저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The effect of low level light therapy on wound
healing: in vitro study with normal and keloid
fibroblast
by
Soo-Eun Jung
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
The effect of low level light therapy on wound
healing: in vitro study with normal and keloid
fibroblast
by
Soo-Eun Jung
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Master of Medicine
Supervised by
You Chan Kim, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Soo-Eun Jung is approved.
SUPERVISORY COMMITTEE
You Chan Kim
Seonghyang Sohn
You Sun Kim
The Graduate School, Ajou University
i -ABSTRACT-
The effect of low level light therapy on wound healing:
in vitro study with normal and keloid fibroblast
Background and Objectives: Low level light therapy (LLLT) is the treatment modality
which is using photobiomodulatory effect of visible to near infrared spectrum light. LLLT is used in clinically to preventing hypertrophic scar or keloid and stimulating wound healing, while the mechanism of their effects remains unclear. Therefore, we investigated the effect of 415 nm, 632 nm, and 830 nm light emitting diodes (LED) to wound healing, especially in normal dermal fibroblasts and keloid dermal fibroblasts.
Material and methods: Normal fibroblasts and keloid fibroblasts were isolated from human.
As preliminary study, we measured the viability of normal and keloid fibroblasts using EZ-CyTox viability assay after 24 hours from irradiation with 3, 10, and 30 J/cm2 of each
wavelength. Proliferation rate of both fibroblasts relative to nonirradiated controls were measured using trypan blue exclusion assay after 24 hours from irradiation with 3, 10, and 30 J/cm2 of each wavelength. After suitable fluence was determinated, further repeated
proliferation experiences were conducted. For migration assay, we made a scratched straight on the center of disc by sterile pipette tip and calculated migration rate by the filled area after 24 hours into the hollow space.
Results: There was no statistically significant effect of LLLT on both normal and keloid
fibroblasts in viability assay. Because irradiation at 3, 10, and 30 J/cm2 of each wavelength
made similar effect to normal fibroblasts, we decided to irradiate normal fibroblasts at fluence of 3 J/cm2. Because keloid fibroblasts increased their proliferation rate after
ii
Normal fibroblasts irradiated with 632 nm at 3 J/cm2 showed significantly higher
proliferation rate (p=0.034) and keloid fibroblasts irradiated with 632 nm at 10 J/cm2 showed
significantly lower proliferation rate (p=0.010). In migration assay, normal fibroblasts showed significantly higher migration rate in 830 nm wavelength and keloid fibroblasts showed no significant difference between wavelengths.
Conclusion: At the aspect of fibroblasts, 632 nm LLLT could accelerate proliferation of
normal fibroblasts, and inhibit proliferation of keloid fibroblasts. Furthermore, 830 nm LLLT could aid wound healing by increasing migration of normal fibroblasts. LLLT could modify proliferation or migration of fibroblasts, and that could stimulate wound healing and prevent hypertrophic scar or keloid.
Keyword: Keloid dermal fibroblast, Low level light therapy, Migration, Normal dermal fibroblast, Proliferation
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF FIGURES ... iv
LIST OF TABLES ... v
I. INTRODUCTION ... 1
II. MATERIAL AND METHODS ... 3
A. Isolation and culture of normal and keloid fibroblasts ... 3
B. Irradiation source ... 3 C. Viability assay ... 3 D. Proliferation assay ... 3 E. Migration assay ... 4 G. Statistical analysis ... 4 III. RESULTS ... 6 A. Viability assay ... 6 B. Proliferation assay... 8 C. Migration assay... 11 IV. DISCUSSION ... 14 V. CONCLUSION ... 17 REFERENCES ... 18 국문요약 ... 22
iv
LIST OF FIGURES
Fig. 1. Viability assay of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at fluence of 3, 10, and 30 J/cm2 ... 7
Fig. 2. Proliferation rate (%) of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at the 3, 10, and 30 J/cm2 ... 8
Fig. 3. Proliferation rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid
fibroblasts. ...10
Fig. 4. Migration assay after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid
v
LIST OF TABLES
Table 1. Viability assay of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at fluence of 3, 10, and 30 J/cm2 ... 6
Table 2. Proliferation rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in
keloid fibroblasts ... 9
Table 3. Migration rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid
1
I. INTRODUCTION
Low level light therapy (LLLT) is using photobiomodulation effect of visible to near infrared spectrum light (Posten et al., 2005). It is thought that photon absorbs at the cytochrome c oxidase enzyme which participates of the oxidative respiration of mitochondria resulting in making a cascade of gene transcription and alteration of various cellular properties (Silveira et al., 2007). Its photobiomodulatory effect is applied in clinically for reducing pain, inflammation and edema, promoting healing of wound, and preventing tissue damage. Nowadays, some clinical observations and small studies are published that LLLT is effective to preventing hypertrophic scar or keloid and stimulating wound healing without any additional pain or discomfort (Barolet and Boucher, 2010; Carvalho et al., 2010; Mamalis et al., 2013).
Wound healing is divided inflammatory phase, proliferation phase, and remodeling phase. After disruption of skin, hemostasis is occurred immediately and inflammatory cells are recruited. Neutrophils kill microbes and macrophages are engulfing the tissue debris. Growth factors, cytokines, and chemokines secreted by inflammatory cells make a complex process of interaction between many cells. During proliferation phase, macrophages secrete platelet-derived growth factors (PDGF), and transforming growth factor (TGF)-β1 and endothelial cells make new vessels. These processes make fibroblasts increase their proliferation and product type III collagen. During remodeling phase, type III collagen is degraded and replaced with type I collagen result in reconstruction of dermis and wound undergoes a contractile response (Profyris et al., 2012). There are no effective modalities accelerating wound healing yet. However, above mentioned, some studies are proved that LLLT is effective to promoting wound healing and preventing hypertrophic scar. LLLT could effect on keratinocytes, inflammatory cells and fibroblasts, and that is resulting in alteration of their motility, proliferation and secretion properties of cytokines (Posten et al., 2005; Peplow et al., 2011). Among these various cells, because fibroblasts play a crucial role in wound healing, LLLT at certain fluences and wavelengths can enhance the release growth factors from fibroblasts and stimulate cell proliferation or migration. Therefore, we planned the study that reveals
2
which wavelength and fluence could enhance fibroblast proliferation and migration. Keloid is contributed by several factors: proliferation of fibroblast, excessive production of collagen synthesis by TGF-β1 and reduction of collagen degeneration (Profyris et al., 2012). Considering excessive production of collagen generation and reduction of collagen degeneration are closely related with fibroblast activity and proliferation, fibroblast is a key cell in keloid and hypertrophic scar. LLLT could mortify keloid fibroblast view in clinical use of LLLT in preventing of recurring keloid after surgical excision or laser ablation of keloid (Barolet and Boucher, 2010). However, studies about the effect of LLLT on fibroblasts from keloid or hypertrophic scar are relatively fewer than those from normal or wounded fibroblasts.
Thus, this study aimed to investigate the effect of blue (415 nm), red (632 nm) and infrared (830 nm) light emitting diodes (LED) to wound healing, especially in normal and keloid dermal fibroblasts.
3
II. MATERIALS AND METHODS
A. Isolation and culture of normal and keloid fibroblasts
Normal fibroblasts were from human foreskin after phimosiectomy and keloid fibroblasts were from keloid-revision surgery. After washing with phosphate buffered saline (PBS) and removing fat, the dermal fibroblasts extracted from skin. Normal and keloid dermal fibroblasts cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal calf serum and 1% streptomycin at 37°C under a humidified atmosphere of 5% carbon dioxide. Fibroblasts were grown as monolayers on plastic Petri dishes and used 10th and 30th passages.
B. Irradiation source
APL® (Medro medical division, Seoul, Korea) was used for this study. This device
produced blue light (415 nm), red light (632 nm), and infrared light (830 nm). The power fluence of each wavelength is 172 mW/cm2 for blue, 205 mW/cm2 for red, 50 mW/cm2
for infrared. During irradiation, cells were maintained in 0.5ml PBS and the distance from light source is 1 cm. After irradiation, PBS was replaced by DMEM.
C. Viability assay
To find out whether LLLT could affect to cell viability, EZ-CyTox cell viability assay kit (Daeillab, Korea) was used. Normal and keloid fibroblasts were seeded at 6 x104
cells/well in 24-well plate and were irradiated with 3, 10, and 30 J/cm2 energy fluence of
LED. After irradiation, the cells were incubated for 24 hours at the atmosphere above mentioned. EZ-CyTox solution (40 μl) was added to each well and the mixtures were incubated for 3 hours at 37°C. Absorbance was measured for 3 hours using an ELISA reader (Molecular Device, Menlo Park, CA, USA) at 450nm.
D. Proliferation assay
Trypan blue exclusion assay was done for measure proliferation rate. Many previous studies about effect of LLLT used very variable fluences of light. As preliminary study to
4
determine the most suitable fluence of irradiation, we conducted 3, 10, and 30 J/cm2
fluence of LED to normal and keloid fibroblasts. Normal fibroblasts were seeded at 1 x 105 cells/well and keloid fibroblasts were seeded at 8.5 x 104 cells/well in 24-well plate.
Because cells were filled up about 80% of plate to giving uniformly irradiation to each cells, the count of fibroblasts per well is different between normal and keloid fibroblasts. After irradiation, the cells were incubated for 24 hours at the atmosphere above mentioned. The DMEM in each well was aspirated and replaced by 0.12% trypsin to detach cells from well. Then, 50 μL of this cell suspension were added to 50 μL of 0.04% trypan blue dye. To counting live cells, 10 μL of the solution were taken to a hemocytometer and examined the microscope (Olympus, Tokyo, Japan). Proliferation rate was defined the ratio of live cell count of irradiation group after 24 hour per that of non-irradiated control. After choosing the most suitable light fluence for both fibroblasts, we repeated the proliferation assay at the determinate light fluence.
D. Migration assay
Normal fibroblasts were seeded at 3 x 105 cells/well and keloid fibroblasts were
seeded at 2.5 x 105 cells/well in 6-well plate for cell migration assay. To make a cell-free
area, each dish was scratched straight on the center by sterile 2 mL yellow pipette tip. After wash-out with PBS, each wavelength of LEDs irradiated to the fibroblasts. After LED irradiation, the cells were incubated and we took photos at the 0, 12, 24 hours at the same point of each well using microscope (Olympus, Tokyo, Japan). We analyzed with the photos, and calculated cell migration area into the hollow space using computer software (Image-Pro Plus, MEDIA CYBERNETICSA, Silver Spring, MD, USA). Migration rate (%) is defined the ratio of migration area into the hollow space after 24 hours per hollow space induced by pipette tip at the initial.
G. Statistical analysis
Data are expressed as mean and standard deviation (SD). A one-way analysis of variance with Dunnett’s multiple compression test using SPSS Statistics Desktop 20.0.0 (IBM, Armonk, NY, USA) was performed. A P value <.05 was considered statistically
5 significant.
6
III. RESULTS
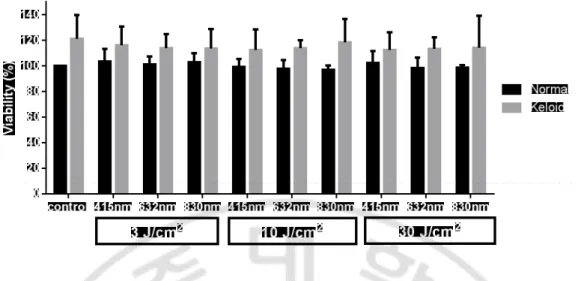
A. Viability assayThree times of experiments are conducted to find out whether LLLT could affect to cell viability. Normal fibroblasts irradiated with 3 J/cm2 in all wavelengths showed slightly
higher viability and normal fibroblasts irradiated with 10 J/cm2 in all wavelengths showed slight lower viability than nonirradiated normal control. Keloid fibroblasts were shown higher viability than normal fibroblasts, regardless of the light fluences and wavelengths. Keloid fibroblasts irradiated with any fluence and any wavelength showed slight lower viability rate than nonirradiated keloid control. However, there was no statistically significant effect of LLLT on both normal and keloid fibroblasts in viability assay (Fig. 1.and Table 1).
Table 1. Viability assay of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at fluence of 3, 10, and 30 J/cm2.
Normal Mean SD Keloid Mean SD Control 100.0 0 121.0 18.9 3 J/cm2 415 nm 103.5 9.9 116.3 14.5 632 nm 101.3 5.9 114.0 10.7 830 nm 102.8 7.1 113.7 15.1 10 J/cm2 415 nm 99.2 6.1 112.5 16.1 632 nm 97.7 6.8 113.9 6.2 830 nm 96.9 3.5 118.4 18.4 30 J/cm2 415 nm 102.3 9.2 112.3 14.0 632 nm 98.2 8.2 113.3 8.9 830 nm 98.7 1.9 114.2 25.1
7
Fig. 1. Viability assay of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at fluence of 3, 10, and 30 J/cm2. Normal fibroblasts
irradiated with 3 J/cm2 in all wavelengths showed slightly higher viability and normal
fibroblasts irradiated with 10 J/cm2 in all wavelengths showed slight lower viability than
nonirradiated normal control. Keloid fibroblasts irradiated with any fluence and any wavelength showed slight lower viability rate than nonirradiated keloid control.
8
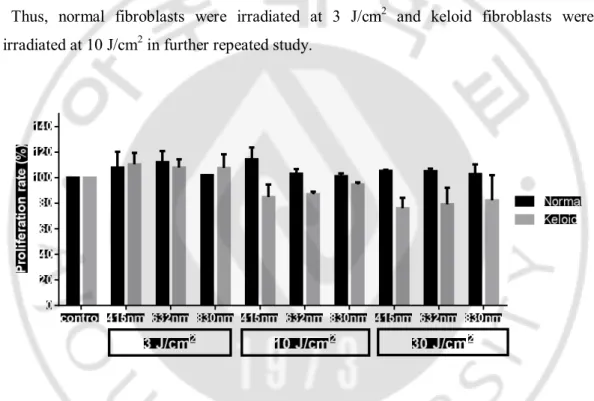
B. Proliferation assay
After performed twice experiences, proliferation rate of normal fibroblasts has a tendency to increase after irradiation in all fluences and all wavelengths. However, there were not dose-dependent results in proliferation rate of normal fibroblast among 3, 10 and 30 J/cm2 irradiation group (Fig. 2.). If irradiation at 3, 10 and 30 J/cm2 made similar
effect on cells, using lowest dose is more suitable and convenient for executing study. Proliferation rate of keloid fibroblasts at 3 J/cm2 in all three wavelengths was higher than
nonirradiated control, while proliferation rate at 10 and 30 J/cm2 is lower than
nonirradiated control (Fig. 2.). If irradiation at 10 and 30 J/cm2 showed similar effect on
cells, using lower dose is more suitable and convenient for executing study.
Thus, normal fibroblasts were irradiated at 3 J/cm2 and keloid fibroblasts were irradiated at 10 J/cm2 in further repeated study.
Fig 2. Proliferation rate (%) of normal and keloid fibroblasts after 24 hours of irradiation with 415, 632, and 830 nm, at the 3, 10, and 30 J/cm2. Proliferation rate of
normal fibroblasts has a tendency to increase after irradiation in all three fluences and all wavelengths. Proliferation rate of keloid fibroblasts is increased at the fluence of 3 J/cm2,
9
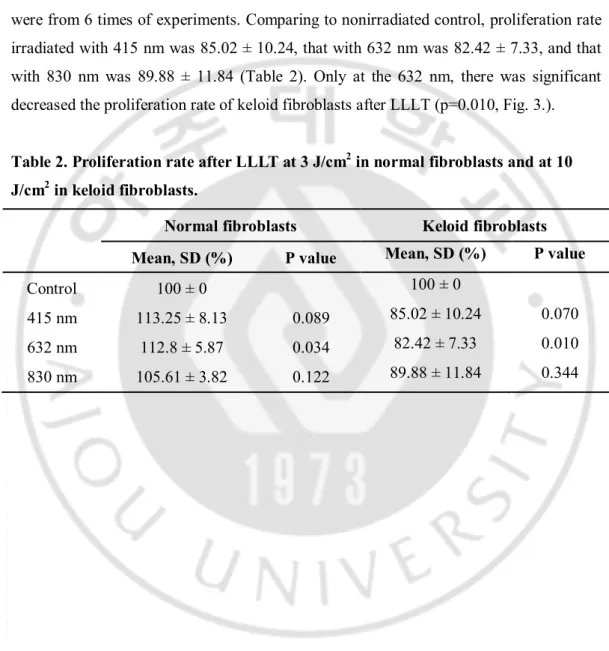
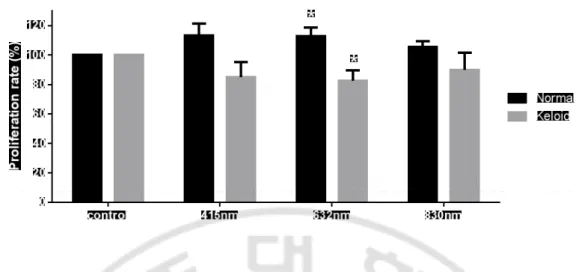
Normal fibroblasts were irradiated by fluence of 3 J/cm2 and results calculated as mean
and standard deviation were from 5 times of experience. Comparing with nonirradiated control, proliferation rate of normal fibroblasts irradiated with 415 nm was 113.25 ± 8.13, that with 632 nm was 112.8 ± 5.87, and that with 830 nm was 105.61 ± 3.82 (Table 2). Only at the 632 nm, there was significantly increase the proliferation rate after LLLT (P=0.034, Fig. 3.). Keloid fibroblasts were irradiated by fluence of 10 J/cm2 and results
were from 6 times of experiments. Comparing to nonirradiated control, proliferation rate irradiated with 415 nm was 85.02 ± 10.24, that with 632 nm was 82.42 ± 7.33, and that with 830 nm was 89.88 ± 11.84 (Table 2). Only at the 632 nm, there was significant decreased the proliferation rate of keloid fibroblasts after LLLT (p=0.010, Fig. 3.).
Table 2. Proliferation rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid fibroblasts.
Normal fibroblasts Keloid fibroblasts
Mean, SD (%) P value Mean, SD (%) P value
Control 100 ± 0 100 ± 0
415 nm 113.25 ± 8.13 0.089 85.02 ± 10.24 0.070 632 nm 112.8 ± 5.87 0.034 82.42 ± 7.33 0.010 830 nm 105.61 ± 3.82 0.122 89.88 ± 11.84 0.344
10
Fig. 3. Proliferation rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid fibroblasts. Normal fibroblasts irradiated with the 632 nm significantly
increased the proliferation rate than control. Keloid fibroblasts irradiated with the 632 nm were significantly decreased the proliferation rate than control.
11
C. Migration assay
Normal fibroblasts were irradiated by fluence of 3 J/cm2 and the results calculated as
mean and standard deviation were from 8 times of experiments. Cell migration rate (%) in control was 66.81 ± 11.57, that in 415 nm was 71.16 ± 11.27, that in 632 nm was 74.64 ± 10.60, and that in 830 nm was 85.44 ± 6.83 (Table 3). Although normal fibroblasts irradiated in any wavelength showed more migration rate than in nonirradiated control, only when irradiated with 830 nm were significant (p=0.013, Fig. 4a. and 4b.). Keloid fibroblasts were irradiated by fluence of 10 J/cm2 and results were
from 20 times of experiments. Cell migration area (%) in control was 75.02 ± 14.64, that in 415 nm was 79.15 ± 11.78, that in 632 nm was 81.74 ± 7.33, and that in 830 nm was 82.86 ± 5.77 (Table 3). Although cells irradiated in each wavelength show more migration than in nonirradiated control, there is no significant difference (Fig. 4a. and 4c.).
Table 3. Migration rate after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid fibroblasts.
Normal fibroblasts Keloid fibroblasts
Mean, SD (%) P value Mean, SD (%) P value
Control 66.81 ± 11.57 75.02 ± 14.64
415 nm 71.16 ± 11.27 0.965 79.15 ± 11.78 0.901 632 nm 74.64 ± 10.60 0.653 81.74 ± 7.33 0.364 830 nm 85.44 ± 6.83 0.013 82.86 ± 5.77 0.184
12 control 415nm 632nm 830nm 0 20 40 60 80 100 M ig ra tio n r a te ( % ) Normal Keloid
*
A13
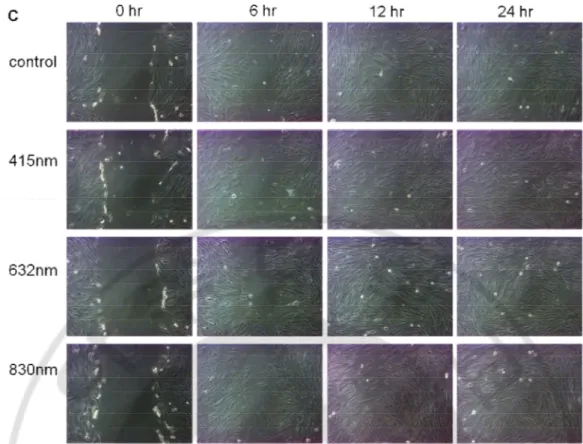
Fig. 4. Migration assay after LLLT at 3 J/cm2 in normal fibroblasts and at 10 J/cm2 in keloid fibroblasts. Migration rate (%) of normal fibroblasts was significant increased
only after irradiation with 830 nm. That of keloid fibroblasts irradiated was increased slightly than nonirradiated control, but it was no statistically significant (A). Photos of normal fibroblasts irradiated with 3 J/cm2 were taken after 0, 6, 12, and 24 hours (B).
Photos of keloid fibroblasts irradiated with 10 J/cm2 were taken after 0, 6, 12, and 24
14
IV. DISCUSSION
According to a definition of LLLT, various wavelength and energy dose are used in each study. Whereas blue light had no effect to normal fibroblast in our study, other study revealed daily irradiation at 410 nm or 420 nm inhibit proliferation of dermal fibroblast at 5-10 J/cm2 (Oplander et al., 2011). As it is reveals that the blue light has
anti-inflammatory effect and phototoxic effect on Propionibacterium acne, LLLT with blue light is used in acne treatment in clinically rather than wound healing (Kwon et al., 2013).
Red light appears to be the most frequently investigated wavelength at the cellular level. Single irradiation of 5 J/cm2 or irradiation of 2.5 J/cm2 on two consecutive days using
632 nm light stimulated migration and proliferation of fibroblasts. Furthermore, higher doses (10 and 16 J/cm2) decreased in cell viability and cell proliferation with a significant
amount of cell damage (Hawkins and Abrahamse, 2006). When normal wounded skin fibroblast cells were irradiated at 632, 830, or 1,064 nm, cells irradiated at 632 nm with 5 J/cm2 showed highest migration rate (Evans and Abrahamse, 2008; Houreld and
Abrahamse, 2008; Houreld and Abrahamse, 2010), while cells irradiated at 830 nm showed higher migration rate in our study. The previous reported study revealed that fibroblasts in diabetic condition increase cell migration, proliferation and collagen production after irradiation with 660 nm diode laser at 5 J/cm2 (Ayuk et al., 2012). The
studies executed with human gingival fibroblasts revealed that irradiation with each 670, 685, 692, 780, or 786 nm light at 2 or 3 J/cm2 accelerated proliferation and migration of
gingival fibroblasts (Almeida-Lopes et al., 2001; Saygun et al., 2008; Basso et al., 2012). On the near-infrared wavelength region, irradiation of 830 and 980 nm influences fibroblasts mitochondrial activity compared to the 2,940 nm wavelength which produces apoptosis (Crisan et al., 2013). Thought in a diabetic condition, wounded fibroblasts showed a significant increase in proliferation after 830 nm diode laser irradiation with a fluence of 5 J/cm2 (Houreld et al., 2010). These different results of proliferation and
migration could be related to the cell type and/or the wavelength used in each study. Also in this study, there is no significant effect of LLLT in EZ-CyTox proliferation assay, contrast to in trypan blue proliferation assay.
15
Although some studies reveal the mechanism of effect of LLLT, most part of mechanism of migration or proliferation of fibroblasts after LLLT are unrevealed. It is thought that photon from LLLT absorbs in cytocrome c enzyme in mitochondria, and makes an intracellular response, such as activating transcription factors, increasing reactive oxygen species, and nitric oxide. Irradiation of red (632 nm, 636 nm, and 638 nm) light is significantly increased keratinocyte growth factor, hepatocyte growth factor, basic fibroblast growth factor, and interlukin (IL)-6 and decreased tumor necrosis factor (TNF)-α, and IL-1β (Evans and Abrahamse, 2008; Sekhejane et al., 2011; Fushimi et al., 2012). Normal wounded fibroblasts increased their proliferation and decreased TNF-α and IL-1β after irradiation at 830 nm with 5 J/cm2 (Houreld et al., 2010). Exposing
fibroblasts to near infrared radiation was shown to induce reactive oxygen species formation and lead to the subsequent increased expression of matrix metalloproteinase (Danno et al., 2001; Schroeder et al., 2008). However, further studies are needed to investigate how these alterations of growth factors and cytokines induce proliferation and migration of fibroblasts.
Studies about LLLT affect on fibroblasts derived from keloid or hypertrophic scar are only a few. Irradiation with 660 nm reduced keloid fibroblast death significantly (Frigo et al., 2010) and stimulated proliferation of fibroblasts derived from hypertrophic scar (Webb et al., 1998). While most studies show LLLT effect to fibroblast to increase proliferation, one study which is used light with 880 nm at 2.4 and 4 J/cm2 revealed that
both normal and hypertrophic scar derived fibroblasts showed mild decrease of cell number (Webb and Dyson, 2003). Furthermore, irradiation with 470 nm LED with 60, 122 and 183 J/cm2 did not affect in proliferation of keloid fibroblasts(Bonatti et al.,
2011). Because studies about the effect of LLLT on keloid or hypertrophic scar derived fibroblasts are relatively fewer than normal fibroblasts, there is no consensus of LLLT effect and further studies are needed to investigate.
Although this study is focused on fibroblasts, LLLT could also promote biostimulatory effects on keratinocytes. LLLT promoted an increase of keratinocyte metabolism, proliferation, and type I collagen and vascular endothelial growth factor (VEGF) gene expression (Basso et al., 2013). LLLT using 638 nm or 518 nm light promoted the
16
migration of keratinocytes, and only 518 nm light make increase gene expression and cytokine secretion of heparin-binding epidermal growth factor-like growth factor and VEGF (Fushimi et al., 2012). In vivo, LLLT may affect both keratinocytes and fibroblasts and it could make synergetic effect to promote wound healing.
This study has some limitations. Each of two proliferation assays was shown different results in this study. It could be explained that effect of LLLT is slight week to detect by EZ-CyTox proliferation assay or more repeated studies are needed. Light dose of normal and keloid fibroblasts is different, because these two cell lines is complete different. So, we conducted the preliminary study to determine most effective dose of each cells and chose the most suitable light dose. Although we only focused on proliferation and migration of fibroblasts in this study, wound healing is a multi-complex process participating various cells, including keratinocyte, endothelial cells and inflammatory cells. Even if specific light promotes proliferation and migration of fibroblast, this contributes a small part of wound healing processing. We cannot prove any specific cytokines, chemokines, molecules, or genes which induced proliferation or migration of fibroblasts. Another consideration is that successful in vitro results do not always translate to the in vivo application. Further studies about molecular and gene levels of LLLT effect which induced proliferation or migration of fibroblasts is needed.
17
V. CONCLUSION
In conclusion, this study demonstrates LLLT effect on normal fibroblasts. Irradiation with 632 nm light could accelerate proliferation of fibroblasts, and irradiation with 830 nm light could enhance wound healing by increasing migration of fibroblasts. Although further studies are needed, when irradiating with 632 nm light, it seems inhibit proliferation of keloid fibroblasts. However, it is likely to occur on a higher dose than the dose irradiated on normal fibroblasts.
18
REFERENCES
1. Almeida-Lopes L, Rigau J, Zangaro RA, Guidugli-Neto J, Jaeger MM: Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29: 179-184, 2001
2. Ayuk SM, Houreld NN, Abrahamse H: Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther 14: 1110-1117, 2012
3. Barolet D, Boucher A: Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: a case series. Lasers Surg Med 42: 597-601, 2010
4. Basso FG, Oliveira CF, Kurachi C, Hebling J, Costa CA: Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28: 367-374, 2013
5. Basso FG, Pansani TN, Turrioni AP, Bagnato VS, Hebling J, de Souza Costa CA: In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent 2012: 719452, 2012
6. Bonatti S, Hochman B, Tucci-Viegas VM, Furtado F, Pinfildi CE, Pedro AC, Ferreira LM: In vitro effect of 470 nm LED (Light Emitting Diode) in keloid fibroblasts. Acta Cir Bras 26: 25-30, 2011
7. Carvalho RL, Alcantara PS, Kamamoto F, Cressoni MD, Casarotto RA: Effects of low-level laser therapy on pain and scar formation after inguinal herniation surgery: a randomized controlled single-blind study. Photomed Laser Surg 28: 417-422, 2010
19
8. Crisan B, Soritau O, Baciut M, Campian R, Crisan L, Baciut G: Influence of three laser wavelengths on human fibroblasts cell culture. Lasers Med Sci 28: 457-463, 2013
9. Danno K, Mori N, Toda K, Kobayashi T, Utani A: Near-infrared irradiation stimulates cutaneous wound repair: laboratory experiments on possible mechanisms. Photodermatol Photoimmunol Photomed 17: 261-265, 2001
10. Evans DH, Abrahamse H: Efficacy of three different laser wavelengths for in vitro wound healing. Photodermatol Photoimmunol Photomed 24: 199-210, 2008
11. Frigo L, Favero GM, Lima HJ, Maria DA, Bjordal JM, Joensen J, Iversen VV, Marcos RL, Parizzoto NA, Lopes-Martins RA: Low-level laser irradiation (InGaAlP-660 nm) increases fibroblast cell proliferation and reduces cell death in a dose-dependent manner. Photomed Laser Surg 28 Suppl 1: S151-156, 2010
12. Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S: Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen 20: 226-235, 2012
13. Hawkins D, Abrahamse H: Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24: 705-714, 2006
14. Houreld N, Abrahamse H: Low-intensity laser irradiation stimulates wound healing in diabetic wounded fibroblast cells (WS1). Diabetes Technol Ther 12: 971-978, 2010
20
proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med Sci 23: 11-18, 2008
16. Houreld NN, Sekhejane PR, Abrahamse H: Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg Med 42: 494-502, 2010
17. Kwon HH, Lee JB, Yoon JY, Park SY, Ryu HH, Park BM, Kim YJ, Suh DH: The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: a double-blind, randomized controlled trial. Br J Dermatol 168: 1088-1094, 2013
18. Mamalis AD, Lev-Tov H, Nguyen DH, Jagdeo JR: Laser and light-based treatment of Keloids - a review. J Eur Acad Dermatol Venereol, 2013
19. Oplander C, Hidding S, Werners FB, Born M, Pallua N, Suschek CV: Effects of blue light irradiation on human dermal fibroblasts. J Photochem Photobiol B 103: 118-125, 2011
20. Peplow PV, Chung TY, Ryan B, Baxter GD: Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29: 285-304, 2011
21. Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M: Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31: 334-340, 2005
22. Profyris C, Tziotzios C, Do Vale I: Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol 66: 1-10; quiz 11-12, 2012
21
23. Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B: Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci 23: 211-215, 2008
24. Schroeder P, Lademann J, Darvin ME, Stege H, Marks C, Bruhnke S, Krutmann J: Infrared radiation-induced matrix metalloproteinase in human skin: implications for protection. J Invest Dermatol 128: 2491-2497, 2008
25. Sekhejane PR, Houreld NN, Abrahamse H: Irradiation at 636 nm positively affects diabetic wounded and hypoxic cells in vitro. Photomed Laser Surg 29: 521-530, 2011
26. Silveira PC, Streck EL, Pinho RA: Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B 86: 279-282, 2007
27. Webb C, Dyson M: The effect of 880 nm low level laser energy on human fibroblast cell numbers: a possible role in hypertrophic wound healing. Journal of Photochemistry and Photobiology B: Biology 70: 39-44, 2003
28. Webb C, Dyson M, Lewis WH: Stimulatory effect of 660 nm low level laser energy on hypertrophic scar-derived fibroblasts: possible mechanisms for increase in cell counts. Lasers Surg Med 22: 294-301, 1998
22 - 국문요약 -
상처 치유에 있어서 저출력 광선치료가 섬유아세포에
미치는 영향
아주대학교 대학원 의학과 정 수 은 (지도교수: 김 유 찬) 연구배경: 저출력 광선치료는 가시광선 및 근적외선의 광학 생조절 (photobiomodulation) 작용을 이용한 치료 방법이다. 기전은 명확히 밝혀져 있지 않으나 통증 및 염증의 경감, 창상 치유 촉진, 손상된 세포 및 세포의 치유 유도 효과를 갖는 것으로 알려져 있다. 현재 임상에서도 저출력 광선 치 료를 비후성 흉터 또는 켈로이드 생성 예방 및 상처 치유 촉진의 목적을 위 해 사용 되고 있다. 연구목적: 따라서 본 연구는 415 nm, 632 nm, 830 nm 파장의 저출력 광선 치료가 정상 그리고 켈로이드에서 추출한 섬유아세포에 미치는 영향에 대해 알아보고자 실험을 진행하였다. 연구방법: 정상 그리고 켈로이드 섬유아세포를 각각 사람에게서 추출하여 배 양하였다. 각 파장 별로 3, 10, 30 J/cm2 의 용량으로 광선을 조사한 후 24 시간 후 세포의 생존률을 EZ-CyTox 키트를 이용하여 측정하였다. 또한 각 파장 별로 3, 10, 30 J/cm2 의 용량으로 광선을 조사하였으며 24시간 후에대조군에 비교하여 섬유아세포의 증식률을 trypan blue exclusion assay 를 이용하여 측정하였다. 세포의 이동률을 측정하기 위해 섬유아세포가 채워진 배지 접시의 한가운데에 파이펫을 이용하여 직선의 인위적인 상처를 생성하 였다. 0, 6, 12, 24 시간 후 동일한 위치에서 사진을 촬영하였으며 0 시간에 비해 채워진 면적을 측정하여 섬유아세포의 이동률을 계산하였다.
23 연구결과: 3, 10, 30 J/cm2 의 광선 치료 시에 정상 및 켈로이드 섬유아세포 의 생존률에 차이가 없었다. 3, 10, 30 J/cm2 의 용량으로 광선을 조사 후 증 식률 비교한 예비실험에서 정상 섬유아세포는 3 J/cm2 의 용량을 조사 시에 증식률이 높아졌으나 켈로이드 섬유아세포의 경우 3 J/cm2 의 용량을 조사하 였을 때 증식률이 대조군에 비해 더 높았다. 따라서 정상 섬유아세포는 3 J/cm2 의 용량으로, 켈로이드 섬유아세포에서는 10 J/cm2 의 용량으로 광선 을 조사하였다. 632 nm 에서 통계학적으로 유의하게 정상 섬유아세포의 증식 률이 증가되었으며 켈로이드 섬유아세포의 증식률은 감소되었다. 830 nm 에 서 정상 섬유아세포의 이동률이 통계학적으로 유의하게 증가하였으나 켈로이 드 섬유아세포의 이동률은 파장별로 유의한 차이를 보이지 않았다. 결론: 632 nm 의 저출력 광선치료는 정상 섬유아세포의 증식을 촉진시키며 켈로이드 섬유아세포의 증식을 억제하는 효과를 나타내었다. 또한 830 nm 의 저출력 광선치료는 정상 섬유아세포의 이동을 증가 시키는 것으로 확인되 었다. 이러한 결과를 토대로 저출력 광선치료가 상처치유 촉진 및 켈로이드 생성 예방의 기초 자료로 이용될 수 있을 것이다. 핵심어: 이동, 저출력 광선치료, 정상 섬유아세포, 증식, 켈로이드 섬유아세포