Agric. Chem. Biotechnol. 46(3), 97-99 (2003)
Article
Antibacterial Activity of Oriental Medicinal Plant Extracts toward Helicobacter pylori
Haeng-Byoung Lee, Hyun-Kyung Lee and Young-Joon Ahn*
School of Agricultural Biotechnology, Seoul National University, Seoul 151-742, Korea Received August 4, 2003; Accepted September 8, 2003
The antibacterial activity of the methanol extracts from 64 oriental medicinal plant species toward Helicobacter pylori ATCC 43504 was examined using an impregnated paper disc bioassay. At 1 mg · disc−1, good anti-H. pylori activity was observed with the extracts of Alpina officinarum rhizome, Alpina oxyphylla fruit, Angelica tenuissina root, Asiasarum heterotropoides root, Lindera strychnifolia root, and Polygonum cuspidatum rhizome. These plants merit further study as potential H. pylori eradicating agents.
Key words : Helicobacter pylori, natural antibacterial agent, medicinal plant. A. officinarum rhizome and A. tenuissima root.
In humans, Helicobacter pylori is a microaerophilic gram- negative bacterium that colonizes the stomachs of about half of the worlds population.1) The H. pylori infection is highly associated with a number of the most important diseases of the upper gastrointestinal tract. These diseases include gastric inflammation, chronic superficial gastritis, duodenal and gastric ulcers, gastric adenocarcinoma, and non-Hodgkins lymphomas of the stomach.14) In developing countries, 70- 90% of the population carries H. pylori, whereas the prevalence of infection in developed countries is lower, ranging from 25 to 50%.4) Most infections by H. pylori are acquired in childhood and persist lifelong if not properly eradicated. Eradication of the H. pylori infection is primarily dependent on the continued application of triple therapies.
These therapies consist of a mixture of two antibiotics, such as amoxicillin, clarithromycin, and/or metronidazole with bismuth or a proton pump inhibitor, which are still the most effective drugs.1) Although effective, repeated use of these chemical drugs have sometimes resulted in the development of resistance1,5-7) and had undesirable effects on nontarget organisms, such as intestinal microorganisms.8,9) These problems have highlighted the need for the development of new strategies for selective H. pylori eradication.
Plants may be an alternative source of material for the H.
pylori eradication because they constitute a rich source of bioactive chemicals. Little research has been undertaken in relation to the eradication of H. pylori, despite the excellent pharmacological actions of oriental medicinal plants.10,11)
This paper describes a laboratory study to assess the potential of plant extracts for use as commercial antibacterial agents. The antibacterial activity of 64 oriental medicinal plant
species in 37 families toward H. pylori was assessed.
Materials and Methods
Helicocbacter pyroli strain. H. pylori ATCC 43504 was obtained from the American Type Culture Collection (Rockville, MD, USA). Stock cultures of the strain were routinely stored at −60oC on Brucella broth (Difco, Detroit, MI, USA) containing 5% bovine calf serum (Hyclone, Longan, UT, USA) and 20% glycerol.
Plants and sample preparation. A total of 64 oriental medicinal plant species in 37 families were purchased from the Boeun Medicinal Herb Shop, Kyungdong Market, Seoul, Korea (Table 1). They were dried in an oven at 40oC for 2 days, then finely powdered. Each 50 g sample of the test plants was twice extracted with 300 ml of methanol at room temperature for 2 days, then filtered. The combined filtrate was concentrated to dryness by rotary evaporation at 40oC.
Table 1 gives the yield of each methanolic extraction.
Microbiological assay. H. pylori ATCC 43504 was incubated microaerobically on Brucella agar that was supplemented with 5% bovine calf serum at 37oC for 3 days in anaerobic jars (Hirayama, Tokyo, Japan). The colonies were suspended in 10 ml of Brucella broth. The inoculum (0.1 ml) was prepared to contain 1×10 7-8 CFU·ml−1 by adjusting the turbidity of the suspension.
An impregnated paper disc bioassay was used for the anti- H. pylori activity of the test materials. The plant samples were tested at doses of 5, 1, 0.5, and 0.1 mg·disc−1. A sample in 0.1 ml of methanol was applied by a microsyringe to the paper discs (ADVANTEC, 8-mm diameter and 1-mm thickness, Toyo Roshi, Japan). After drying in a fume hood, the discs were placed on the agar surface that was inoculated with H.
pylori. All of the plates were incubated at 37oC for 3 days under microaerophilic conditions in anaerobic jars. Diameters
*Corresponding author
Tel: 82-2-880-4702; Fax: 82-2-873-2319 E-mail: yjahn@snu.ac.kr
98 Haeng-Byoung Lee et al.
of the inhibition zones were recorded. The control discs received 0.1 ml of methanol. All of the inhibition tests were replicated at least three times.
The antibacterial activity was classified as follows: very strong response, zone diameter à30 mm; strong response, zone diameter 21-29 mm; moderate response, zone diameter 16-20 mm; weak response, zone diameter 11-15 mm; and no response, zone diameter ß10 mm.
Results and Discussion
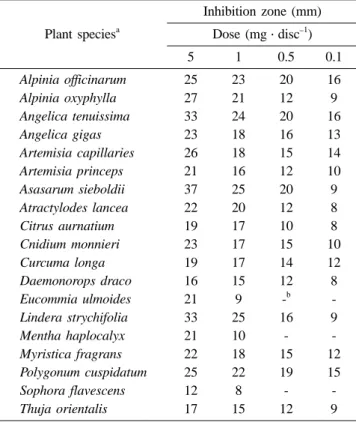
When the methanol extracts of the 64 test plant samples were bioassayed by the impregnated paper disc, significant differences were observed in the antibacterial activity against
H. pylori (Table 2). Potencies varied according to plant species and dose. Of these, 14 plant species at 5 mg·disc−1 showed potent antibacterial activity toward H. pylori (inhibition zone, >20 mm). At 1 mg·disc−1, strong anti-H.
pylori activity was produced from extracts of Alpina officinarum rhizome, Alpina oxyphylla fruit, Angelica tenuissina root, Asiasarum heterotropoides root, Lindera strychnifolia root, and Polygonum cuspidatum rhizome. At 0.1 mg·disc−1, the extracts of A. officinarum rhizome and A.
tenuissima root gave good antibacterial activity toward H.
pylori. These plant species might be good candidates for naturally-occurring H. pylori eradicating agents.
Differential susceptibility of H. pylori to the plant extracts from the same plant species was also observed (Table 2). The Table 1. List of 64 medicinal plant species tested for Helicobacter pylori ATCC 43504
Family Plant species Tissue
Useda Yield
(%)b Family Plant species Tissue
Useda Yield
(%)b Amaranthaceae Achyranthes bidentata Ro 12.1 Labiatae Elsholtzia ciliata Wp 3.6
Amarylidaceae Curculigo orchioides Rh 8.8 Mentha haplocalyx Wp 11.3
Apiaceae Angelica gigas Ro 14.8 Prunella vulgaris subsp. asiatica Sp 9.7
Angelica tenuissima Ro 12.0 Wp 5.9
Cnidium monnieri Fr 6.5 Schizonepeta tenuifolia Wp 6.9
Notopterygium incisum Rh 11.1 Lauracea Lindera strychnifolia Ro 7.2
Araceae Pinellia ternate Tu 5.6 Liliaceae Ophiopogon japonicus Tu
Aristolochiaceae Asiasarum heterotropoides Ro 5.0 Meliaceae Melia azedarach Fr 10.2
Brassicaceae Brassica hirta Se 0.4 Melia toosendan Fr 2.6
Chenopodiaceae Kochia scoparia Fr 5.0 Moraceae Morus alba Fr 5.0
Compositae Artemisia capillaries Wp 13.3 Lf 10.0
Artemisia princeps var. orientalis Wp 15.5 Ro 3.9
Aster tataricus Ro, Rh 31.9 St 9.7
Atractylodes lancea Rh 19.8 Myristicaceae Myristica fragrans Se 14.9
Atractylodes ovata Rh 9.1 Oleaceae Forsythia suspense Fr 16.8
Taraxacum mongolicum Wp 8.2 Palmae Daemonorops draco Re 63.6
Tussilago farfara Fl 15.6 Plantaginaceae Plantago asiatica Se 1.2
Xanthium strumarium Fr 1.4 Polygonaceae Polygonumcuspidatum Rh 24.9
Convolvulaceae Pharbitis nil Se 7.6 Polypodiaceae Pyrrosia lingua Wp 3.8
Cornaceae Cornus officinalis Fr 56.3 Rosaceae Prunus mume Fr 20.8
Cupressaceae Thuja orientalis Lf 16.8 Sanguisorba officinalis Ro 13.4
Cynomoriaceae Cynomorium songaricum Wp 16.7 Rutaceae Citrus aurnatium Fr 7.4
Dipsacaceae Dipsacus asperoides Ro 38.5 Phellodendron amurense Ba 17.0
Ebenaceae Diospyros kaki Lf 12.2 Sapindaceae Euphoria longana Ar 23.1
Eucommiaceae Eucommia ulmoides Ba 8.5 Saururaceae Houttuynia cordata Wp 12.0
Euphorbiaceae Ricinus communis Se 43.3 Scrophulariaceae Rehmannia glutinosa var. purpurea Ro 17.6
Fabaceae Astragalus membranaceus Ro 9.7 Solanaceae Lycium chinense Fr 14.6
Dolichos lablab Se 4.6 Solanum nigrum Wp 7.3
Kalopanax pictum Ba 13.7 Valerianaceae Patrinia scabiosaefolia Ro 15.9
Sophora angustifolia Ro 10.7 Zingiberaceae Alpinia officinarum Rh 9.2
Sophora japonica Fl 6.2 Alpinia oxyphylla Fr 10.1
Sophora japonica Fr 21.9 Amomum kravanh Fr 1.3
Spatholobus suberectus Ca 11.0 Amomum villosum Fr 3.8
Gramineae Lophatherum gracile Wp 9.0 Curcuma longa. Rh 11.1
aAr, aril; Ba, bark; Ca, caulis; fl, flower; Fr, fruit; Lf, leaf; Se, seed; St, stem; Re, resin; Rh, rhizome; Ro, root; Sp, spike; Tu, tuber;
and Wp, whole plant.
b(Dried weight of methanol extract/dried weight of sample) ×100.
Anti-Helicobacter pylori activity of medicinal plants 99
anti-H. pylori activity was more pronounced in the A.
tenuissima root extract than in the A. gigas root. Similar differences in the response of H. pylori to two Artemisia species and two Artractylodes species were likewise observed.
These results suggest that quantitative and/or qualitative chemical composition among the plant species may be different.
Plant extracts and phytochemicals have potential as products for H. pylori eradication because many of them are selective, often biodegrade to nontoxic products, and may be applied to humans in the same way as other conventional chemical drugs.12-15) Additionally, some plant-derived materials are highly effective against drug-resistant H.
pylori.16)
The results of this study indicate that some plant extracts that are described could be useful for eradicating H. pylori.
Further research is necessary to establish whether humans exert the anti-H. pylori activity in vivo after consumption of these materials.
Acknowledgments. This work was supported by grants from the NaturoBiotech Co. Ltd. and the Ministry of Education for the Brain Korea 21 Project of the Korean Government to YJA.
References
1. Dunn, B. E., Cohen, H. and Blaser, M. J. (1997) Helico- bacter pylori. Clin. Microbiol. Rev. 10, 720-741.
2. Pounder, R. E. and Ng, D. (1997) The prevalence of Heli- cobacter pylori infection in different countries. Aliment.
Pharmacol. Ther. 9, S33-S39.
3. Blaser, M. J. (1992) Helicobacter pylori: its role in disease.
J. Clin. Infect. Dis. 15, 381-393.
4. Taylor, D. N. and Parsonnet, J. (1995) Epidemiology and natural history of H. pylori infections. In Infections of the Gastrointestinal Tract, Blaser, M. J., P. F. Smith, J. Ravdin, H. Greenberg and R. L. Guerrant (eds.), pp. 551-564, Raven Press, New York, USA.
5. Goddard, A. F. and Longan, R. P. H. (1996) Antimicrobial resistance and Helicobacter pylori. J. Antimicrob.
Chemother. 37, 639-643.
6. Graham, D. Y., Boer, W. A. de and Tytgat, G. N. (1996) Choosing the best anti-Helicobacter pylori therapy: effect of antimicrobial resistance. Am. J. Gastroenterol. 91, 1072- 1076.
7. Karim, Q. N. and Logan, R. P. H. (1996) Emerging pat- terns of Helicobacter pylori (H. pylori) antimicrobial resis- tance In Europe. Gut 39, A51.
8. Ahn, Y. J., Park, S. J., Lee, S. G., Shin, S. C. and Choi, D.
H. (2000) Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostrid- ium spp. J. Agric. Food Chem. 48, 2744-2748.
9. Zoppi, G., Cinquetti, M., Benini, A., Bonamini, E. and Minelli, E. B. (2001) Modulation of the intestinal ecosys- tem by probiotics and lactulose in children during treat- ment with ceftriaxone. Curr. Ther. Res. Clin. E. 62, 418- 435.
10. Tang, W. and Eisenbrand, G. (1992) In Chinese Drugs of Plant Origin, Springer, New York, USA.
11. Namba, T. (1993) In Coloured Illustrations of Wakan-Yaku (The Crude Drugs in Japan, China and the Neighboring Countries), Hoikusha, Osaka, Japan.
12. Mitsher, L. A., Leu, R. P., Bathala, M. S., Wu, W. N., Beal, J. L. and White, R. (1972) Antimicrobial agents from higher plants. I: Introduction, rationale and methodology.
Lloydia 35, 157-166.
13. Fabry, W., Okemo, P. and Ansborg, R. (1996) Activity of East African medicinal plants against Helicobacter pylori.
Chemotherapy 42, 315-317.
14. Cowan, M. M. (1999) Plant product as antimicrobial agent.
Clin. Micorobiol. Rev. 12, 564-582.
15. Yesilada, E., Gurbuz, I. and Shibata, H. (1999) Screening of Turkish anti-ulcerogenic folk remedies for Helicobacter pylori anti-activity. J. Ethnopharmacol. 66, 289-293.
16. Fukai, T., Marumo, A., Kaitou, K., Kanda, T., Terada, S.
and Nomura, T. (2002) Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 71, 1449-1463.
Table 2. Antibacterial activity of 19 selected medicinal plant extracts toward Helicobacter pylori ATCC 43504 using the impregnated paper disc bioassay
Plant speciesa
Inhibition zone (mm) Dose (mg·disc−1)
5 1 0.5 0.1
Alpinia officinarum 25 23 20 16
Alpinia oxyphylla 27 21 12 9
Angelica tenuissima 33 24 20 16
Angelica gigas 23 18 16 13
Artemisia capillaries 26 18 15 14
Artemisia princeps 21 16 12 10
Asasarum sieboldii 37 25 20 9
Atractylodes lancea 22 20 12 8
Citrus aurnatium 19 17 10 8
Cnidium monnieri 23 17 15 10
Curcuma longa 19 17 14 12
Daemonorops draco 16 15 12 8
Eucommia ulmoides 21 9 -b -
Lindera strychifolia 33 25 16 9
Mentha haplocalyx 21 10 - -
Myristica fragrans 22 18 15 12
Polygonum cuspidatum 25 22 19 15
Sophora flavescens 12 8 - -
Thuja orientalis 17 15 12 9
aPlants showing >20 mm of inhibition zone at 10 mg·disc−1.
bNot determined.