman CW: Transforming growth factor-beta 1 is in plaques in Alzheimer and down pathologies. Neuroreport 4: 69-72, 1993
59. Van Muiswinkel FL, Veerhuis R, Eikelenboom P: Amyloid beta protein primes cultured rat microglial cells for an enhanced phorbol 12-myristate 13-acetate-induced respiratory burst activity. J Neurochem 66: 2468-2476, 1996
60. Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S: The role of glial cells in Parkinson's disease. Curr Opin Neurol 14: 483-489, 2001
61. Wang L, Goillot E, Tepper RI: IL-10 inhibits alloreactive cytotoxic T lymphocyte generation in vivo. Cell Immunol 159: 152-169, 1994
62. Wilkinson BL, Landreth GE: The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation 3: 30, 2006
63. Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S: NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A 100: 6145-6150, 2003
64. Xia MQ, Hyman BT: Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J Neurovirol 5: 32-41, 1999
- 국문요약 -
알츠하이머 동물모델 (APP/PS1 Transgenic Mice)에서
IL-1β, TNF-α, iNOS, IL-10, ROS 생성에 대한 연구
아주대학교 대학원 의학과 이 환 구
(지도교수: 이 용 범)
본 연구에서는 알츠하이머 동물모델 (APP/PS1 Transgenic Mice) 대뇌피질 에서 염증성 매개물질인 IL-1β, TNF-α, iNOS, ROS와 IL-10의 발현을 관찰 하였다. 유전자이식 생쥐에서 우리는 6개월부터 17개월에 이르기까지 섬유성 아밀로이드 베타 (fibrillar β-amyloid)의 축적이 늘어난다는 것을 확인하였다. 마이크로글리아와 아스트로사이트의 활성화는 아밀로이드 베타의 침착과 같은 양상으로 계속해서 증가되며, 노인성 반 (senile plaque)과 면밀하게 접근되어 나타나며, 14개월에서 17개월의 마이크로글리아와 아스트로사이트는 형태학적 으로 손상되어 있으며 과도하게 활성화되어 있었다. IL-1β, TNF-α, iNOS의 mRNA와 단백질 레벨은 3개월부터 확인할 수 있으며 시간에 따라 증가된다. IL-10의 mRNA와 단백질 레벨은 6개월째 유전자이식 생쥐에서 확인할 수 있 는데, 이는 염증성 매개물질들의 발현보다 3개월 늦은 것이다. 이중면역형광 염색 (double immunoflorescence staining)으로 IL-1β, TNF-α, IL-10의 발
현이 Iba1-면역양성을 보이는 마이크로글리아에서 대부분 배치됨을 관찰하였 다. 활성산소종 (ROS) 생성은 8개월부터 나타나며 14개월과 17개월 사이에서 두드러지게 증가되었다. NADPH oxidase의 주요 구성물질인 gp91phox 단백질 은 Iba1-면역양성을 보이는 마이크로글리아에서 대부분 배치됨을 관찰하였다. 또한, 14개월과 17개월의 대뇌피질에서 NeuN-양성적 뉴론과 MAP2-면역반 응적인 수지상 돌기가 상당히 감소되어 있음을 관찰하였다. 이 결과들은 알츠 하이머병 뇌조직에서 섬유성 아밀로이드 베타 (fibrillar β-amyloid)의 축적에 따라, 활성화된 마이크로글리아가 염증성 매개물질의 과도한 발현과 NADPH oxidase 활성화에 의한 활성산소종 생성을 유도하여 뉴론의 손상을 야기시킬 수 있음을 보여준다. 핵심어: 알츠하이머 질병, 인터루킨-10, 마이크로글리아, IL-1β, TNF-α, iNOS, 활성산소종 38