Efficacy of Add-back Therapy with Tibolone for Prevention of Bone Mineral Density Loss in Women Treated with
GnRH Agonist for Endometriosis
Bo Yon Lee, Min Hyung Jung, Yong Il Ji1, Heung Yeol Kim2
Department of Obstetrics and Gynecology, School of Medicine, Kyung Hee University, Kyung Hee Medical Center, Seoul, Department of Obstetrics and Gynecology, Cheong-Ju St. Mary Hospital1, Cheong-Ju,
Department of Obstetrics & Gynecology, College of Medicine, Kosin University2, Busan, Korea
자궁내막증 환자에서 GnRH Agonists 치료시 동반되는 골밀도 감소 예방을 위한 Tibolone Add Back 요법의 효용성
경희대학교 의학전문대학원 경희의료원 산부인과학교실, 청주성모병원 산부인과학교실1, 고신대학교 의과대학 산부인과학교실2
이보연․정민형․지용일1․김흥열2
목적: 본 연구 내막증으로 진단되어 GnRH agonist로 치료하는 환자들에서 골밀도 감소를 예방하기 위한 티볼론 추가 치료의 효과를 판단하고자 한다.
방법: 복강경 수술 및 병리학적으로 자궁내막증으로 진단되고 6차례의 3.75 mg goserelin acetate 투여를 종결한 환자 179명의 의무기록을 후향적으로 검토하였다. 25명의 환자는 치료 전 및 치료 후 골밀도 검사가 시행되지 않아 제외하 였다. 요추 1-4번과 대퇴골의 골밀도를 dual energy X-ray absorptiometry를 이용하여 GnRH agonist 치료 전후로 2회 측 정하였다. 97명에서 티볼론 추가 요법을 시행하였고, 57명에서는 시행하지 않았다.
결과: 평균 연령은 40.61±6.89세이며 평균 체질량 지수는 23.43±3.14 (kg/m2)이었다. GnRH agonist 치료 전의 골밀도 수치와 비교해 요추와 대퇴골의 평균 골밀도 감소치는 추가요법을 시행한 군과 시행하지 않은 군에서 각각 0.060±0.024 및 0.047±0.022이었다. 요추의 평균 골밀도 감소는 추가 요법을 시행한 군에서 통계적으로 낮게 나타나 골밀도 소실을 예방할 수 있다 (P=0.044).
결론: 티볼론 추가요법은 6차의 GnRH agonist 치료에서 발생할 수 있는 골밀도의 감소를 특히 요추에서 예방할 수 있다.
중심단어: GnRH agonist, 골밀도, 추가요법, 티볼론
Received: January 20, 2009, Revised: February 3, 2009 Accepted: February 27, 2009
Corresponding Author : Heung Yeol Kim, Obstetrics & Gynecology, College of Medicine, Kosin University
Tel: +51-990-6226, Fax: +51-990-3300 E-mail: hykyale@yahoo.com
Endometriosis is an estrogen-dependent inflammatory disease that affects 5 to 10% of women of reproductive
age in the United States1. Its defining feature is the presence of endometrium-like tissue in sites outside the uterine cavity, primarily on the pelvic peritoneum and ovaries. The main clinical features are chronic pelvic pain, pain during intercourse, and infertility1. Treatment for this disorder has involved a variety of approaches, but foremost among these therapeutic modalities has been the use of pharmacotherapy. A wide assortment of
drugs has been included oral contraceptives, progesto- gens, and gestrinone. However, the mainstay of phar- macological therapy for the past 15 years has been a class of drugs collectively referred to as gonado- tropin-releasing hormone (GnRH) agonists2. The GnRH agonists have been heavily utilized for all aspects of endometriosis. However, both patients and physicians have major concerns regarding the efficacy of these drugs and the potential adverse effects. Long-term use
(>6 months) may increase bone turnover, resulting in significant bone loss as a consequence of hypoestro- genism, which may not recover after the treatment.
Furthermore, the rate of BMD loss continues in a linear fashion, making therapeutic courses beyond 6 months potentially concerning3. Given these facts, the US FDA has specifically recommended that GnRH agonists should not be administered in the absence of add-back therapy for more than a 6-month treatment interval2,4. However, with add-back therapy, duration of treatment of up to 1 year has been validated as efficacious with a low rate of BMD loss2. There is a report that the BMD significantly decreased from baseline after 24 weeks of treatment of leuprolide acetate depot (P<
0.01), and remained significantly below the baseline (P<0.01) at 12 months after the treatment period5. Several different agents have been considered as regimen of add-back therapy to prevent bone loss in women with endometriosis treated with GnRH agonist, including etidronate, progesterons alone, estrogen and progesteron combinations, tibolone, parathyroid hormone, calcitonin, and physical exercise5,6. Unresolved issues from these studies include ideal regimens and whether the add- back therapy prevents bone loss without compromising efficacy.
Tibolone, a synthetic weak estrogenic steroid struc- turally related to noretinodrel, has intrinsic estrogenic, androgenic, and progestogenic properties. It is success- fully used in the treatment of climacteric complaints and in the prevention of bone loss in postmenopausal women, without significant endometrial stimulation7-11.
It may represent a useful drug for add-back supple- mentation as an alternative to estrogen–progestin thera- py during GnRH agonists treatment. The objective of the present study wasto examine the utility of the tibolone for add-back in a group of relatively young women on prolonged GnRH agonist for endometriosis.
MATERIALS AND METHODS
From Jan 2006 through Dec 2008, we retrospectively reviewed the medical records of 179 women who had been given a laparoscopic proven diagnosis of endome- triosis and 6 cycles of goserelin acetate 3.78 mg treatment at department of obstetrics and gynecology of Kyung Hee medical center. After twenty five patients were excluded because either or both pre- and post- treatment bone mineral density (BDM) examination was not carried out, 164 patients were analyzed. The BMD of the lumbar spine (L1-L4) and femur neck was measured by dual energy X-ray absorptiometry (DEXA;
GE Lunar Expert, USA; or GE Lunar Prodigy 9.30, USA) two times Pre- (just before the GnRH agonist injection) and post-GnRH agonist treatment (1 months later from finishing last injection, almost 7 months later from first shot). Tibolone 2.5 mg daily add-back therapy was given to 97 patients for 6 months and remaining 57 patients were not received. The data were statistically analyzed with SPSS 13.0 for windows (SPSS Inc, Chicago, Ill., USA). The statistical signi- ficance of differences between groups was determined by student t-test, and P<0.05 was significant statis- tically.
RESULTS
We studied 164 women treated with GnRH-agonist for endometriosis. The patients were classified into two groups (with/without tibolone 2.5 mg daily add-back therapy for 6 months; 97 patients versus 57 patients respectively). Subject characteristics at the time of the
Table 1. Characteristics of the study group
GnRH agonist without tibolone
(n=57)
GnRH agonist with tibolone
(n=97)
P value*
Age (yrs) Parity Height (cm) Body weight (kg) Body mass index (kg/m2)
40.07±7.28 1.41±1.04 157.39±4.90 58.83±8.52 23.79±3.38
40.89±6.68 1.65±0.91 157.34±5.29 57.37±7.36 23.24±2.99
0.38 0.71 0.94 0.18 0.20 Values are given as mesn±SD
*: Difference between groups was determined by using the Student t-test.
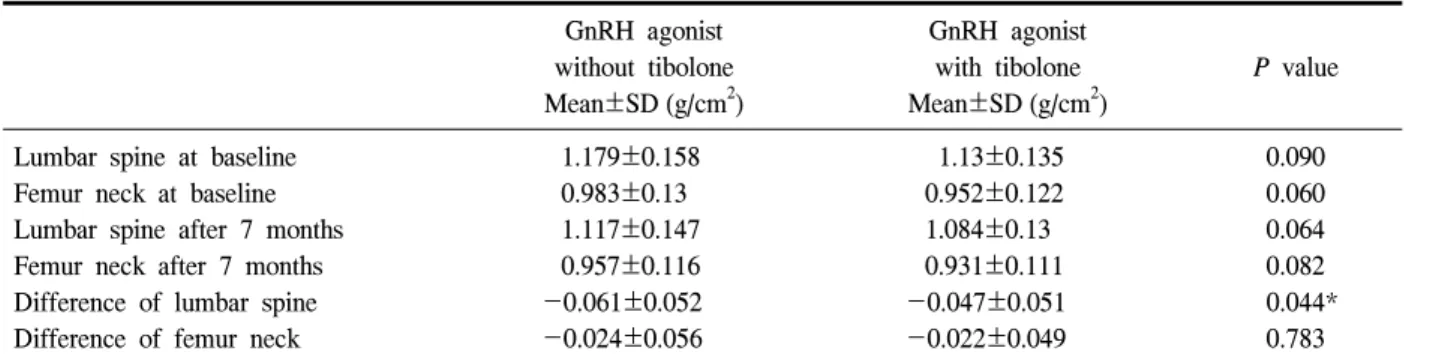
Table 2. The variation of BMD in lumbar spine and femur neck
GnRH agonist
without tibolone Mean±SD (g/cm2)
GnRH agonist with tibolone Mean±SD (g/cm2)
P value
Lumbar spine at baseline Femur neck at baseline Lumbar spine after 7 months Femur neck after 7 months Difference of lumbar spine Difference of femur neck
1.179±0.158 0.983±0.13 1.117±0.147 0.957±0.116 -0.061±0.052 -0.024±0.056
1.13±0.135 0.952±0.122 1.084±0.13 0.931±0.111 -0.047±0.051 -0.022±0.049
0.090 0.060 0.064 0.082 0.044*
0.783
*: Statistically significant between groups (P<0.05).
bone density assessment are presented in Table 1. The mean age was 40.61±6.89 years and mean BMI was 23.43±3.14 (kg/m2). There was no significant diffe- rence between the groups in age, height, body weight and bone mass index. The mean decrease of BMD (g/cm2) of lumbar spine and femur neck was 0.052 and 0.022, respectively. Compared with the baseline value, the mean decrease of BMD of lumbar spine and femur neck was 4.52% and 2.32%, respectively. The change of BMD in lumbar spine and femur neck was shown in Table 2. The pre-treatment mean (±SD) BMD of the lumbar spine and femur neck was 1.179±0.158 g/cm2 and 0.983±0.130 g/cm2 in the GnRH agonist without add-back therapy group and 1.130±0.135 g/cm2 and 0.952±0.122 g/cm2 in GnRH agonist with add-back therapy group. The difference between two groups was not statistically significant (P=0.09, P=0.06 respec-
tively). The post-treatment mean (±SD) BMD of the lumbar spine and femur neck was 1.117±0.147 g/cm2 and 0.957±0.116 g/cm2 in the GnRH agonist without add-back therapy group and 1.084±0.130 g/cm2 and 0.931±0.111 g/cm2 in GnRH agonist with add-back therapy group. The difference between groups was not statistically significant (P=0.06, P=0.08 respectively).
The change in BMD of lumbar spine and femur neck between baseline and after 7 months was -0.061±
0.052 g/cm2 and -0.024±0.056 g/cm2 in GnRH agonist without add-back therapy group and -0.047±0.051 g/cm2 and -0.022±0.049 g/cm2 in GnRH agonist with add-back therapygroup. Compared with baseline value, the mean decrease of BMD of lumbar spine and femur neck was 4.52% and 2.32%, respectively. The mean decrease of BMD of lumbar spine and femur neck between baseline and after 7 month was down to
4.16% and 2.31% in GnRH agonist with add-back therapy group, compared with 5.17% and 2.44% in GnRH agonist without add-back therapy group. The mean decrease of BMD was significantly grater in lumbar spine (P=0.044) but not in femur neck (P=0.783) in GnRH agonist without add-back therapy group.
DISCUSSION
Gonadotropin-releasing hormone (GnRH) agonists are widely used in the medical treatment of various gynecologic problems related to sex hormones, such as endometriosis, adenomyosis, uterine leiomyoma, pre- menstrual syndrome and precocious puberty12. The concept of add-back therapy to prevent hypoestrogenic effects of GnRH aginists was explored since the late 1980s13. The first studies reported partial relief of hypoestrogenic symptoms by combining GnRH agonists with progestins14. The rationale for add-back therapy is the “estrogen threshold hypothesis” first suggested by Barbieri15. The hypothesis states that low levels of circulating estradiol result in regression of ectopic endometriotic implants and that adding back small amounts of estrogen will reduce the side effects of GnRHagonist, such as vasomotor symptoms and bone loss with preserving therapeutic efficacy. GnRH agonists without add-back therapy may induce adverse effects on the microstructure of cancellous bone, which are incomplete recovery after therapy and may induce loss of the trabecular component of bone, leading to increased risk for fracture in later life16. The lumbar spine are susceptible to hypoestrogenism because they are composed of trabecular bone more than 70%, whereas the skeleton of limbs are less sensitive17. Some study reported that bone mineral density loss of lumbar spine recovered completely 2 years after completion of 6 months of GnRH agonist therapy without add-back in patients with endometriosis18. Because bone mineral density does not measure the microstructure of
cancellous bone, increased risk for fracture may exist despite demonstrable restorationof bone mineral density.
Most important complication of longer than 6 months of GnRH agonist treatment is loss of BMD which may be irreversible after completion of treatment19. A more recent randomized study reported the success of tibolone administered in association with GnRH agonist in preventing the hypoestrogenic symptoms and preser- vation of lumbar spine bone density without reducing the therapeutic effect of GnRH agonist in premeno- pausal women with laparoscopically diagnosed endo- metriosis20,21. Some authors suggested that tibolone had a protective effect on cortical bone similar to the estro- gen-progestin add-back regimen22. Lack of endometrial stimulation in tibolone may be superior to estrogen- progestogen add-back20. Tibolone has the advantage of improving libido and mood in comparison with other hormonal therapies, and has a high compliance rate because of its low incidence of side effects23. Tibolone add-back therapy may be a safe alternative to estrogen-progestogen add-back in endometriosis and expand their role prolonging the duration of GnRH agonist treatment. The time of beginning add-back therapy preferred concomitant initiation with GnRH agonist rather than delay until the agonist takes effect or until the patient complains of side effects, based on the results of different randomized trials3. Progesteron component in tibolone has a synergistic effect with estrogen on BMD, resulting in greater bone density than estrogen alone24. Moghissi et al25. and others have shown a positive effect on BMD by add-back therapy.
However, add-back therapy with estrogen reduces the positive effect of GnRH agonist on endometriosis26. So low dose of estrogen that doesn’t stimulate endo- metriosis is needed in add back therapy with GnRH agonist. Some studies with a long follow-up of 12 months or longer have reported that BMD returns to the baseline level25. whereas others report no change as compared with the value at the treatment end point27. Orwoll et al4. and Pierce et al28. found that there is
only a partial return to baseline BMD levels after 12~
15 months after cessation of therapy, even 6 years after treatment despite 2 mg estradiol and 1 mg norethistero- nacetate daily. However, most recent studies describe BMD returns to the baseline level by 6 months after the end of treatment. Some of the patients were followed up for treatment periods of up to 6 years with maintained clinical improvement of endometriosis and with preservation of bone density6. The bone loss induced by GnRH agonist is greater than that after natural menopause, averaging 1% per month during 6 months of treatment19. In this study, mean decrease of BMD of lumbar spine and femur neck was 4.52% and 2.32%, respectively, in agreement with existing studies29. Adding back tibolone, the mean decrease of BMD of lumbar spine and femur neck was down to 4.16% and 2.31%, compared with 5.17% and 2.44% respectively without add-back group. The present study shows a statistically significant protective effect of tibolone on BMD at lumbar spine in add-back group (P=0.044).
Though there was no significant difference in degree of BMD decrease according to types of GnRH agonist in this study, the decrease of BMD is slightly different according to types of GnRH agonist. During treatment of GnRH agonist, none of these studies reported clinical symptoms such as low-back pain or fractures associated with decreased BMD. The reduction of BMD in women with GnRH agonist is considered to proceed silently, so physician have to check BMD routinely during GnRH agonist treatment even though patients receive add-back therapy.
We did not evaluate the effect on the vasomotor symptoms or improvement of endometriotic symptoms induced by GnRH treatment, but emphasized the protective effects on bone loss of add-back therapy by using tibolone. Long term follow-up after end of GnRH agonist treament or laparoscopic re-evaluation about endometriosis may be necessary for accurate analysis.
Comparison add-back therapy by using tibolone with estrogen-progesteron regimen, may display advantage of
tibolone, but in this study, we did not do that. So we had better proceed further study by this concept. The frequency of endometriosis has increased in recent years, although this is probably the result of greater use of diagnostic laparoscopy and better recognition of the more subtle laparoscopic signs of endometriosis. The incidence of endometriosis may also increase according that women married late in life, treatment of endo- metriosis needs to be safe enough to prolong duration of agonist therapy. This may be achieved by add-back therapy, using by tibolone without endometrial stimu- lation.
CONCLUSION
In conclusion, this study shows that tibolone administered in association with GnRH agonist reduces the decrease of BMD in women with laparoscopic proven endometriosis. GnRH induced hypoestrogenism may explain the more severe loss in lumbar spine and add-back therapy may be an effective prevention of BMD loss in lumbar spine. Women, usually those with severe disease before treatment, may benefit from prolonged GnRH agonist administration, preferably with add-back therapy. It is important to remember that GnRH agonist treatment induce somehow decrease of BMD, even though add-back therapy has protective effect on bone loss.
ABSTRACT
Objectives: To determine the effect of tibolone add- back therapy for prevention of bone mineral density loss in women treated with GnRH agonist for their endometriosis.
Methods: Medical records of 179 patients who had been given a laparoscopically or pathologically proven diagnosis of endometriosis and finished the 6 cycles of goserelin acetate 3.78 mg treatment were reviewed retrospectively. Twenty five patients were excluded
because either or both pre- and post-treatment bone mineral density (BDM) examination was not carried out. The BMD of the lumbar spine (L1-L4) and femur neck was measured by dual energy X-ray absorp- tiometry two times (Pre- and post-GnRH agonist treatment). Tibolone add-back therapy was given to 97
patients and remaining 57 patients were not received.
Results: The mean age was 40.61±6.89 years and mean body mass index was 23.43±3.14 (kg/m2).
Comparing to pretreatment value, the mean decrease of BMD of lumbar spine and femur neck in without add-back group and with add-back group was 0.060±0.024 and 0.047±0.022, respectively. The mean decrease of BMD of lumbar spine was prevented significantly in add-back group (P=0.044).
Conclusions: Tibolone add-back therapy could prevent BMD loss occurs during 6 cycles of GnRH agonist treatment especially in lumbar spine.
Key Words: GnRH agonist, Bone mineral density, Add-back therapy, Tibolone
REFERENCES
1. Giudice LC, Kao LC. Endometriosis. Lancet 2004;
364:1789-99.
2. Olive DL. Optimizing gonadotropin-releasing hor- mone agonist therapy in women with endome- triosis. Treat Endocrinol 2004;3:83-9.
3. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add- back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol 1998;91:
16-24.
4. Orwoll ES, Yuzpe AA, Burry KA, Heinrichs L, Buttram VC Jr, Hornstein MD. Nafarelin therapy in endometriosis: long-term effects on bone mineral density. Am J Obstet Gynecol 1994;171:1221-5.
5. Matsuo H. Bone loss induced by GnRHa treatment in women. Nippon Rinsho 2003;61:314-8.
6. Mitwally MF, Gotlieb L, Casper RF. Prevention
ofbone loss and hypoestrogenic symptoms by estrogen and interrupted progestogen add-back in long-term GnRH-agonist down-regulated patients with endometriosis and premenstrual syndrome.
Menopause 2002;9:236-41.
7. Riggs BL. Tibolone as an alternative to estrogen for the prevention of postmenopausal osteoporosis in selected postmenopausal women. J Clin Endo- crinol Metab 1996;81:2417-8.
8. Uryan II, Taskin O, Erden F, Buhur A, Burak F, Ozekici U, et al. Effectiveness and Long-Term Safety of Prolonged Gosereline and Tibolone in Women with Endometriosis. J Am Assoc Gynecol Laparosc 1996;3:S51-2.
9. Edmonds DK. Add-back therapy in the treatment of endometriosis: the European experience. Br J Obstet Gynaecol 1996;103(Suppl 14):10-3.
10. Bedaiwy MA, Casper RF. Treatment with leupro- lide acetate and hormonal add-back for up to 10 years in stage IV endometriosis patients with chronic pelvic pain. Fertil Steril 2006;86:220-2.
11. Cheung TH, Lo KW, Yim SF, Lam C, Lau E, Haines C. Dose effects of progesterone in add-back therapy during GnRHa treatment. J Reprod Med 2005;50:35-40.
12. Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med 2001;345:266-75.
13. Gargiulo AR, Hornstein MD. The role of GnRH agonists plus add-back therapy in the treatment of endometriosis. Semin Reprod Endocrinol 1997;15:
273-84.
14. Friedman AJ, Barbieri RL, Doubilet PM, Fine C, Schiff I. A randomized, double-blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril 1988;
49:404-9.
15. Barbieri RL. Hormone treatment of endometriosis:
the estrogen threshold hypothesis. Am J Obstet Gynecol 1992;166:740-5.
16. Compston JE, Yamaguchi K, Croucher PI, Garrahan NJ, Lindsay PC, Shaw RW. The effects of gonadotrophin-releasing hormone agonists on iliac crest cancellous bone structure in women with endometriosis. Bone 1995;16:261-7.
17. Yu Q, Lin S, He F, Li B, Lin Y, Zhang T, et al.
Clinical manifestations of low bone mass in amenorrhea patients with elevated follicular sti- mulating hormone. Chin Med J (Engl) 2002;115:
1376-9.
18. Paoletti AM, Serra GG, Cagnacci A, Vacca AM, Guerriero S, Solla E, et al. Spontaneous reversi- bility of bone loss induced by gonadotropin- releasing hormone analog treatment. Fertil Steril 1996;65:707-10.
19. Pickersgill A. GnRH agonists and add-back the- rapy: is there a perfect combination? Br J Obstet Gynaecol 1998;105:475-85.
20. Taskin O, Yalcinoglu AI, Kucuk S, Uryan I, Buhur A, Burak F. Effectiveness of tibolone on hypoestro- genic symptoms induced by goserelin treatment in patients with endometriosis. Fertil Steril 1997;67:
40-5.
21. Franke HR, van de Weijer PH, Pennings TM, van der Mooren MJ. Gonadotropin-releasing hormone agonist plus “add-back” hormone replacement therapy for treatment of endometriosis: a prospective, ran- domized, placebo-controlled, double-blind trial. Fertil Steril 2000;74:534-9.
22. Lindsay R, Hart DM, Kraszewski A. Prospective double-blind trial of synthetic steroid (Org OD 14) for preventing postmenopausal osteoporosis. Br Med J 1980;280:1207-9.
23. Nathorst-Boos J, Hammar M. Effect on sexual life-a comparison between tibolone and a conti- nuous estradiol-norethisterone acetate regimen. Ma- turitas 1997;26:15-20.
24. Vanin CM, MacLusky NJ, Grynpas MD, Casper RF. The effect of three hormone replacement regi- mens on bone density in the aged ovariectomized rat. Fertil Steril 1995;63:643-51.
25. Moghissi KS, Schlaff WD, Olive DL, Skinner MA, Yin H. Goserelin acetate (Zoladex) with or without hormone replacement therapy for the treatment of endometriosis. Fertil Steril 1998;69:1056-62.
26. Gnoth CH, Godtke K, Freundl G, Godehardt E, Kienle E. Effects of add-back therapy on bone mineral density and pyridinium crosslinks in patients with endometriosis treated with gonadotropin-
releasing hormone agonists. Gynecol Obstet Invest 1999;47:37-41.
27. Nencioni T, Penotti M, Barbieri-Carones M, Ortolani S, Trevisan C, Polvani F. Gonadotropin releasing hormone agonist therapy and its effect on bone mass. Gynecol Endocrinol 1991;5:49-56.
28. Pierce SJ, Gazvani MR, Farquharson RG. Long- term use of gonadotropin-releasing hormone analogs and hormone replacement therapy in the manage- ment of endometriosis: a randomized trial with a 6-year follow-up. Fertil Steril 2000;74:964-8.
29. Lindsay PC, Shaw RW, Bennink HJ, Kicovic P.
The effect of add-back treatment with tibolone (Livial) on patients treated with the gonadotropin- releasing hormone agonist triptorelin (Decapeptyl).
Fertil Steril 1996;65:342-8.