Introduction
Despite being the most common spinal vascular malformation, spinal arteriovenous fistula (AVF) is often
underdiagnosed. Diagnosis of this disease is difficult because symptoms are nonspecific and similar to other radiculopathy related to spinal stenosis. However, with delay in diagnosis and treatment, symptoms slowly progress and become more severe. Jellema et al. 1 reported a 15month median delay in diagnosis for 62 of 80 patients with AVF. Here we report an unusual case wherein a spinal AVF patient with progressive weakness in the bilateral lower limbs had been incorrectly diagnosed due to his symptoms and medical
항암치료를 시행한 환자의 척수 경막 동정맥루에 의한 흉부 척수병증에 대한 증례보고
전소연
1
, 조이찬1
, 임성훈1
, 홍보영1
, 인연권2
, 김준성1
가톨릭대학교 성빈센트병원 1재활의학과, 2영상의학과
A Rare Case of Thoracic Myelopathy Caused by
Spinal Dural Arteriovenous Fistula in Chemotherapy Treated Patient
So Yeon Jun 1 , Leechan Jo 1 , Seong Hoon Lim 1 , Bo Young Hong 1 , Yon Kwon Ihn 2 , Joon-Sung Kim 1
Departments of
1Rehabilitation Medicine and
2Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
Received January 2, 2017
Revised (1st) February 11, 2017, (2nd) March 31, 2017 Accepted April 5, 2017
Corresponding Author: Joon-Sung Kim
Department of Rehabilitation Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, 93 Jung-bu-daero, Paldal-gu, Suwon 16247, Korea
Tel: 82-31-249-7650, Fax: 82-31-249-4481, E-mail: svpmr@chol.com
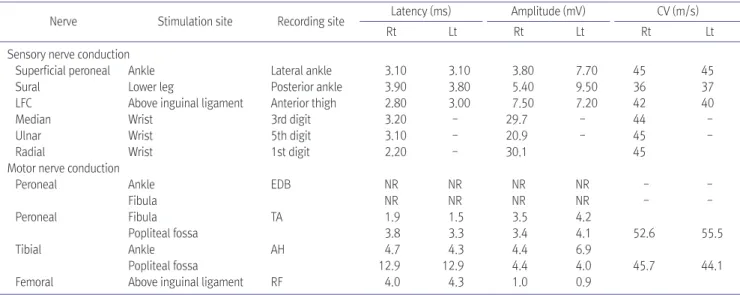
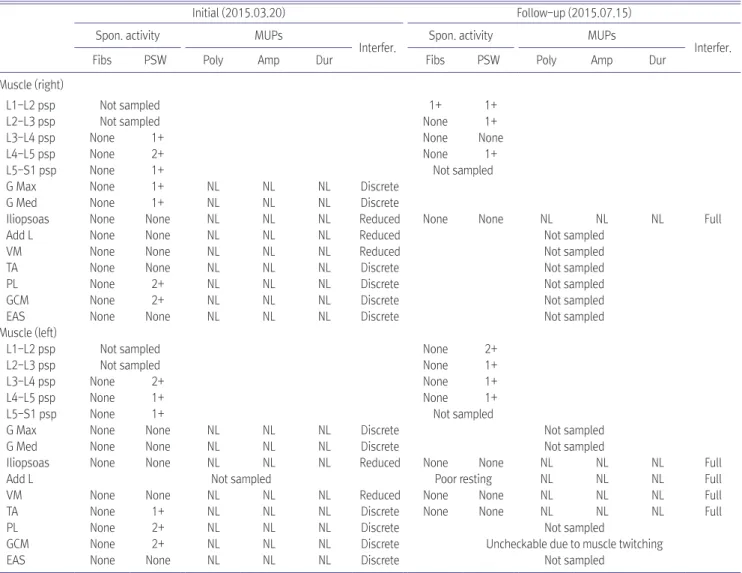
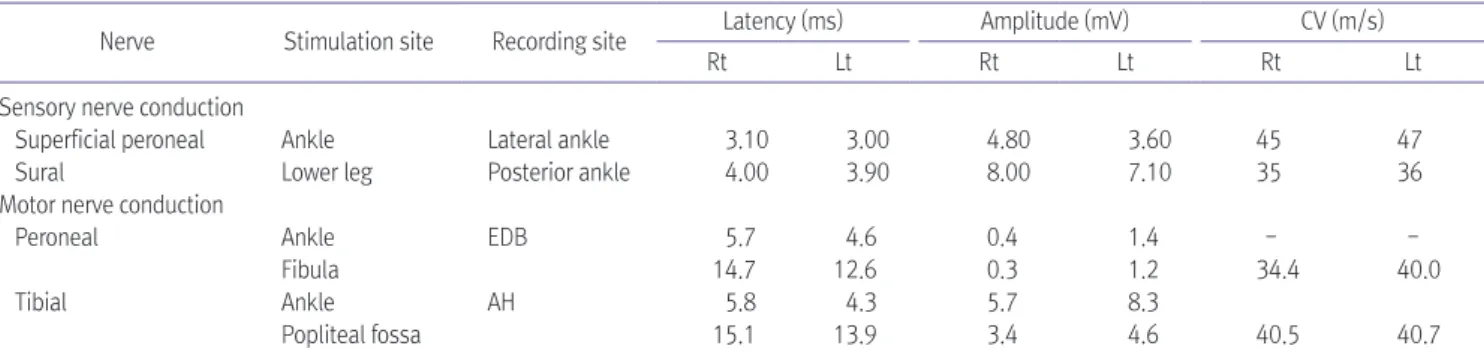
Spinal arteriovenous fistula is the most common vascular malformation of the spinal cord and causes progressive impairment like paraplegia or tetraplegia. Diagnosis of the disease is often challenging because its heterogeneous presentation mimics those of other neurological disorders, such as lumbosacral radiculopathy or peripheral polyneuropathy. Here we report a case of thoracic myelopathy resulting from spinal arteriovenous fistula in a patient treated with chemotherapy. In our clinic, he was diagnosed with spinal arteriovenous fistulas through magnetic resonance imaging and spinal angiography. Electromyography was used to evaluate the clinical features of the patient.
Subsequently, he underwent surgery and was transferred to our outpatient department to continue rehabilitation therapy. Eventually, the motor power grade in his lower extremities improved from poor to fair and he was able to walk independently with a wheel walker.