관련 문서
This book contains hundreds of complete, working examples illustrating many common Java programming tasks using the core, enterprise, and foun- dation classes APIs.. Java Examples
However, recent reports suggested that dynamin has been implicated in altering cell membrane shape during cell migration associated with cytoskeleton
Although Korea has been playing a leading role in the development of fusion power generation and Korea is playing a leading role in the world stage,
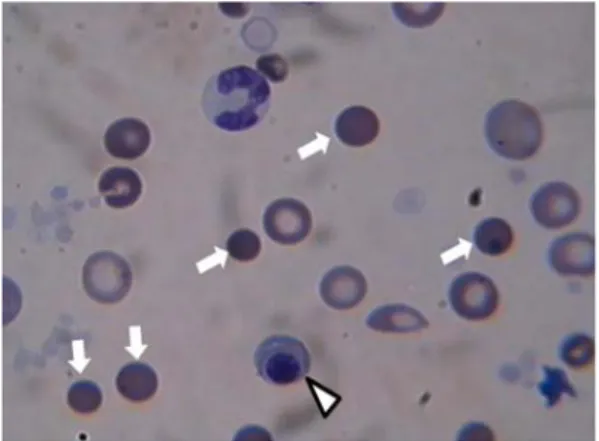
Background : Janus tyrosine kinase 2 (JAK2) V617F mutation has been described in a high proportion of patients with Philadelphia
Purpose: We investigated recent aspects of causative organism and antimicrobial susceptibility of urinary tract infections(UTI).. Materials and Methods:
Since then, studying to steppe routes has been made in various fields, allowing a balanced understanding of the role of nomadic imperial history, which
• Common polymorphism in MAO-A gene resulting in relatively low-activity enzyme, has been associated with increased risk for violent or antisocial behavior as well as for
(A) Interestingly, this has often been the case with English animal expressions.. As you may have observed, they make irregular twists and turns in their