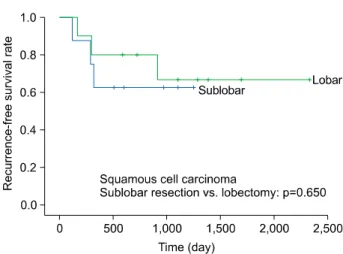
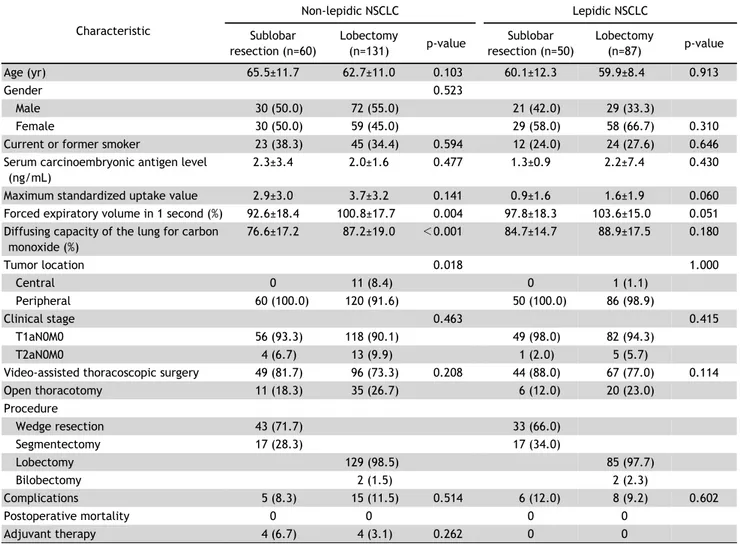
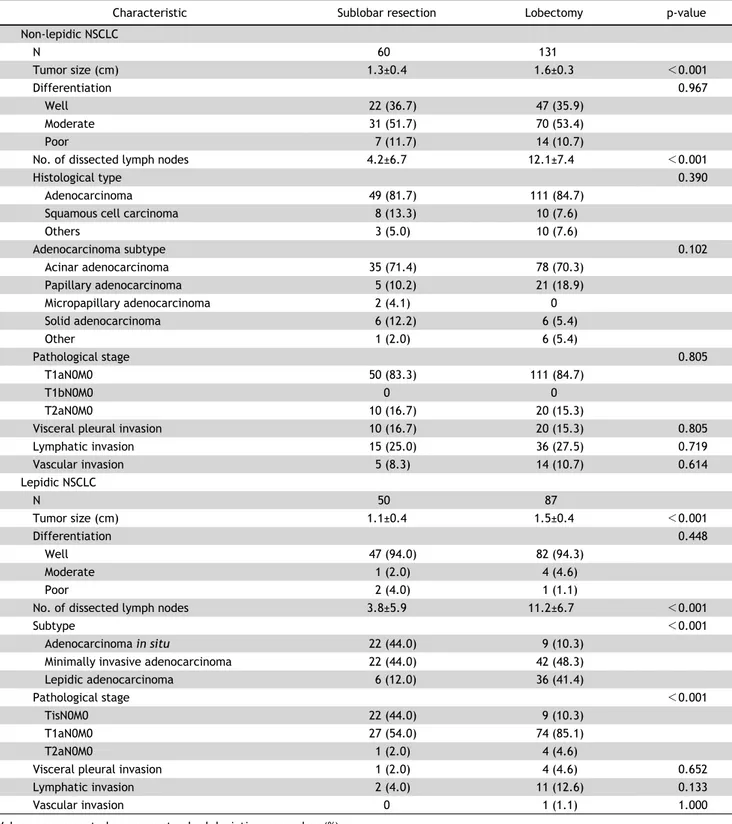
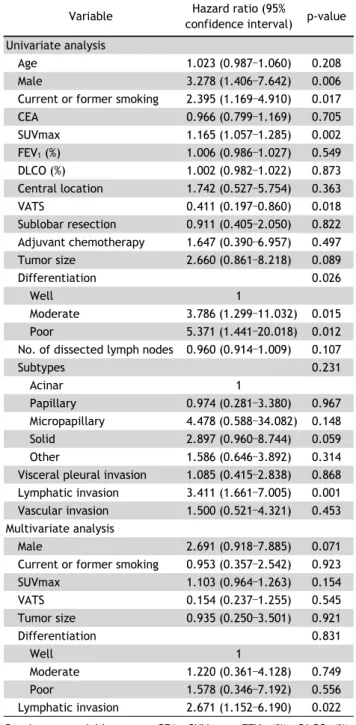
Lobectomy versus Sublobar Resection in Non-Lepidic Small-Sized Non-Small Cell Lung Cancer
전체 글
수치

관련 문서
The HbA1c level is correlated with cardiovascular risk factor in non diabetic elderly population and we suggest that HbA1c may predict cardiovascular risk as
In this study, we developed a non-toxic titanium alloy with low elastic modulus in order to improve biocompatibility, and the surface characteristics of
Overall survival in elderly extensive-stage small cell lung cancer patients received no less than four cycles of the first-line chemotherapy according to
• Phase II clinical trials of PI3Kδ inhibitor (idelalisib) showed dramatic response in patients with previously treated indolent non-hodgkin’s
Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable
Patients with breast cancer; ovarian cancer; renal cell carcinoma; pancreatic neuroendocrine cancer; colorectal cancer; head and neck cancer; non-small cell lung
Method We obtained blood samples from 49 patients who underwent surgical treatment for esophageal squamous cell cancer (ESCC) We performed ctDNA analysis with
따라서 본 연구에서는 비흡수성 차단막으로써 사용되고 있는 고가의 고어텍스 소재 의 치과의료용 차단막을 대체하기 위하여