Department of Energy Chemical Engineering
Kyungpook National University, Sangju 386, Gajang-dong Sangju 742-711, Korea
Solution Thermodynamics
CHME232-001
Instructor: Prof. Moon Gab Kim E-mail: mg_kim@knu.ac.kr
Chapter 10.
Vapor/Liquid Equilibrium :Introduction
10.1 The nature of equilibrium
• A static condition in which no changes occur in the
macroscopic properties of a system with time.
• At the microscopic level, conditions are not static.
The average rate of passage of molecules is the same in both directions, and no net interphase transfer of material occurs.
• An isolated system consisting of liquid and vapor phases in intimate contact eventually reaches a final state wherein no tendency exists for change to occur within the system.
Measures of Composition(1)
The three most common measures of composition are
mass fraction, mole fraction, and molar concentration.
• Mass or mole fraction is defined as the ratio of the mass or number of moles of a particular chemical species in a mixture or solution to the
total mass or number of moles of the mixture or solution: xi=mi/m=[mi dot]/[m dot] or
Measures of Composition(2)
The three most common measures of composition are
mass fraction, mole fraction, and molar concentration.
• Molar concentration is defined as the ratio of the mole fraction of a particular chemical species in a mixture or solution to its molar volume:
Ci=xi/V
This quantity has units of moles of i per unit volume.
• For flow processes convenience suggests its expression as a ratio of rates. Multiplying and dividing by molar flow rate [ni dot] gives:
Ci=[ni dot]/q
where rate [ni dot] is molar flow rate of species i , and q is volumetric flow rate.
• The molar mass of a mixture or solution is the mole-fraction-weighted sum of the molar masses of all species present:
Measures
of
composition
Mass or
mole fraction
concentration
Molar
Molar mass for
a mixture or
solution
m
m
m
m
x
i i i
V
x
C
i
i
i
i
i
M
x
M
Measures of composition
10.2 Phase Rule for Intensive Variables
• The intensive state of a PVT system containing N chemical species and p phases in equilibrium is characterized by
the intensive variables (total number of systemic intensive variables) = temperature T, pressure P, and (N-1) mole fractions for each phase.
• The number of variables that is independently fixed in a system at equilibrium = the number of variables that characterize the intensive state of the system - the number of
independent equations connecting the variable:
• Degrees of freedom = Total number of systemic intensive
variables – number of independent equations relating all the
10.2 Phase Rule for Intensive Variables
• Phase Rule Variables:
The system is characterized by T, P and (N-1) mole fractions for each phase(p)
Requires knowledge of 2 + (N-1)p variables
• Phase Rule Equations: At equilibrium, i =
i = i p for all N species These relations provide (p-1)N equations
The difference is F = 2 + (N-1)
p
- (
p
-1)N
= 2-
p
+N
• Phase rule : For a system of p phases and N species, the degree of freedom is:
F = 2 - p + N
Phase rule vs. Duhem’s theorem
• Duhem’s rule :
phase rule variables phase rule equations
additional [(N-1)p] constraint on the mass of each species;
Total # of variables: 2+(N-1)p+p = 2+Np
Total # of independent equations: (p-1)N+N = Np
for any closed system formed initially from given masses of
prescribed chemical species, the equilibrium state is completely determined when any two independent variables are fixed.
Two ? When phase rule F = 1, at least one of the two variables must be extensive, and when F = 0, both must be extensive.
(
1
)(
)
(
1
)(
)
2
2
N
N
N
F
p
p
p
Intensive phase-rule variable
extensive variable(masses or mole numbers)
Phase equili. eqn. Material bal. eqn.
[2 (
1)( )]
(
1)
(
1)
2
기포점(bubble point) 기포점 곡면 이슬점(dew point) 이슬점 곡면 온도축에 수직평면 : A-L-B-D-E-A 일정한 T에서의 Pxy선도 압력축에 수직평면 : H-I-J-K-L-H 일정한 P에서의 Txy선도
10.3 VLE: QUALITATIVE
BEHAVIOR
• Under surface- sat. V states (P-T-y1) • Upper surface- sat. L states (P-T-x1) • Liquid at F, reduces pressure at
constant T & composition along FG, the first bubble appear at L – bubble point • As pressure reduces, more & more L
vaporizes until completed at W; point where last drop of L (dew) disappear – dew point
red curve blue curve
(bubble P line)
평형상태에 있는 상의 조성을 연결하는 선; Tie line
온도축에 수직평면 : 일정한 T에서의 Pxy선도
온도축에 수직평면 : A-L-B-D-E-A 일정한 T에서의 Pxy선도압력축에 수직평면 : 일정한 P에서의 Txy선도
압력축에 수직평면 : H-I-J-K-L-H 일정한 P에서의 Txy선도
조성축에 수직; 일정한 조성에서의 P-T diagram
일정한 조성의 혼합물 각각에 대한 P-T 거동 Tie line, P-T평 면에 수직 임계점의 위치; 조성에 의존 general neither. Next slideretrograde condensation(역행 응축)
최대 압력 최대 온도 BD선을 따라 기포점->이슬점 까지 증발 액체상태 분율 0.2 0.1 포화증기 Application to operation of deep natural-gas wells liquid and vaporvapor
혼합물의 경우 압력이 감소함 (일반적으로는 기화현상발생) 에도
x
1– y
1diagram for several pressures for same system
X1=77mol% P=1263 psia Fig.12.6에서 점 M
Azeotropic mixtures: (액상의 경우)
P-x; Neg. dev.; 이종 간의 분자간 인력>동종 Pos. dev. ; 동종 간의 분자간 인력>이종 특히 동종간의 인력이 너무 크면;
T-x; Neg. dev.; 이종 간의 분자간 인력 <동종 Pos. dev. ; 동종 간의 분자간 인력 <이종
10.4 Simple models for VLE
• The simplest are Raoult’s law and Henry’s law. • Raoult’s law:
the vapor phase is an ideal gas (apply for low to moderate pressure) the liquid phase is an ideal solution (apply when the species that are
chemically similar)
» only to species for which a vapor pressure is known
=> Temp. of species is within subcritical region
» although it provides a realistic description of actual behavior for a small class of systems, it is valid for any species present at a mole
fraction approaching unity, provided that the vapor phase is an
ideal gas. ) ..., , 2 , 1 (i N P x P yi i isat
Correlation of Vapour Pressure Data
Pisat, or the vapour pressure (of component i, is commonly represented by Antoine’s Equation:
- For acetonitrile (Component 1):
- For nitromethane (Component 2):
These functions are the only component properties needed to characterize ideal VLE behaviour
224
C
/
T
47
.
2945
2724
.
14
kPa
/
P
ln
1sat
209 C / T 64 . 2972 2043 . 14 kPa / P ln 2sat C
T
B
A
P
ln
isat
* Vapor pressure = the partial pressure of the vapor over the
liquid, measured at equilibrium
VLE Calculations using Raoult’s Law
Raoult’s Law for ideal phase behaviour relates the
composition of liquid and vapour phases at
equilibrium through the component vapour pressure,
P
isat.
Given the appropriate information, we can apply
Raoult’s Law to the solution of 5 types of
problems:
»Dew Point: Pressure and Temperature
»Bubble Point: Pressure and Temperature
»P,T Flash
P
P
x
y
isat i i
25
• Raoult’s law
1 1 1 2 2 2 1 2 1 1 2 2 1 2 1 2 1 2 1 1 2 2 1 1 2 21,
1
(
)
sat i i i sat sat sat sat sat satsat sat sat
i i i
y P
x P
partial pressure
y P
x P
y P
x P
y P
y P
x P
x P
y
y
x
x
P y
y
x P
x P
P
x P
x P
x P
)
• Raoult’s law
1 2 1 2 1 2 1 2 1 2 1 2 1 21
(
)
1
1
1
/
ii sat sat sat sat sat
i
i
sat sat sat
i sat i i i
y P
y P
y P
y
y
x
x
x
P
P
P
P
P
P
P
P
y
y
y
P
P
P
P
y
P
1 2 ( 1 2) 1 1 2 2 sat i i i sat sat sat i i i y P x P P y P y P P y y x P x P P x P
•
As we are going to see later in the course, the aforementioned
VLE calculations are also applicable to non-ideal or/and
multi-component mixtures
•
For now, we are going to employ them only for the
calculation of the state and composition of binary and ideal
mixtures
•
The calculations revolve around the use of 2 key equations:
1) Raoult’s law for ideal phase behaviour:
2) Antoine’s Equation
VLE Calculations – Introduction (cont’d)
sat i i i i
y
P
x
P
P
*
*
i i i sat iC
T
B
A
P
)
ln(
(1)
(2)
Gibbs Phase Rule - Application
•Phase Rule Variables: 2 + (N-1)p variables
• Phase Rule Equations: (p-1)N equations
The difference is :
F = 2 + (N-1)p - (p-1)N = 2- p +N
Binary system Ternary system Quaternary system Variables 2+(2-1)*2 = 4 T, P, x1, y1(4) 2+(3-1)*2 = 6 T, P, x1, x2, y1, y2(6) 2+(4-1)*2 = 8 T, P,
x1, x2, x3, y1, y2, y3(8) Equations Pyi = xiPiS (i=2) 2 Pyi = xiPiS (i=3) 3 Pyi = xiPiS (i=4) 4 F 2 - 2 + 2 = 2 T and x1(y1) or P and x1(y1) or T and P 2 - 2 + 3 = 3 T, x1(y1) & x2(y2) or P, x1(y1) & x2(y2) or T, P and xi(yi) 2 - 2 + 4 = 4 T, x1(y1), x2(y2) & x3(y3) or P, x1(y1), x2(y2) & x3(y3) or T, P, xi(yi) & xk(yk)
Summary : VLE Calculations
Specified/Known
Variables Uknown Variables Calculation
T, x P, y BUBL P
T, y P, x DEW P
P, x T, y BUBL T
P, y T, x DEW T
P, T x, y P, T Flash
• Complete knowledge of the thermodynamic state of a
mixture at equilibrium requires
calculation of its P, T, and composition
• The type of calculation that we need to perform is subject to
the variables we are looking to evaluate
VLE in Binary Systems
• For a two phase (p=2), binary system (N=2): F = 2- 2 + 2 = 2 • Therefore, for the binary case, two intensive variables must be
specified to fix the state of the system.
• We can examine VLE behaviour as a function of pressure and
composition. Acetonitrile(1) - Nitromethane(2) @ 75C 40 50 60 70 80 90 0.0 0.2 0.4 x1,y1 0.6 0.8 1.0 P r e s s u r e , k P a y1 x1
VLE in Binary Systems
Alternately, we can specify a system pressure (often atmospheric) and examine VLE behaviour as a function of temperature and
composition.
Acetonitrile(1) Nitromethane(2) @ 70kPa
65.0 70.0 75.0 80.0 85.0 90.0 0.00 0.20 0.40 0.60 0.80 1.00 x1,y1 T e m p , d e g C y1 x1
Dewpoint and Bubblepoint
calculation with Raoult’s law
•
Bulb P: Calculate y
i
and P, given x
i
and T
•
Dew P: Calculate x
i
and P, given y
i
and T
•
Bulb T: Calculate y
i
and T, given x
i
and P
- Calculate and from Antoine’s Equation
- For the vapour-phase composition (bubble) we can write:
y
1+y
2=1 (3)
- Substitute y
1and y
2in Eqn (3) by using Raoult’s law:
- Re-arrange and solve Eqn. (4) for P
- Now you can obtain y
1from Eqn (1)
- Finally, y
2= 1-y
1BUBL P (y, P) Calculation (T, x
1known)
sat 1
P
P
2sat 1 P P x 1 P P x P P x P Px1* 1sat 2* 2sat 1* 1sat ( 1 )* 2sat
sat i i
x P
y
P
1 2 1 2(
P
P
)
x
P
P
sat
sat
sat1
i i y(4)
, sat sat i i i i i i i x P P y P x P y P Example 10.1
Binary system acetonitrile(1)/nitromethane(2) conforms closely to Raoult’s law. Vapor pressure for the pure species are given by the following Antoine equations:
00 . 209 64 . 972 , 2 2043 . 14 ln 00 . 244 47 . 945 , 2 2724 . 14 ln 0 2 0 1 C t kPa P C t kPa P sat sat
a) Prepare a graph showing P vs. x1(=0.6) and P vs. y1 at temperature 750C a) BUBL P calculations;
) ( 1 2 1 2 P P x A PP sat sat sat
At 750C, the saturated pressure is given by Antoine equation; 83.21 41.98
2 1 sat sat P P
Substitute both values in (A) to find P;
kPa P P 72 . 66 6 . 0 98 . 41 21 . 83 98 . 41 1
i yi i sat i iP x P
1 2 1 2 P P x PP sat sat sat
P P x y sat 1 1 1
The corresponding value of y1 is found from Eq. 10.1. sat i i iP x P y
66.72
0.7483 21 . 83 6 . 0 1 1 1 P P x y satIdeal Phase Behaviour: P - x - y Diagrams
T=75ºC x1=0.6 P=66.72 kPa y1=0.7483 Given asBUBL P
To be calc’ed;- Calculate and from Antoine’s Equation
- For the liquid-phase composition (dew) we can write:
x
1+x
2=1 (5)
- Substitute x
1and x
2in Eqn (5) by using Raoult’s law:
(6)
- Re-arrange and solve Eqn. (6) for P
- Now you can obtain x
1from Eqn (1)
- Finally, x
2= 1-x
1DEW P (x, P) Calculation (T, y
1known)
sat 1
P
P
2sat1
P
P
y
1
P
P
y
P
P
y
P
P
y
sat 2 1 sat 1 1 sat 2 2 sat 1 1*
*
*
(
)
*
1
i i x ) ..., , 2 , 1 ( / 1 N i P y P i sat i i
i i sat iy P
x
P
, sat i i i i i i sat i y P P y P x P x P satP
x
P
y
P
*
*
i sat i i P y P 1 1 ixi sat sat P y P y P 2 2 1 1/ / 1 sat P P y x 1 1 1 1 1 2 2 1 (B) sat sat P y P y P For y1=0.6; kPa P 59.74 98 . 41 4 . 0 21 . 83 6 . 0 1
DEW P calculation
, by Eq. 10.3;
4308 . 0 21 . 83 74 . 59 6 . 0 1 1 1 sat P P y xAt 750C, the saturated pressure is given by Antoine equation; 83.21 41.98
2 1 sat sat P P
Substitute both values in (B) to find P;
The corresponding value of y1 is found from Eq. 10.1. sat i i iP x P
Ideal Phase Behaviour: P - x - y Diagrams
T=75ºC y1=0.6 P=59.74 kPa x1=0.4308 Given asDEW P
To be calc’ed;• Since T is an unknown, the saturation pressures for the mixture components cannot be calculated directly.
Therefore, calculation of T, y1 requires an iterative approach, as follows:
1. Re-arrange Antoine’s equation so that the saturation temperatures of the components at pressure P can be calculated:
2. (7)
3. Select a temperature T' so that ( ) 4. Calculate Pisat from each Antoine equation
BUBL T (y, T) Calculation (P, x
1known)
i i i sat i
C
P
A
B
T
)
ln(
1(T')
2(T')
sat satP
and P
sat 2 sat 1T
T
T
'
, sat sat i i i i i i i x P P y P x P y P i i i sat i C T B A P ) ln(5. Solve Eqn. (4) for pressure P’
6. If , then P' =P; If not, try another T' –value
Go back step 2.
7. Calculate y
1from Raoult’s law
1 P P x 1 P P x P P x P P
x1* 1sat 2* 2sat 1* 1sat ( 1 )* 2sat
(4)
2 1 2 1
'
sat(
sat sat)
P
P
P
P
x
P
'
P
sat i i ix P
y
P
Same as before, calculation of T, x
1requires an iterative approach:
1. Re-arrange Antoine’s equation so that the saturation temperatures
of the components at pressure P can be calculated from Eqn. (7):
2. Select a temperature T' so that
3. Calculate from Antoine’s Eqn.
4. Solve Eqn. (6) for pressure P'
5. If , then P' =P; If not, try another T' -value
6. Calculate x
1from Raoult’s law
DEW T (x, T) Calculation (P, y
1known)
sat 2 sat 1
T
T
T
'
1(T')
2(T')
sat satP
and P
P
'
P
i i i sat i C P A B T ) ln( i i sat iy P
x
P
1 1 2 21
/
sat/
satP
y P
y
P
, sat i i i i i i sat i y P P y P x P x P i i i sat i C T B A P ) ln(•
Bulb T: Calculate y
i
and T (given x
i
and P)
•
Dew T: Calculate x
i
and T (given y
i
and P)
(
1 2)
sat sat
p
p
by
2 1 2 sat P P x x (C)
(B) ) ..., , 2 , 1 (i N P x P i sat i i
1 2 1 2 1 2 ln ln sat ln sat B B P P A A t C t C 1 2(
p
satp
sat)
2 1 2 1 1 1 1 1 2 1 1 2 2 2 1 2 ( ) 1 ( 1) 1 ( 1)sat sat sat sat sat sat sat P P P P x P P x x x x x x P P P x x P 1 2 sat sat p p 1 1 1 1 ln / / sat B P kPa A t C C 2 2 2 2 ln / / sat B P kPa A t C C 양변 P2sat로 나누고
DEW T (x, T)
BULB T (y, T)
1 2 1 2 2 1 1 2 1 1 2 1 2 1 1 2 1 2 1 2 1 2 1 1 1 1 1 1 2 1 1 1 = 1 1 , 1 ( )sat sat sat
sat sat sat sat sat sat sat
sat sat sat sat
sat P y y y P y P y y P P P P P P P P y y P y y y y P P P P P P y y
1 2
1 ( ) 2 P P x A PP sat sat sat
) ..., , 2 , 1 ( / 1 N i P y P i sat i i (B) (A)
BULB T (y, T)
con’t
• step 1: calc t
• step 2: Calc
• step 3: Calc by eqn. B←
• step 4: Calc t for 2-comp by Antoine eqn.
• Determine a new by eqn. C
• Calc yi…
2 satP
2 2 2ln
2 satB
t
C
A
P
2 1 2 satP
P
x
x
1 2 1 2 1 2 1 2 ln ln Psat ln Psat A A B B t C t C
sat 2 sat 1 T T T ' i i i sat i C P A B T ) ln( 1 2 1 ( ) 2 sat sat t T T←
←
sat i i ix P
y
P
(known; x
i, P)
(C)
Iteration procedure
Lecture 1 45
DEW T (x, T) by
(known; y
i, P)
1(
1 2)
satP
P y
y
←
(C)
(10.3)
where
Iteration procedure
• Calc
Calc by eqn. B
• Calc t for 1-comp by Antoine eqn.
• Determine a new by eqn. C
• Calc xi
1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C 1 satP
1 1 1ln
1 satB
t
C
A
P
1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C
sat 2 sat 1 T T T ' i i i sat i C P A B T ) ln( 1 2 1 ( ) 2 sat sat t T T←
←
) ..., , 2 , 1 ( / 1 N i P y P i sat i i
1 / 2 sat sat P P
1(
1 2)
satP
P y
y
i i saty P
x
P
1 2(
p
satp
sat)
Binary system acetonitrile (1)/nitromethane(2) conforms closely to Raoult’s law. Vapor pressures for the pure species are given by the following Antoine equations:
(a) Prepare a graph showing P vs. x1 (bubble point-line) and P vs. y1 (dew
point-line) for a temperature of 75°C.
(b) Prepare a graph showing T vs. x1 (bubble point-line) and T vs. y1 (dew
point-line) for a pressure of 70 kPa.
00 . 224 / 47 . 2945 2724 . 14 / ln 1 C t kPa Psat 00 . 209 / 64 . 2972 2043 . 14 / ln 2 C t kPa Psat
Example 10.1
)
...,
,
2
,
1
(
i
N
P
x
P
y
i
i isat
)
...,
,
2
,
1
(
i
N
P
x
P
i sat i i
1
i i y Binary system 1 2 1 2(
P
P
)
x
P
P
sat
sat
sat1
i i x ) ..., , 2 , 1 ( / 1 N i P y P i sat i i
For dewpoint calculation
- Liquid phase composition is unknown
Calculation procedure :
For bubblepoint calculation
- vapor phase composition is unknown
sat i i
x P
y
P
i i sat iy P
x
P
BULB P DEW P 1 1 2 21
/
sat/
satP
y P
y
P
(a) i) BULB P : known properties ; T(= 75°C) and x1(= 0.6) unknown properties : P and y1
1 2
1
2
(
P
P
)
x
P
P
sat
sat
sat21
.
83
1
satP
At 75°CP
2sat
41
.
98
1)
98
.
41
21
.
83
(
98
.
41
x
P
e.g. x1 = 0.6 0.7483 72 . 66 ) 21 . 83 )( 6 . 0 ( 1 1 1 P P x y sat 72 . 66 PTherfore, at 75°C, a liquid mixture of 60 mol-% (1) and 40 mol-% (2) is in equilibrium with a vapor containing 74.83 mol-% (1) at pressure of 66.72 kPa.
Ref. => Fig. 10.11 => next slide
Example 10.1(solution)
by Antoine eqn. 00 . 224 / 47 . 2945 2724 . 14 / ln 1 C t kPa Psat 00 . 209 / 64 . 2972 2043 . 14 / ln 2 C t kPa Psat (A)
(a) ii) DEW P : known properties ; T(= 75°C) and y1(= 0.6) unknown properties : P and x1
21 . 83 1 sat P At 75°C 98 . 41 2 sat P e.g. y1 = 0.6 1 1 1
(0.6)(59.74)
83.21
0.4308
saty P
x
P
59.74
P
Therfore, at 75°C, a vapor mixture of 60 mol%(1) and 40 mol%(2) is in equilibrium with a liquid containing 43.08 mol% (1) at pressure of 59.74 kPa. => Liquid-phase composition at point c'
Ref. => Fig. 10.11 => next slide
Example 10.1
by Antoine eqn. 1 2 1 21
Sat SatP
y
y
P
P
1 59.74 0.6 / 83.21 0.4 / 41.98 P kPa Ideal Phase Behaviour: P - x - y Diagrams
T=75ºC x1=0.6 P=66.72 kPa y1=0.7483 T=75ºC y1=0.6 P=59.74 kPa x1=0.4308 Given as Given as DEW P BUBL PCalculation procedure : T – x – y calculation
sat sat sat
P
P
P
P
x
2 1 2 1
Simple method of plotting T – x – y
ln
sat i i i iB
t
C
A
P
Select t satP
1 satP
2 t vs. x1 t vs. y1• Given p, xi (or yi) for all components Find T, yi (or xi) for all components
Select t between and
t
1satt
2sat1 2 1 2 1
(
)
(
)
sat sat sat satP
P
P
y
P P
P
1 2 1 2(
P
P
)
x
P
P
sat
sat
sat1 1 2 2
1
/
sat/
satP
y P
y
P
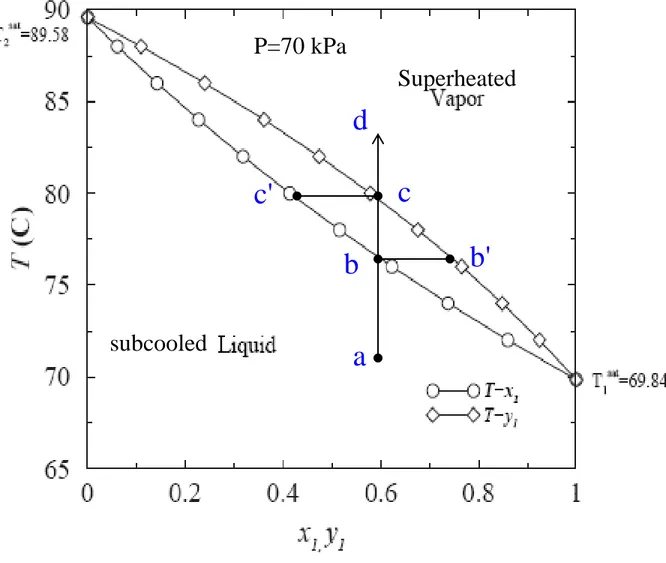
Fig. 10.12 txy diagram of acetonitrile(1)-nitromethane(2) at 70kPa d c b' b c' a subcooled Superheated P=70 kPa
53
BULB T(known; x
i, P) by
=>1st see next slideIteration procedure
• Calc
• Calc by eqn. B←
• Calc t for 2-comp by Antoine eqn.
• Determine a new by eqn. C
• Calc yi…
1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C 2 satP
22972.64
209.00
14.2043 ln
satt
P
2 1 2 satP
P
x
x
1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C
sat 2 sat 1 T T T ' i i i sat i C P A B T ) ln( 1 2 1 ( ) 2 sat sat t T T←
←
(0.6)(87.17) 0.7472 70 sat i i i x P y P (b) BUBL T, having x1=0.6, P = 70 kPa
2945.47 2972.64 ln 0.0681 224.00 209.00 t t t = 76.42ºC = 87.17 kPaP1sat
(B)
(C)
(C)
Check (t
n-1-t
n)
1 1 1 1 2 2 2 2 sat sat ij sat sat y x P P P y x P P P
2 1 2 1 1 1 1 2 2 2 2 2 1 1 2 1 1 1 2 ( ) 1 ( 1) , 1 ( 1) 1 ( 1)sat sat sat
sat sat
sat sat sat
sat sat P P P P x P P P x P P P P x P P P x P x x x x P x x 1
(
1 2)
satP
P y
y
1 2 1 2 2 1 1 2 1 2 1 1 2 1 2 1 2 1 2 1 1 1 21
1
1
/
/
(
)
sat sat sat sat sat satsat
sat sat sat sat sat sat
P
y
y
y P
y P
P
P
P P
P
y
y P
P
y
y P
P
P
P
P y
y
(B)
2 1 2 satP
P
x
x
DEW T(known; y
i, P) by
1(
1 2)
satP
P y
y
←
Iteration procedure
• Calc
Calc
• Calc t for 1-comp by Antoine eqn.
• Determine a new
• Calc x
i 1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C 1 satP
12945.47
224.00
14.2724 ln
satt
P
1 2 1 2 1 2 1 2 ln lnPsat ln Psat A A B B t C t C
sat 2 sat 1 T T T ' i i i sat i C P A B T ) ln( 1 2 1 ( ) 2 sat sat t T T←
←
1(
1 2)
satP
P y
y
(0.6)(70) 0.4351 96.53 i i sat i y P x P (b) DEW T, having y1=0.6, P = 70 kPa
2945.47 2972.64 ln 0.0681 224.00 209.00 t t t = 79.58ºC = 96.53 kPaP1sat
←
Check (t
n-1-t
n)
VLE in Binary Systems
Alternately, we can specify a system pressure (often atmospheric) and examine VLE behaviour as a function of temperature and
composition.
Acetonitrile(1) Nitromethane(2) @ 70kPa
65.0 70.0 75.0 80.0 85.0 90.0 0.00 0.20 0.40 0.60 0.80 1.00 x1,y1 T e m p , d e g C y1 x1 x1=0.6, P = 70 kPa y1=0.7472, T = 76.42ºC
BUBL T
y1=0.6, P = 70 kPa X1=0.4351, T = 79.58ºCDEW T
Gas-Liquid Equilibrium – Henry’s Law
• In the previous discussions, both components could exist
as a pure liquid at the temperatures of interest
• In other words we were interested in vapor-liquid
equilibria
• How can we extend this discussion to include gas-liquid
equilibria,
for species that do not condense
Henry’s law
Raoult’s law를 사용하기 위해서는 의 값이 필요. 적용온도(25ºC)가 임계온도 이하여야 함(∵ 증기압계산이 가능함) 적용온도 >> 공기의 임계온도(-140.95℃) => 증기압을 계산할 수 없음 Ex) 공기(1) 중의 물(2) 증기의 분율(y2); Raoult’s law을 물에 적용 하여 구함 » 가정 : 공기가 액상 중에 용해되지 않는다 물(액체물은 순수한 것으로 가정)에 대한 Raoult’s law ; Ex) 물(2)속에 용해된 공기(1)의 분율(x1); 적용온도 (25ºC) >> 공기의 임계온도 => Raoult’s law을 적용할 수 없음. ∴ Henry’s law를 이용하여 해결 - 기상을 이상기체로 간주(충분히 낮은 압력에 적용 가능) 액상 중에 아주 희박한 용질로서 존재하는 성분에 대해 적용(예, 물속의 공기 등) 기상 중의 성분의 분압은 그 성분의 액상 몰분율에 직접 비례한다. H x P y sat iP
2 2 2 (1)(3.166) 0.0312 101.33 sat x P y P 물의 증기압, at 25ºCair H2O x1(액상,air) y2(기상,water) x2(액상,물) y1(기상,공기) 1 1 1 sat y P x P
Henry’s law
•Henry’s law :
기상 중의 성분의 분압은 그 성분의 액상 몰분율에 직접 비례한다. » ; Henry’s constant • 25ºC 대기압 하에서의 공기(1)/물(2) 계에 대해 y1=1-y2=1-0.0312=0.9688인 공기(1)에 Henry’s law를 적용하면, •Check unit !! i H 5 1 1 1
(0.9688)(101.33)
1.35 10
72,950
y P
x
H
i i iP
x
H
y
From Table 10.1x1: mole fraction of air in water
■Example 10.2 Assuming that carbonated water contains only CO2 (species 1) and H2O (species 2) , determine the compositions of the vapor and liquid phases in a sealed can of “soda” and the pressure exerted on the can at 10°C. Henry’s
constant for CO2 in water at 10°C is about 990 bar. Henry’s law for species 1: y1P x1H1
Raoult’s law for species 2: sat
P x P y2 2 2 sat P x H x P 1 1 2 2 Assuming x1 = 0.01
(0.01)(990) (0.99)(0.01227)
9.912 bar
P
1 1 1P x H y assuming y1 ≡ 1.0 01 . 0 1 x sat P x P y2 2 2 0012 . 0 2 y 9988 . 0 1 y Justified the assumption
almost pure CO
Justified the assumption
CO2 : species 1
H2O : species 2
From steam table at 10°C
1 1 9.912 0.0100 990 P x H 2 2 2 (0.99)(0.01227) 0.0012 9.912 sat x P y P
10.5 VLE modified Raoult’s law
Account is taken of deviation from solution ideality in the liquid phase by a factor inserted into Raoult’s law:
) ... , 3 , 2 , 1 (i N P x P yi i
i isat The activity coefficient, f (T, x
i)
i sat i i i P x P
i sat i i i P y P
/ 1Example 10.3 For the system methanol (1)/methyl acetate (2), the following
equations provide a reasonable correlation for the activity coefficients:
2 1 2 2 2
ln
(2.771 0.00523 )
Ax
T x
2 2 1 2 1 ln (2.771 0.00523 ) Ax T x
The Antoine equations provide vapor pressures:
424 . 33 ) ( 31 . 3643 59158 . 16 / ln 1 K T kPa Psat 424 . 53 ) ( 54 . 2665 25326 . 14 / ln 2 K T kPa Psat Calculate
(a): P and {yi} for T = 318.15 K and x1 = 0.25 (b): P and {xi} for T = 318.15 K and y1 = 0.60 (c): T and {yi} for P = 101.33 kPa and x1 = 0.85 (d): T and {xi} for P = 101.33 kPa and y1 = 0.40
Solution 10.3
(a) for T = 318.15, and x1 = 0.25 51 . 44 1 sat P P2sat 65.64 50 . 73 ) 64 . 65 )( 072 . 1 )( 75 . 0 ( ) 51 . 44 )( 864 . 1 )( 25 . 0 (
i sat i i i P x P
sat i i i iP x P y
y1 0.282 y2 0.718 By Antoine eqn. 864 . 1 1 2 1.072 2 2 1 (2.771 0.00523 ) ln T x 2 1 2 (2.771 0.00523 ) ln T x=> no difference with simple Raoult’s law
γ=f(x,T)
BUBL P calc
A iterative process is applied, with 1 1 2 1
i sat i i i P y P
/ 1 sat P P y x 1 1 1 1
x2 1 x 1Converges at: P 62.89 kPa 1 1.0378 2 2.0935 x1 0.8169
1 1 1 2 2 2
1
sat satP
y
P
y
P
(b): for T = 318.15 K and y1 = 0.60 Initial values 2 2 1 (2.771 0.00523 ) ln T x 2 1 2 (2.771 0.00523 ) ln T x 2nd step, 51 . 44 1 sat P P2sat 65.64 By Antoine eqn.γ=f(x,T)
=> no x
i Check (Pn-1-Pn) Approx. valueDEW P Calc
424 . 33 ) ( 31 . 3643 59158 . 16 / ln 1 K T kPa Psat 424 . 53 ) ( 54 . 2665 25326 . 14 / ln 2 K T kPa Psat(c): for P = 101.33 kPa and x1 = 0.85
by
with α
71 . 337 1 sat T T2sat 330.08A iterative process is applied, with T (0.85)T1sat (0.15)T2sat 336.57
... 1 2 ... 1 1 1 2 2 sat
P
P
x
x
1.0236 2.1182 y 0.670 y 0.330 ln( ) sat i i sat i i i B T C A P 101.33 P kPa 1 2 1 2 1 2 1 2 ln ln sat ln sat B B P P A A t C t C 1 2 ln Psat Psat 2nd step sat i i i i x P y P γ=f(x,T)
=> no T
Check (Psat n-1-Psatn) New T 2 2 1 (2.771 0.00523 ) ln T x 2 1 2 (2.771 0.00523 ) ln T xBULB T calc
(d): for P = 101.33 kPa and y1 = 0.40 71 . 337 1 sat T T2sat 330.08
A iterative process is applied, with T (0.40)T1sat (0.60)T2sat 333.13
1 1 2 1 ... 1 sat P
i sat i i i P x P i i i sat i C K T B A kPa P ) ( / ln Converges at: T 326.70K 1.3629 1.2523 x 0.4602 x 0.5398 ... 2 sat P ... 1 x x2 ... ... 1 2 ... ... 1 sat P 1 2 1 1 2 ln sat y y P P Saturation Temps. are the same as part (c)
Initial Values; 1 2 ln Psat Psat 1 i i sat i y P x P 2 2 1 (2.771 0.00523 ) ln T x 2 1 2 (2.771 0.00523 ) ln T x 1 1 1 ln 1 sat B T C A P γ=f(x,T) => no x,T 1 2 1 1 2 ln sat y y P P Check (Tn-1-Tn)
DEW T calc
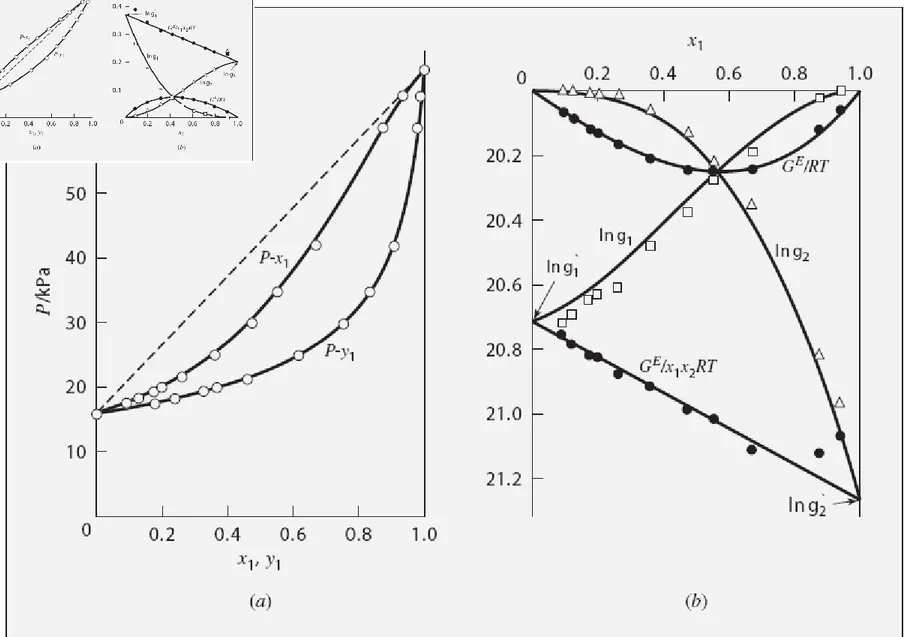
(e): the azeotropic pressure and the azeotropic composition for T = 318.15 K
Define the relative volatility:
2 2 1 1 12 x y x y Azeotrope y1 x1 y2 x2 12 1 sat i i i i y P x
P sat sat P P 2 2 1 1 12 1 1 1 1 1 12 1 2 2 2 2 1 2 0.224 exp( ) exp(2.771 0.00523 )sat sat sat
x sat sat sat
P P P P P Ax P T 2 2 1, 1 exp(Ax2 ) 2 1 1, 2 exp(Ax1 ) 1 2 1 1 1 2 1 12 0 2 2 2 2 exp( ) exp(2.771 0.00523 ) 2.052
sat sat sat
x sat sat sat
P P Ax P T P P P
Since α12 is a continuous function of x1: from 2.052 to 0.224, α12 = 1 at some point There exists the azeotrope!
1 1 1 12 sat sat P P 1 2 65.64 1.4747 az sat az sat P 2 1 2
ln
Ax
2 ln
AxFrom Antoine eq.
1 2 , 0 1 when x x when x, 2 0 x11 2 2 exp(Ax1 ) 2 1 exp(Ax2) 1 1 1 1 sat y x P P
1 2 2 1 1 12 sat sat P P 1 2 2 1 65.64 1.4747 44.51 az sat az sat P P 2 2 1
(
2
.
771
0
.
00523
)
ln
T
x
2 1 2(
2
.
771
0
.
00523
)
ln
T
x
2 2 1 2 1 2 1 2 1 2 2 1 1 1 1 ln ( )( ) ( ) (2.771 0.00523 )(1 2 ) ln1.4747 0.388 1.107(1 2 ) 0.388 0.325 Ax Ax A x x x x A x x T x x x
az azy
x
1
0
.
325
1 1exp(
22)
exp(1.107 (1 0.325) )
21.657
azAx
1 1 (1.657)(44.51) 73.76 az az sat P P kPa 2 1 2ln
Ax
2 2 1 ln
Ax sat i i i i y P x
P10.6 VLE from K-value correlations
A convenient measure, the K-value:
the “lightness” of a constituent species, i.e., of its tendency to favor the vapor phase.
When Ki > 1, species i => higher concentration in vapor phase
When Ki < 1, species i => higher concentration in liquid phase,
and is considered a “heavy” constituent The Raoult’s law and modified Raoult’s law :
For bubble point calc,
for dew point calc i i i x y K , 1 sat i i i i sat i i i i i i i i i i i i i y P x P y P K y K x x P y y x x K K sat i i i sat i i i i
y P x P
y
P
K
x
P
1 i i y K 1
i i iK x
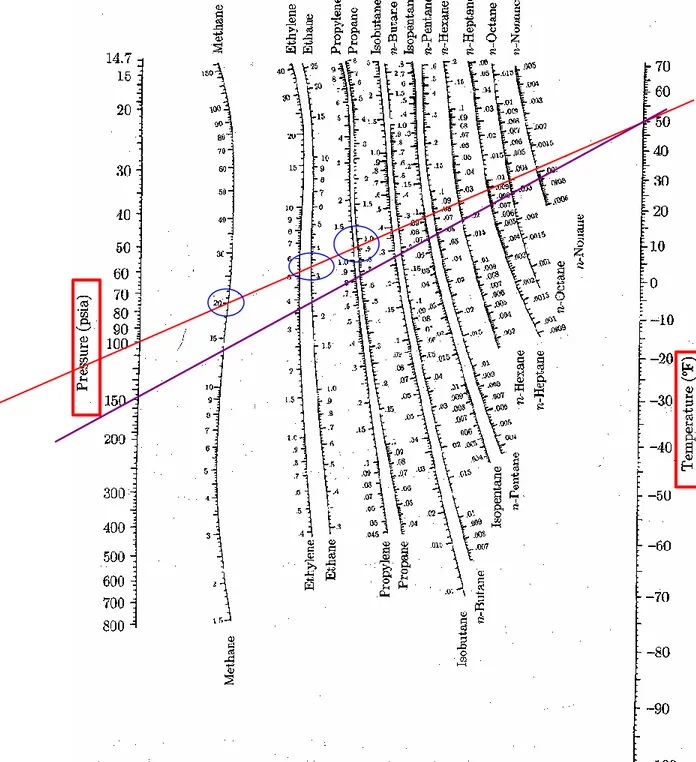
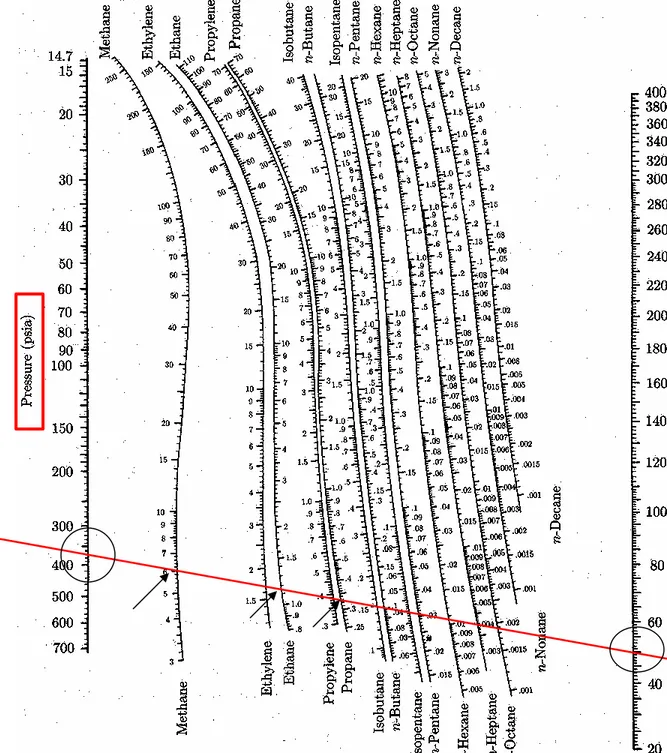
Fig. 10.13 K-values for
systems of light hydocarbons.
Example 10.4 For a mixture of 10 % methane, 20 % ethane, and 70
mol-% propane at 50°F, determine: (a) the dewpoint pressure, (b) the bubblepoint
pressure. The K-values are given by Fig. 10.13.
(a) at its dewpoint, only an insignificant amount of liquid is present:
P = 100 (psia) P = 150 (psia) P = 126 (psia)
Species yi Ki yi /Ki Ki yi /Ki Ki yi /Ki
Methane 0.10 20.0 0.005 13.2 0.008 16.0 0.006 Ethane 0.20 3.25 0.062 2.25 0.089 2.65 0.075 Propane 0.70 0.92 0.761 0.65 1.077 0.762 0.919 Σ(yi /Ki) = 0.828 Σ(yi /Ki) = 1.174 Σ(yi /Ki) = 1.000
(b) at bubblepoint, the system is almost completely condensed:
P = 380 (psia) P = 400 (psia) P = 385 (psia)
Species xi Ki xi Ki Ki xi Ki Ki xi Ki Methane 0.10 5.60 0.560 5.25 0.525 5.49 0.549 Ethane 0.20 1.11 0.222 1.07 0.214 1.10 0.220 Propane 0.70 0.335 0.235 0.32 0.224 0.33 0.231 Σ(xi Ki) = 1.017 Σ(xi Ki) = 0.963 Σ(xi Ki) = 1.000 액상의 경우, dew point 보다 낮은 압력에서는 Sxi<1, 높은 압력에서는 Sxi>1
1
i i iK x
1 i i i y K Fig. 10.14 K-values for
systems of light hydocarbons.
Example 10.4
Calculation by Excela) dewpoint pressure
species yi Ki yi/Ki Ki yi/Ki Ki yi/Ki
Methane 0.1 20 0.005 13.2 0.008 16 0.006
Ethane 0.2 3.25 0.062 2.25 0.089 2.65 0.075
Propane 0.7 0.92 0.761 0.65 1.077 0.762 0.919
∑(yi/Ki)= 0.827 1.173 1.000
b) bubblepoint pressure
species xi Ki Ki*xi Ki Ki*xi Ki Ki*xi
Methane 0.1 5.6 0.560 5.25 0.525 5.49 0.549
Ethane 0.2 1.11 0.222 1.07 0.214 1.1 0.220
Propane 0.7 0.335 0.235 0.32 0.224 0.33 0.231
∑(Ki*xi)= 1.017 0.963 1.000
P=100(psia) P=150(psia) P=126(psia)
T, P Flash Calculation
A liquid at a pressure equal to or greater than its bubble point pressure “flashes” or partially evaporates when the pressure is reduced,
producing a two-phase system of vapor and liquid in equilibrium.
Consider a system containing one mole of non reacting chemical species: The general nature of a flash calculation is :
Temperature-Pressure Flash: (T, P, z) => (V, x, y)
zi
F
y
i
V
x
i
L
0 x, y 1 VAPOR LIQUID P P B P A A B T = const. x y b r bubble dewFlash Calculations
Liquid VapourF
V
L
T, P
F can be a liquid, a gas or a two phase mixture This is called a flash calculation, because if the pressure is reduced enough, the liquid changes to vapor in a “flash”Flash Calculations
Given a temperature and pressure and an overall composition, => we can calculate xi and yi
Consider a system containing 1 mole of liquid:
• zi = the overall composition
• L =moles of liquid, with mole fractions xi • V =moles of vapor, with mole fractions yi
•
The material balance equations are:
. Note: The solution V = 1 is always a trivial solution.
1 ( 1, 2, , ) i i i L V z x L y V i N (1 ) ( 1, 2, , ) (1 ) 1 (1 ) 1 (1 ) (1 ) 1 ( 1) (1 ) i i i i i i i i i i i i i i i i i i i i i i z x V y V i N y z V y V K z y V V K z z K z K z K y V K V V K V V K V V K ( 1, 2, , ) 1 ( 1) i i i i z K y i N V K L을 제거
and solving for
i i i i x y K y 1 1 ( 1) i i i i z K V K 1
i i y Liquid VapourF
V
L
T,
P
, sat sat i i i i i i i y P y P x P K x P Ref.Flash calculations(Summary)
The moles of liquid
1
V
L
z
i
x
iL
y
iV
The moles of vapor
The liquid mole fraction
The vapor mole fraction
V
y
V
x
z
i
i(
1
)
i ) 1 ( 1 i i i i K V K z y 1 ) 1 ( 1
i i i K V K zzi
F
y
i
V
x
i
L
0 x, y 1 VAPOR LIQUID P P B P A A B T = const. x y b r bubble dewExample 10.5 The system acetone (1)/acetonitrile (2)/nitromethane(3) at 80°C and 110 kPa has the overall composition, z1 = 0.45, z2 = 0.35, z3 = 0.20, Assuming that Raoult’s law is appropriate to this system, determine L, V, {xi}, and {yi}. The vapor pressures of the pure species are given.
First, do a BUBL P calculation, with {zi} = {xi} :
kPa P x P x P x
Pbubl 1 1sat 2 2sat 3 3sat (0.45)(195.75)(0.35)(97.84)(0.20)(50.32) 132.40
Second, do a DEW P calculation, with {zi} = {yi} :
kPa P y P y P y
Pdew sat sat sat 101.52
/ / / 1 3 3 2 2 1 1
Since Pdew < P = 110 kPa < Pbubl, the system is in the two-phase region, sat i i i i y P K x P 1 1.7795 195.75 110 K 8895 . 0 2 K K3 0.4575 1 ) 1 ( 1
i i i K V K z mol V 0.7364 ) 1 ( 1 i i i i K V K z y 5087 . 0 1 y 3389 . 0 2 y 1524 . 0 y mol V L 1 0.2636 i i i i i y K x y x K 2859 . 0 1 x 3810 . 0 2 x 3331 . 0 x1
st2
nd3
rdExample 10.5
Calculation by Excel A B C Vapor Pressure Acetone 14.3145 2756.22 228.06 214.32 195.75 Acetonitrile 14.895 3413.1 250.523 96.41 97.84 Nitromethane 14.7513 3331.7 227.6 50.40 50.32 Temp/℃ 80 P/kPa 110 calc V 0.8107 0.7365overall composition Eqn. 10.17 -7.6126E-08 -5.49479E-08
z1 0.45 z2 0.35 L 0.1893 0.2635 z3 0.2 y1 0.4957 0.5087 Pbubl/kPa 140.2694 132.40 1) y2 0.3409 0.3389 Pdew/kPa 103.1141 101.52 2) y3 0.1634 0.1524 K1 1.9484 1.7795 x1 0.2544 0.2859 K2 0.8765 0.8895 x2 0.3889 0.3810 K3 0.4582 0.4575 x3 0.3566 0.3331
Antoine Constants Given Values in Textbook
3)
4)
5)
Example 10.6
반복법에 의한 해
P/kPa 200 yi for ν= yi for ν= yi for ν= yi for ν=
해찾기에 의한 결 과 xi=yi/Ki for v=0.273 zi Ki 0.3500 0.2500 0.2700 0.2730 0.2500 Methane 0.1 10 0.2410 0.3077 0.2915 0.2893 0.3077 0.0308 Ethane 0.2 1.76 0.2780 0.2958 0.2921 0.2915 0.2958 0.1681 Propane 0.7 0.52 0.4375 0.4136 0.4182 0.4189 0.4136 0.7955 Σyi 0.9565 1.0171 1.0018 0.9997 1.0171 0.9943 Σxi
Calculation by Excel
Example
Assuming Roult’s Law to be valid, prepare (a)a Pxy diagram for T=90oC, and
(b)a Txy diagram for P=90 kPa
for a mixture of 1-chlorobutane (1) /chlorobenzene (2) Antoine Coefficients:
A B C
1-chlorobutane (1) 13.9600 2826.26 224.10