저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Genotype- versus phenotype-directed
personalization of antiplatelet treatment in
Koreans with coronary artery disease
undergoing coronary stenting
by
Sung Gyun Ahn
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Genotype- versus phenotype-directed
personalization of antiplatelet treatment in
Koreans with coronary artery disease
undergoing coronary stenting
by
Sung Gyun Ahn
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Ph. D. of Medicine
Supervised by
Seung-JeaTahk, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Sung Gyun Ahn is approved.
SUPERVISORY COMMITTEE
Joon-Han Shin
Seung-Jae Tahk
Myong-Ho Yoon
So-Yeon Choi
Junghan Yoon
The Graduate School, Ajou University
December, 13
th, 2013
감사의 글
“소심심고 (素心深考),소박한 마음으로 돌아가서 다시 깊이 생각하라” 수학자 히로나가헤이스케가‘학문의 즐거움’에서 소개한 내용이다. 본인은 1996 년 원주의과대학 졸업 후 수원, 페루우아누꼬, 포항 등에서 내과 전문의, 국제협력의사, 심장내과 분과전문의로서 경험을 쌓고, 졸업 후 14 년이 지난 2000 년에 모교에서 부름을 받아 교수로서 삶을 영위할 수 있는 특권을 누리게 되었다. 토지의 저자 박경리 선생께서 아끼셨던 대지의 근원, 원주 (原州)의 흙을 매일 밟으면서 살고 있음에 감사한다. “Standing on the shoulders of giants”아이작 뉴턴의 고백처럼지금껏인생과 학문분야에서많은 거장과 스승들을 만났고 그들의 어깨 위에서 더 깊고 넓은 학문의 세계를 맛볼 수 있었다.먼저 페루까지 국제전화를해서심장내과 연구강사로받아주시고,심혈관중재학의 A 부터 Z 까지 지도해 주신진정한마에스트로탁승제 교수님, 본인의 첫 SCI 논문의 책임저자로 도움을 주셨던 늘 한결같은 신준한 교수님, 심장내과 chief 전공의 시절부터형님리더십을 보여주셨던 윤명호 교수님, 내과의사로서 첫 걸음마를 잘띌 수 있게 도와주셨던 최소연 교수님께 진심으로 감사를 드린다. 이젠 삶의 터전이 된 원주에서 가족처럼 따뜻하게 맞아주셨고, 지금도많은 도움을 주고 계신 윤정한, 이승환, 유병수, 김장영, 안민수, 윤영진, 이준원 교수님과 이지현 연구강사께도 감사를 드린다.귀찮은 서류작업에 많은 도움을 주신 배현경선생께도 감사의 마음을 전한다. 삶의 이유 중 하나인 가족의 헌신과 배려가 없었다면 지금의 나는 불가능했을 것이다. 보석 같은 아내 장이선과 자랑스런 세 아이들 의진, 의민, 희원이에게 나의 사랑을 듬뿍 전한다. 아들을 위해 매일 눈물로 기도하시고, 아낌없이 주는 나무가 되어주신어머님과, 항상저의 편이 되셔서 격려해주실 뿐만 아니라 때때로 용돈까지 챙겨 주시는 마음 넓은 장인어르신 내외께도 진심으로 감사를 드린다. 지면부족으로 일일이 열거하지 못한 분들께는 앞으로 살면서 차근차근 보답하겠노라고 다짐한다. 마지막으로 나의 주, 나의 하나님께 이 모든 영광을 돌린다.i
- ABSTRACT -
Genotype- versus phenotype-directed personalization of antiplatelet
treatment in Koreans with coronary artery disease undergoing
coronary stenting
Sung Gyun AhnDepartment of Medical Sciences The Graduate School, Ajou University
(Supervised by Professor Seung-Jea Tahk)
We evaluated the effectiveness of genotype- versus phenotype-directed individualization for use of P2Y12 inhibitors to decrease high on-treatment of platelet
reactivity (HOPR). Sixty-five patients undergoing percutaneous coronary intervention for non-ST elevation acute coronary syndromes were randomly assigned to genotype- or phenotype-directed treatment. All patients were screened for the CYP2C19 *2, *3, or *17 alleles by using Verigene CLO assay (Nanosphere, Northbrook, IL, USA). On-treatment platelet reactivity was measured using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). 21 CYP2C19 *2 or *3 carriers (65.6%) or 11 patients with HOPR (33.3%), defined as ≥230 of P2Y12 reaction unit (PRU), were given 90 mg ticagrelor twice
daily; non-carriers or patients without HOPR were given 75 mg clopidogrel daily. The primary endpoint was the percentage of patients with HOPR after 30 days of treatment. PRU decreased following both genotype- and phenotype-directed therapies (242±83 vs. 109±90,
ii
p<0.001 in the genotype-directed group; 216±74 vs. 109±90, p=0.001 in the phenotype-directed group). Five subjects (16.2%) in genotype-phenotype-directed group and one (3.3%) in the phenotype-directed group had HOPR at day 30 (p=0.086). All patients with HOPR at the baseline who received ticagrelor had a PRU value of <230 after 30 days of treatment. Conversely, clopidogrel did not lower the number of patients with HOPR at the baseline. Tailored antiplatelet therapy according to point-of-care genetic and phenotypic testing is feasible and effective in decreasing HOPR after 30 days.
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FIGURES ··· ⅳ LIST OF TABLES ··· ⅴ ABBREVIATION ··· ⅵ . Ⅰ INTRODUCTION ··· 1 . Ⅱ SUBJECTS AND METHODS ··· 3A. SUBJECTS ··· 3
1. Trial design and study population ··· 3
B. METHODS ··· 5
1. VerifyNowP2Y12 test ··· 5
2. Verigene CLO assay ··· 5
3. Phenotype- versus genotype-guided antiplatelet regimen ··· 6
4. Definitions and endpoints ··· 6
5. Statistical analysis ··· 7 . Ⅲ RESULTS ··· 8 . Ⅳ DISCUSSION ··· 17 . Ⅴ CONCLUSION ··· 21 REFERENCES ··· 22 국문요약 ··· 29
iv
List of Figures
Fig. 1. Schematic diagram of the study. CYP: cytochrome, PCI: percutaneous coronary intervention, PRU: P2Y12 reaction unit, ST-ACS: ST-elevation acute coronary
syndrome ··· 4
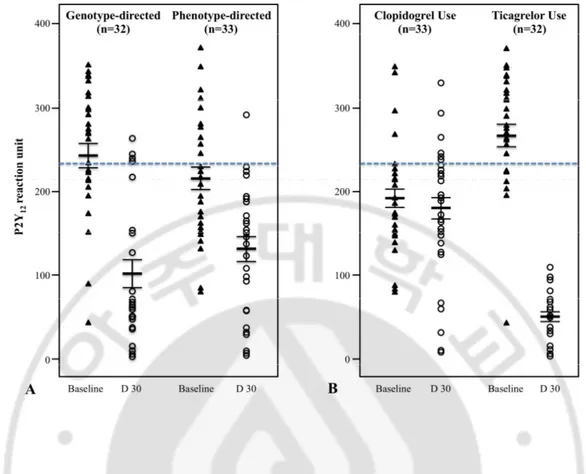
Fig. 2. Comparison of on-treatment of platelet reactivity between the baseline and at 1 month follow-up according to the personalization strategy for antiplatelet therapy (A), and the type of antiplatelet agents (B) ··· 16
v
List of Tables
Table 1. Baseline characteristics ··· 9
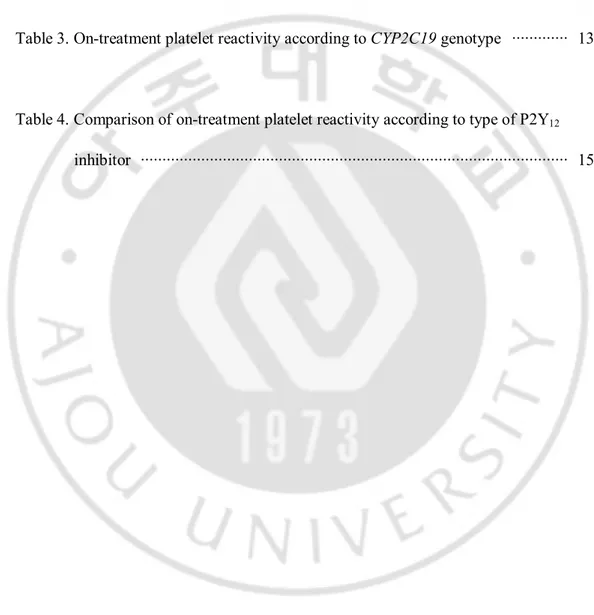
Table 2. Comparison of on-treatment platelet reactivity ··· 12
Table 3. On-treatment platelet reactivity according to CYP2C19 genotype ··· 13
Table 4. Comparison of on-treatment platelet reactivity according to type of P2Y12
vi
ABBREVIATION
ACS = Acute coronary syndromes DAPT = Dual antiplatelet therapy DES = Drug-eluting stent
CV = Cardiovascular
CYP = Cytochrome
HOPR = High on-treatment platelet reactivity
MI = Myocardial infarction
1
-I. INTRODUCTION
The advent of P2Y12 inhibitors with antiplatelet effect has expanded the use of
drug-eluting stent (DES) for occlusive coronary artery disease. Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel has been considered as the standard treatment in patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI), because DAPT has been shown to reduce the incidence of myocardial infarction (MI) or death from cardiovascular (CV) causes (Yusulf et al., 2001; Levine et al., 2011). However, despite of great efficacy of DAPT including clopidogrel, incident ischemic events still occured substantially in patients with ACS (Tang et al., 2007).
Clopidogrel is a prodrug that is converted to its active metabolite principally by cytochrome (CYP) P450 2C19, at which time it inhibits platelet activation by irreversible binding with P2Y12 receptor. Genetic polymorphism in ABCB1, CYP2C19 may affect the
absorption and metabolism, respectively of clopidogrel, and consequently alter its pharmacodynamics. As a result, there exists high inter-individual variability in the responsiveness to clopidogrel and on-treatment platelet reactivity (OPR). High OPR (HOPR) with clopidogrel is associated with an increased risk of ischemic events with patients with ACS undergoing PCI (Lee K et al., 2009; Marcucci et al., 2009; Ahn et al., 2012).
Newer potent P2Y12 inhibitors, such as prasugrel or ticagrelor have been shown to
be superior to clopidogrel in terms of their ability to decrease events in patients with ACS. However, their benefits were limited because of increased episodes of bleeding (Wiviott et al., 2007; Wallentin et al., 2009). Personalization of antiplatelet treatment for ACS may be
2
-necessary to attain the maximum inhibition of platelet activation with minimum risk of bleeding. The recent development of point-of-care assay kits, VerifyNow P2Y12 (Patti et al., 2008; Price, 2009; Price et al., 2011) or Verigene CLO assays (Buchan et al., 2011), for the assessment of platelet function and CYP2C19 polymorphism, respectively, has enabled the immediate assignment of individualized antiplatelet treatment. However, as yet, studies using high-dose clopidogrel or prasugrel have not conclusively demonstrated a clinical benefit of the phenotype- (Price et al., 2011; Trenk et al., 2012) or genotype-directed personalization of antiplatelet therapy (Roberts et al., 2012) based on residual platelet reactivity, or the presence of CYP2C19 loss-of-function alleles
Therefore, the aim of this study was to investigate the feasibility of point-of-care genotypic and phenotypic testing to guide individualized antiplatelet therapy using ticagrelor in Korean patients with non-ST elevation ACS undergoing PCI. We also compared the effectiveness of genotype-guided versus phenotype-guided antiplatelet therapy in terms of decreasing the number of patients with HOPR after 30 days.
3
-II. SUBJECTS AND METHODS
A. Subjects
1. Trial design and study population
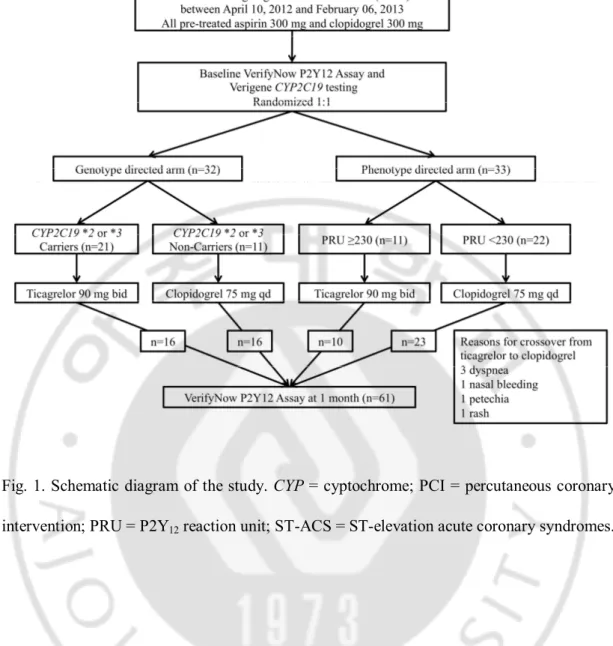
The present study was a single center, prospective, randomized, proof-of-concept trial. The study flow is shown in Fig. 1. The study protocol was approved by the Ethical Review Board of Wonju Severance Christian Hospital (Wonju, South Korea). Informed consent was obtained from all participants.
Between April 10, 2012, and February 06, 2013, 65 patients aged 18-75 were randomly assigned to genotype- or phenotype-directed treatment according to a random-number table. Patients were eligible if they had undergone PCI for non-ST-elevation ACS. The patients presenting with ST-segment elevation MI and who had severe left ventricular dysfunction (ejection fraction, <30%), or cardiogenic shock were excluded from the study. Other exclusion criteria were bradyarrhythmia, a history of transient ischemic or cerebrovascular attacks, a platelet count of <150,000/mL, hematocrit <30%, and a creatinine clearance rate of <30 ml/min. Patients who received a periprocedural thombolytic agent or glycoprotein IIb-IIIa inhibitor, or who planned to use oral anticoagulants or other antiplatelet agent such as cilostazol, were also excluded.
4
-Fig. 1. Schematic diagram of the study. CYP = cyptochrome; PCI = percutaneous coronary intervention; PRU = P2Y12 reaction unit; ST-ACS = ST-elevation acute coronary syndromes.
5
-B. Methods
1. VerifyNow P2Y12 test
OPR was measured using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). Blood samples were obtained 12 to 24 hours after PCI and after a 30-day follow-up period. Each sample was placed in a tub containing 3.2% citrate, and the P2Y12 reaction unit
(PRU) was assessed within 2 hours, as previously described (Price, 2009). The HOPR was defined as PRU value ≥230 based on previous studies involving Caucasian subjects (Patti et al., 2008; Bonello et al., 2010). Although studies conducted among Korean subjects revealed that HOPR of an approximate PRU of 270 is necessary to predict future cardiovascular event (Ko et al., 2011; Ahn et al., 2012; Park et al., 2012), we selected a PRU value of 230 as the cutoff, in order to include more patients who might benefit from phenotype-guided antiplatelet therapy.
2. Verigene CLO assay
All patients were screened for the CYP2C19 *2, *3, or *17 allele by using the Verigene CLO assay (Nanosphere, Northbrook, IL, USA). The Verigene CLO assay is an automated sample-to-result microarray-based assay in which DNA extracted from whole blood samples is hybridized to allele-specific probes immobilized on a glass slide. The detection of captured DNA is achieved using nanoparticle-conjugated probes that have been demonstrated to provide excellent sensitivity and that eliminate the need for a target amplification step prior to array hybridization (Jannetto et al., 2010). The Verigene CLO assay accurately identified homozygous and heterozygous *2 and *3 phenotypes with
6
-specificity of 100% and a final call rate 99.7%. The assay automated and can produce a result in approximately 3.5 hours (Saracini et al., 2012).
3. Phenotype- versus genotype-guided antiplatelet regimen
Regardless of their prior exposure to clopidogrel, all patients received 300 mg of aspirin and 300 mg of clopidogrel before arriving at the catheterization room. A bolus of unfractionated heparin (70 U/kg) was administered immediately before coronary angiography though the introducer sheath. A second bolus of unfractionated heparin (70 U/Kg) was administered immediately before the PCI. Additional heparin was administered to achieve an activated clotting time of 250–300 s. PCI was performed using balloon predilatation, followed by DES deployment via transradial or transfemoral arteries. The choice regarding the specific type of DES was left to be determined by the operator’s discretion. All patients were given 100 mg of aspirin daily after PCI. Patients with HOPR or who were CYP2C19 *2 or *3 carriers were also given 90 mg of ticagrelor twice daily. Patients without HOPR or who were non-carriers were given of 75 mg clopidogrel daily (Fig. 1).
4. Definitions and endpoints
The primary endpoint was the percentage of patients with HOPR after 30 days of DAPT. The secondary endpoints were 1) PRU, ∆PRU, %IPA, and ∆%IPA, 2) the correlation between the presence of CYP2C19 *2 or *3 carriers and HOPR at baseline, 3) the incidence of bleeding by Bleeding Academic Research Consortium (BARC) definition (Mehran et al., 2011), 4) major adverse cardiac events (MACE) defined as the composite of death from CV
7
-causes, non-fatal myocardial infarction, ischemia-driven target lesion revascularization or stent thrombosis.
5. Statistical analysis
The proportion of patients with HOPR after 1 month of DAPT in the genotype-directed group is assumed to be 5% (Roberts et al., 2012). On a ratio basis of 80%, we calculated that we needed 44 subjects per group to detect a 20% difference the groups. The test statistic used was the two-sided Z test with pooled variance. The significance level of the test was targeted at 0.05. Assuming a dropout rate of 14%, we would therefore need 50 patients each in the genotype- and phenotype-directed groups, forming a total of 100 patients. Enrollment was ceased at 65 patients because the number of Verigene CLO assay kits was insufficient. Analysis was based on intent-to-treat or per-treatment where necessary. All continuous variables are presented as means±SD and were analyzed using the Student’s t-test. Categorical variables are presented as frequencies (percentage), and were analyzed using the Chi-square test or Fisher’s exact test. We compared the change in platelet reactivity between baseline and after 1 month in the genotype-guided and phenotype-guided groups with Student’s t-test. The significance level was defined as p-value <0.05. All analyses were performed with SPSS 20.0 (SPSS Inc., Chicago, Ill, USA).
8
-III. RESULTS
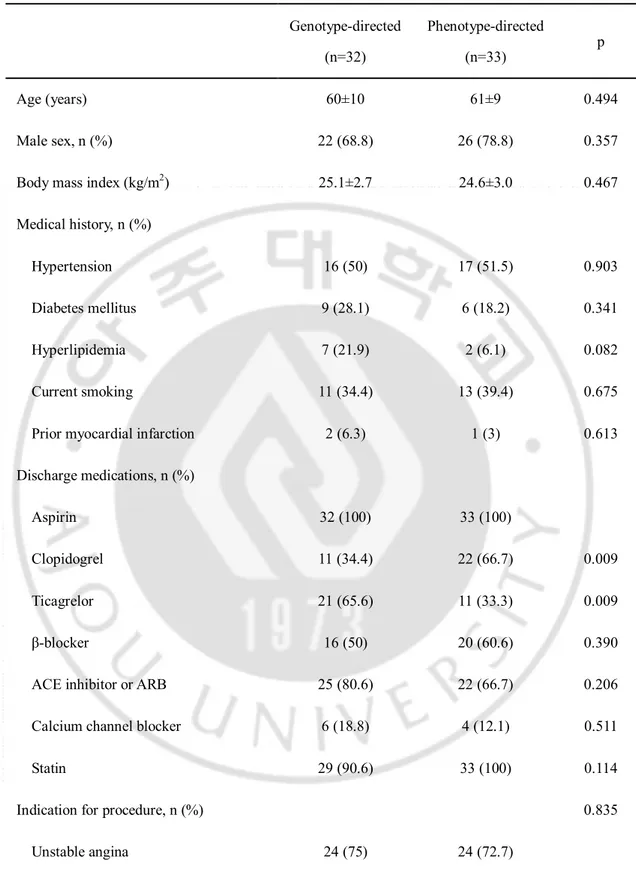
Of the 32 patients of the genotype-directed group, 21 CYP2C19 *2 or *3 carriers (65.6%) were administered ticagrelor and 11 CYP2C19 *2 or *3 non-carriers (34.4%) were administered clopidogrel. Of the 33 patients of the phenotype-directed group, 11 patients with HOPR (33.3%) were administered ticagrelor and 22 patients without HOPR (66.7%) were administered clopidogrel. In the genotype-directed group, there were five crossover cases from ticagrelor to clopidogrel because of dyspnea (2 patients), nasal bleeding (1 patient), petechia on the whole body (1 patient), and generalized rash (1 patient). In the phenotype-directed group, one crossover from ticagrelor to clopidogrel occurred due to dyspnea. Sixty-one patients (95.3%) had follow-up Verifynow P2Y12 assay, 31 and 30 in the genotype- and phenotype-directed groups. respectively (Fig. 1). Baseline clinical and procedural characteristics between the groups are presented in Table 1. The two groups were balanced with respect to age, sex, and histories of diabetes mellitus, hypertension, hyperlipidemia, current smoker, and MI. The distribution of discharge medication use between the two groups was similar, except for use of clopidogrel and ticagrelor. Clopidogrel was taken more frequently and ticagrelor less frequently by patients in the phenotype-guided group. (clopidogrel: 34.4% vs. 66.7%, p=0.009, genotype vs. phenotype, p=0.009; ticagrelor: 65.6% vs. 33.3%, genotype vs. phenotype, p=0.009). The indications for PCI, left ventricular ejection fraction, and distribution of vessels stented did not differ between the groups. Procedural variables including the extent of coronary artery disease, stent number, total stent length, and mean stent diameter were also similar.
9
-Table 1. Baseline characteristics
Genotype-directed (n=32) Phenotype-directed (n=33) p Age (years) 60±10 61±9 0.494 Male sex, n (%) 22 (68.8) 26 (78.8) 0.357 Body mass index (kg/m2) 25.1±2.7 24.6±3.0 0.467
Medical history, n (%)
Hypertension 16 (50) 17 (51.5) 0.903 Diabetes mellitus 9 (28.1) 6 (18.2) 0.341 Hyperlipidemia 7 (21.9) 2 (6.1) 0.082 Current smoking 11 (34.4) 13 (39.4) 0.675 Prior myocardial infarction 2 (6.3) 1 (3) 0.613 Discharge medications, n (%)
Aspirin 32 (100) 33 (100)
Clopidogrel 11 (34.4) 22 (66.7) 0.009 Ticagrelor 21 (65.6) 11 (33.3) 0.009 β-blocker 16 (50) 20 (60.6) 0.390 ACE inhibitor or ARB 25 (80.6) 22 (66.7) 0.206 Calcium channel blocker 6 (18.8) 4 (12.1) 0.511
Statin 29 (90.6) 33 (100) 0.114
Indication for procedure, n (%) 0.835 Unstable angina 24 (75) 24 (72.7)
- 10 -
NSTEMI 8 (25) 9 (27.3)
Left ventricular ejection fraction (%) 61±10 64±9 0.323
Vessels stented 0.900
Left main artery 2 (6.3) 1 (3) Left anterior descending artery 15 (46.9) 16 (48.5) Circumflex artery 6 (18.8) 6 (18.2) Right coronary artery 9 (28.1) 10 (30.3) Procedural variables
Vessels/patent 1.3±0.5 1.3±0.5 0.943 Stents/patient 1.8±1.1 1.5±0.8 0.271 Total stent length (mm) 40±26 35±26 0.420 Stent diameter (mm) 3.2±0.4 3.4±1.0 0.478
Values are expressed as number (%) or mean±SD. ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; NSTEMI = non-ST-segment elevation myocardial infarction.
- 11 -
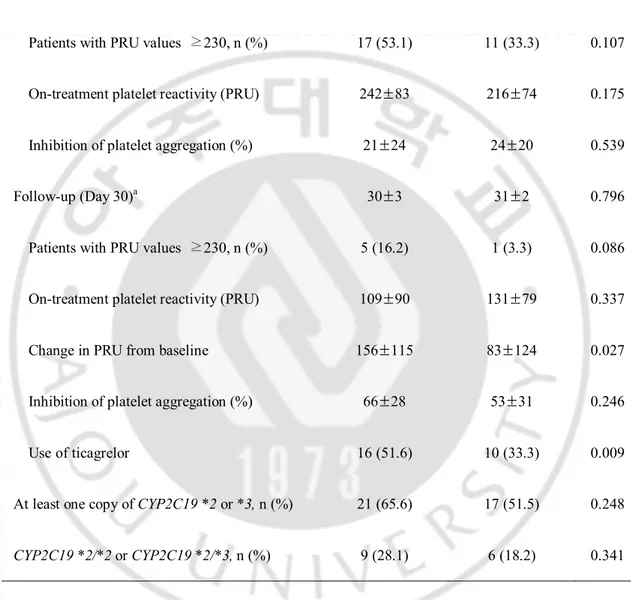
The comparison of OPR according to the personalization strategy of DAPT is as shown in Table 2. At the baseline, there were no differences in OPR, %IPA and the percentage of patients with HOPR between the groups. OPR decreased following both genotype- and phenotype-directed therapies (242±83 vs. 109±90, p<0.001 in the genotype-directed group; 216±74 vs. 109±90, p=0.001 in the phenotype-directed group). At day 30, the percentage of patient with HOPR tended to be higher in the genotype-directed group (16.2% vs. 3.3%, p=0.086). At the 30-day follow-up, PRU and %IPA were similar between the groups. However, ∆PRU was higher in the genotype- versus the phenotype-directed group (156±115 vs. 83±124, p=0.027). This may be due to the fact that more patients were administered ticagrelor in the genotype-directed group (51.6% vs. 33.3%, p=0.009).
Approximately 35% of patients were heterozygous for CYP2C19 *2 or *3 carriers. 23% were homozygous for CYP2C19 *2 or were carriers of CYP2C19 *2/*3 (Table 3). Baseline OPR was higher in patients expressing CYP2C19 *2/*2 or CYP2C19 *2/*3 compared to that in patients expressing wild-type CYP2C19*1/*1 or CYP2C19*1/*17 and in patients heterozygous for CYP2C19 *2 or *3 (280±50 vs. 214±80, p=0.004). However, no difference was found in the baseline OPR between CYP2C19 *2 or *3 non-carriers and those heterozygous for CYP2C19 *2 or *3 (217±84 vs. 209±78, p=0.880). After 30 days, ∆OPR from the baseline to follow-up was higher among patients expressing CYP2C19 *2/*2 or
- 12 -
Table 2. Comparison of on-treatment platelet reactivity
Genotype-directed (n=32) Phenotype-directed (n=33) p Baseline
Patients with PRU values ≥230, n (%) 17 (53.1) 11 (33.3) 0.107
On-treatment platelet reactivity (PRU) 242±83 216±74 0.175
Inhibition of platelet aggregation (%) 21±24 24±20 0.539
Follow-up (Day 30)a 30±3 31±2 0.796
Patients with PRU values ≥230, n (%) 5 (16.2) 1 (3.3) 0.086
On-treatment platelet reactivity (PRU) 109±90 131±79 0.337
Change in PRU from baseline 156±115 83±124 0.027
Inhibition of platelet aggregation (%) 66±28 53±31 0.246
Use of ticagrelor 16 (51.6) 10 (33.3) 0.009
At least one copy of CYP2C19 *2 or *3, n (%) 21 (65.6) 17 (51.5) 0.248
CYP2C19 *2/*2 or CYP2C19 *2/*3, n (%) 9 (28.1) 6 (18.2) 0.341
a61 patients (95.3%) had follow-up VerifyNow P2Y12 assay: 31 in the genotype-directed
group and 30 in the phenotype-directed group. CYP = cytochrome; PRU = P2Y12 reaction
- 13 -
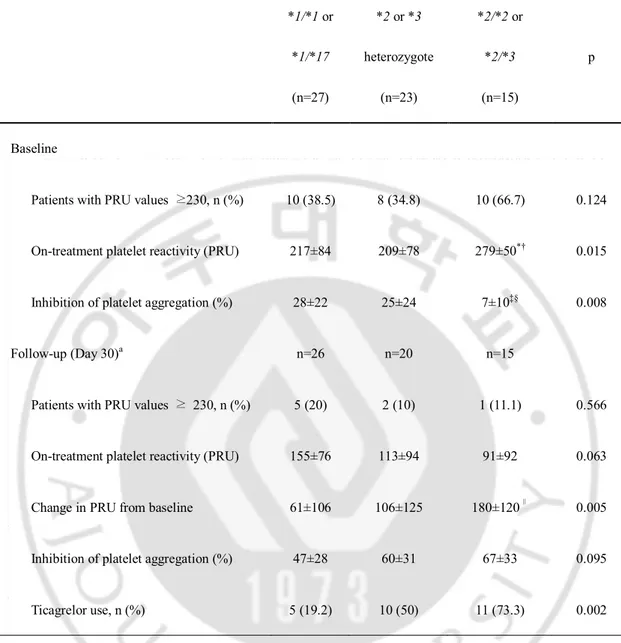
Table 3. On-treatment platelet reactivity according to CYP2C19 genotype *1/*1 or *1/*17 (n=27) *2 or *3 heterozygote (n=23) *2/*2 or *2/*3 (n=15) p Baseline
Patients with PRU values ≥230, n (%) 10 (38.5) 8 (34.8) 10 (66.7) 0.124
On-treatment platelet reactivity (PRU) 217±84 209±78 279±50*† 0.015
Inhibition of platelet aggregation (%) 28±22 25±24 7±10‡§ 0.008
Follow-up (Day 30)a n=26 n=20 n=15
Patients with PRU values ≥ 230, n (%) 5 (20) 2 (10) 1 (11.1) 0.566
On-treatment platelet reactivity (PRU) 155±76 113±94 91±92 0.063
Change in PRU from baseline 61±106 106±125 180±120∥ 0.005
Inhibition of platelet aggregation (%) 47±28 60±31 67±33 0.095
Ticagrelor use, n (%) 5 (19.2) 10 (50) 11 (73.3) 0.002
aFollow-up VerifyNow P2Y12 assay was not available in one patient with CYP2C19 *1/*1 or CYP2C19 *1/*17 and 3 patients with CYP2C19 *2 or *3 heterozygote. *p=0.039 vs.
CYP2C19 *1/*1 or CYP2C19 *1/*17. †p=0.019 vs. CYP2C19 *2 or *3 heterozygote. ‡p=0.007 vs. CYP2C19 *1/*1 or CYP2C19 *1/*17. §p=0.046 vs. CYP2C19 *2 or *3
heterozygote. ∥p = 0.004 vs. CYP2C19 *1/*1 or CYP2C19 *1/*17. Abbreviations as in
- 14 -
The number of patients with HOPR at baseline was greater in those allocated ticagrelor; however, none had HOPR after 30 days of treatment. The number of patients with HOPR did not change after 30 days of clopidogrel treatment (Table 4). Baseline OPR was significantly higher in patients slated to receive ticagrelor. After 30 days of treatment, OPR was lower (49±30 vs. 179±77, p <0.001) in patients taking ticagrelor compared to clopidogrel (Fig. 2); accordingly, ∆PRU from baseline to follow-up was higher (84±9 vs. 34±26, p <0.001) in patients given ticagrelor. There were no MACEs in either group during the 1-month follow-up period. There were two nuisance BARC type 2 bleeding events in the genotype-directed group related to ticagrelor use.
- 15 -
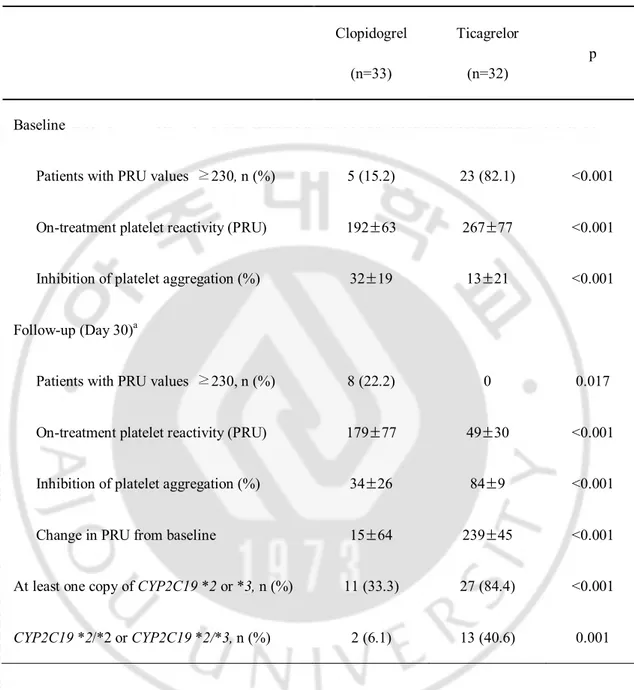
Table 4. Comparison of on-treatment platelet reactivity according to type of P2Y12
inhibitor Clopidogrel (n=33) Ticagrelor (n=32) p Baseline
Patients with PRU values ≥230, n (%) 5 (15.2) 23 (82.1) <0.001
On-treatment platelet reactivity (PRU) 192±63 267±77 <0.001
Inhibition of platelet aggregation (%) 32±19 13±21 <0.001
Follow-up (Day 30)a
Patients with PRU values ≥230, n (%) 8 (22.2) 0 0.017
On-treatment platelet reactivity (PRU) 179±77 49±30 <0.001
Inhibition of platelet aggregation (%) 34±26 84±9 <0.001
Change in PRU from baseline 15±64 239±45 <0.001
At least one copy of CYP2C19 *2 or *3, n (%) 11 (33.3) 27 (84.4) <0.001
CYP2C19 *2/*2 or CYP2C19 *2/*3, n (%) 2 (6.1) 13 (40.6) 0.001
aAt 30 days, 36 patients were given clopidogrel and 25 patients were given ticagrelor.
- 16 -
Fig. 2. Comparison of on-treatment of platelet reactivity (OPR) between baseline versus 1–month follow-up (D 30) according to the personalization strategy for antiplatelet therapy (A) and the type of antiplatelet agent (B). OPR decreased following both and phenotype-directed therapy (242±83 vs. 109±90, p <0.001 in the genotype-directed group; 216±74 vs. 109±90, p=0.001 in the phenotype-genotype-directed group). Five subjects (16.2%) in the genotype-directed group and one (3.3%) in the phenotype-directed group had HOPR after 30 days of treatment (p=0.086). After 30 days of treatment, OPR was lower in patients taking ticagrelor compared to clopidogrel (49±30 vs. 179±77, p <0.001).
- 17 -
IV. DISCUSSION
This was the first study that demonstrated the feasibility and effectiveness of antiplatelet therapy tailored by point-of-care genetic or platelet function testing to decrease the OPR in patients undergoing PCI. The major findings in the present study were that OPR was effectively decreased following both genotype- and phenotype-directed therapy and compared to clopidogrel, ticagrelor caused higher decrease in OPR regardless of the
CYP2C19 genotype.
Heightened platelet reactivity contributes to the occurrence of ischemic events especially in certain clinical subsets such as ACS, chronic kidney disease, or diabetes mellitus. HOPR with clopidogrel treatment has also been proposed to be a predictor of ischemic CV events in patients undergoing DES implantation. To date, there is little consensus on the treatment options to overcome HOPR. Studies examining the effect of high-versus standard-dose clopidogrel have yielded discrepant findings. Mehta et al. noted a reduction in CV events and stent thrombosis in patients given double-dose clopidogrel, whereas Price et al. found no effect on CV outcomes (Mehta et al., 2010; Price et al., 2011). Price et al. attributed this difference to the selection only of patients with HOPR in their study, whereas Mehta et al. enrolled patients regardless of platelet reactivity. Although the addition of cilostazol to DAPT lowered PRU levels more than DAPT, the effect of triple versus double therapy on composite CV outcomes was inconsistent in patients undergoing PCI (Chen et al., 2009; Suh et al., 2011). The more potent antiplatelet agents, prasugrel or ticagrelor, have been shown to be superior to clopidogrel in terms of reducing CV events, but with a higher occurrence of bleeding episodes (Wiviott et al., 2007; Wallentin et al.,
- 18 -
2009), which is associated with a higher risk of CV events (Eikelboom et al., 2006). In the present study, ticagrelor substantially reduced OPR after 30 days. However, two patients ceased taking ticagrelor use because of nasal bleeding and the appearance of petechiae on the whole body. Because of the concern of the increased risk of bleeding, the use of parasugrel or ticagrelor is not yet widespread in Korea, even among the ACS population after PCI. Further study is therefore warranted on the effects of reduced doses of prasugrel or ticagrelor on the clinical endpoints in Korean ACS patients.
The use of point-of-care testing to tailor antiplatelet therapy according to risk stratification for bleeding and ischemia represents an ideal strategy for ACS patients. Among these tests, the VerifyNow P2Y12 assay to measure OPR is increasingly being used given the evidence suggesting an association between HOPR and CV events (Patti et al., 2008; Bonello et al., 2010). However, there are several shortcomings to measuring OPR: 1) OPR is a surrogate marker only representing the inhibition of P2Y12 receptor mediated platelet
activation, not whole platelet activity; 2) OPR cannot be measured in patients treated with glycoprotein IIb-IIIa inhibitors or thrombolysis; 3) PRU value varies with elapsed time following ACS or PCI (Ahn et al., 2011; Campo et al., 2011); 4) the cutoff for HOPR varies according to patient ethnicity (Ko et al., 2011; Ahn et al., 2012); and 5) the relationship between low OPR and bleeding events has yet to be definitively established. Moreover, trials of personalization of antiplatelet therapy according to the result of VerifyNow P2Y12 assay have failed to demonstrate any improvement in outcomes (Price et al., 2011; Collet et al., 2012; Trenk et al., 2012).
- 19 -
By comparison, genotyping can be carried out under any clinical circumstances with no limitations with respect to timing. In previous studies, CYP2C19 * or *3 carriers have been shown to have higher OPR and poor clinical outcomes, compared with CYP2C19
*2 or *3 non-carriers (Shuldiner et al., 2009). However, genotyping has also its drawbacks.
Clopidogrel is a pro-drug that is converted to an active metabolite in two hepatic steps involving CYP1A2, 2C9, 2C19, 3A4/5, not only by 2C19. To date, point-of-care clopidogrel genetic testing is limited to CYP2C19 single nucleotide polymorphism (Buchan et al., 2011; Roberts et al., 2012); yet the CYP2C19 *2 genotype only accounts for approximately 12% of the variation in clopidogrel response (Shuldiner et al., 2009). The feasibility of genotype-guided prasugrel use has been proven in a proof-of-concept trial (Roberts et al., 2012), but the clinical benefit of genotype-guided antiplatelet therapy has yet to be demonstrated.
Although genetic testing and platelet function testing may provide additive information in selecting an antiplatelet regimen for patients undergoing PCI, their routine use is not recommended in current practice guidelines (Levine et al., 2011). Genotyping may be an improved guide for chronic antiplatelet therapy, while platelet function testing is more useful in acute settings. Nevertheless, the benefits of genotype- and phenotype-directed individualization of antiplatelet therapy to improve clinical outcomes requires validation though further randomized controlled trials.
A potential limitation of the study relates to the early termination of the trial due to the unavailability of the Verigene CLO assay during the study. Furthermore, crossover rate from ticagrelor to clopidogrel is higher in the genotype- versus the phenotype-directed group, possibly due to the higher number of patients allocated ticagrelor in the genotype-directed
- 20 -
group. Moreover, there was no standard care control group comparing genotype- versus phenotype-directed antiplatelet therapy. Finally, our analyses were based on a single determination of PRU value, which is subject to random measurement error and may have underestimated the strength of the associations.
- 21 -
V. CONCLUSION
In summary, tailoring the use of P2Y12 inhibitors based on point-of-care genetic or
phenotypic testing is feasible and seems to be efficacious in terms of decreasing HOPR after 30 days.
- 22 -
REFERENCES
1. Ahn SG, Lee SH, Sung JK, Kim JY, Yoon J: Intra-individual variability of residual platelet reactivity assessed by the VerifyNow-P2Y12 assay in patients with clopidogrel resistance after percutaneous coronary intervention. Platelets 22: 305-307, 2011
2. Ahn SG, Lee SH, Yoon JH, Kim WT, Lee JW, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH: Different prognostic significance of high on-treatment platelet reactivity as assessed by the VerifyNow P2Y12 assay after coronary stenting in patients with and without acute myocardial infarction. JACC Cardiovasc Interv 5: 259-267, 2012
3. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA, Working Group on High On-Treatment Platelet R: Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll
Cardiol 56: 919-933, 2010
4. Buchan BW, Peterson JF, Cogbill CH, Anderson DK, Ledford JS, White MN, Quigley NB, Jannetto PJ, Ledeboer NA: Evaluation of a microarray-based genotyping assay for the rapid detection of cytochrome P450 2C19 *2 and *3 polymorphisms from whole blood using nanoparticle probes. Am J Clin Pathol 136: 604-608, 2011
5. Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, Marchesini J, Bernardi F, Ferrari R, Valgimigli M: Prospective evaluation of on-clopidogrel platelet
- 23 -
reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol 57: 2474-2483, 2011
6. Chen KY, Rha SW, Li YJ, Poddar KL, Jin Z, Minami Y, Wang L, Kim EJ, Park CG, Seo HS, Oh DJ, Jeong MH, Ahn YK, Hong TJ, Kim YJ, Hur SH, Seong IW, Chae JK, Cho MC, Bae JH, Choi DH, Jang YS, Chae IH, Kim CJ, Yoon JH, Chung WS, Seung KB, Park SJ, Korea Acute Myocardial Infarction Registry I: Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 119: 3207-3214, 2009
7. Collet JP, Cuisset T, Range G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrie D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monsegu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Barthelemy O, Beygui F, Silvain J, Vicaut E, Montalescot G, Investigators A: Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 367: 2100-2109, 2012
8. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S: Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114: 774-782, 2006
9. Jannetto PJ, Buchan BW, Vaughan KA, Ledford JS, Anderson DK, Henley DC, Quigley NB, Ledeboer NA: Real-time detection of influenza a, influenza B, and respiratory syncytial virus a and B in respiratory specimens by use of nanoparticle probes. J Clin Microbiol 48: 3997-4002, 2010
- 24 -
10. Ko YG, Suh JW, Kim BH, Lee CJ, Kim JS, Choi D, Hong MK, Seo MK, Youn TJ, Chae IH, Choi DJ, Jang Y: Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J 161: 383-390, 2011
11. Lee K, Lee SW, Lee JW, Kim SY, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH: The significance of clopidogrel low-responsiveness on stent thrombosis and cardiac death assessed by the verifynow p(2)y(12) assay in patients with acute coronary syndrome within 6 months after drug-eluting stent implantation. Korean Circ J 39: 512-518, 2009
12. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH: 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124: 2574-2609, 2011
13. Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF: Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up.
- 25 -
14. Mega JL, Hochholzer W, Frelinger AL, 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS: Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 306: 2221-2228, 2011
15. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H: Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123: 2736-2747, 2011
16. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S, investigators C-Ot: Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 376: 1233-1243, 2010
17. Park KW, Park JJ, Jeon KH, Kang SH, Oh IY, Yang HM, Cho HJ, Lee HY, Kang HJ, Koo BK, Oh BH, Park YB, Kim HS: Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc Ther 30: 5-11, 2012
- 26 -
18. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G: Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol 52: 1128-1133, 2008
19. Price MJ: Bedside evaluation of thienopyridine antiplatelet therapy. Circulation 119: 2625-2632, 2009
20. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ: Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 305: 1097-1105, 2011
21. Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, Dick A, Marquis JF, O'Brien E, Goncalves S, Druce I, Stewart A, Gollob MH, So DY: Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 379: 1705-1711, 2012
22. Saracini C, Vestrini A, Galora S, Armillis A, Abbate R, Giusti B: Pharmacogenetics of clopidogrel: comparison between a standard and a rapid genetic testing. Genet Test Mol
- 27 -
23. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA: Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302: 849-857, 2009
24. Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, Koo BK, Cho YS, Youn TJ, Chae IH, Choi DJ, Rha SW, Bae JH, Kwon TG, Bae JW, Cho MC, Kim HS: Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol 57: 280-289, 2011
25. Tang EW, Wong CK, Herbison P: Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 153: 29-35, 2007
26. Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Muller U, Richardt G, Jakubowski JA, Neumann FJ: A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 59: 2159-2164, 2012
27. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF,
- 28 -
Harrington RA, Investigators P, Freij A, Thorsen M: Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045-1057, 2009
28. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, Investigators T-T: Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001-2015, 2007
29. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation.
- 29 - - 국문요약 -
관상동맥스텐트 시술을 받은 한국인 관상동맥질환 환자에서
유전자형 혹은 표현형에 따른 개인형 맞춤 항혈소판제 치료
아주대학교 대학원의학과 안 성 균 (지도교수: 탁 승 제) 유전자형 혹은 표현형 현장검사에 의한 개인형 맞춤 항혈소판 치료 후, 잔여 혈소판 활성도가 얼마나 효과적으로 감소되는지를 비교하였다. 2012 년 4 월 2013 년 2 월까지 ST 분절 비상승 급성 관동맥증후군으로 관상동맥 스텐트 시술을 받은 65 명의 환자들을 유전자형 혹은 표현형 맞춤 치료군으로 무작위 할당하였다. 모든 환자에서 Verigene CLO assay (Nanosphere, Northbrook, IL, USA)를 통해 CYP2C19*2, *3, 혹은 *17 단일 염기다형성을 측정하였고, 클로피도그렐에 대한 혈소판 활성도는 VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA)를 사용하여 측정하였다. 높은 혈소판 반응성 (High on-treatment platelet reactivity; HOPR)은 클로피도그렐 사용 후 P2Y12 반응단위(P2Y12 Reaction Unit; PRU)가 230 이상인 경우로 정의하였다. CYP2C19 *2, *3 유전자형을 나타내는 21 명의 환자 (65.6%)들과, HOPR 을 보이는 11 명의 환자들(33.3%)에게 티카그렐러 90mg 을 하루에 두번씩 투여하였고, CYP2C19
- 30 - 75mg 을 하루에 한번씩 투여하였다. 일차 종점은 항혈소판제 치료 30 일 이후에 HOPR 을 가지고 있는 환자들의 비율이었다. 30 일째 유전자형 맞춤 치료군에서 5 명의 환자 (16.2%)에서, 표현형 맞춤 치료군에서는 1 명(3.3%)의 환자에서 HOPR 을 가지고 있었다 (p=0.086). 양군 모두 시작보다 1 달 후 HOPR 의 빈도가 의미 있게 감소하였으며 (유전자형 맞춤 치료군: 53.1% vs. 16.2%, 치료 1 일 vs. 치료 30 일, p=0.022; 표현형 맞춤 치료군: 33.3% vs. 3.3%, 치료 1 일 vs. 치료 30 일, p=0.012), PRU 도 의미 있게 감소하였다 (유전자형 맞춤 치료군: 242±83 vs. 109±90, 치료 1 일 vs. 치료 30 일, p<0.001; 표현형 맞춤 치료군: 216±74 vs. 109±90, 치료 1 일 vs. 치료 30 일, p=0.001). 티카그렐러를 투여 받은 모든 환자에서 치료 후 30 일에 HOPR 은 관찰되지 않았다. 반면, 클로피도그렐 투여 받은 환자에서는 치료 하루와 30 일 사이에 높은 혈소판 활성도에 차이가 없었다. 결론적으로 유전자형 혹은 표현형 현장검사에 의한 개인형 맞춤 항혈소판제 치료는 임상에서 쉽게 적용할 수 있으며, 치료 30 일 후 HOPR 을 감소시키는 데에 효과적이다. 핵심어: 항혈소판 약제, 유전자형 검사, 혈소판 기능검사, 현장검사