관련 문서
FBG: fasting blood glucose, TC: total cholesterol, TG: triglyceride, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol,
Serum uric acid levels and risk for vascular disease in patients with metabolic syndrome... Prevalence if the metabolic syndrome in a Turkish
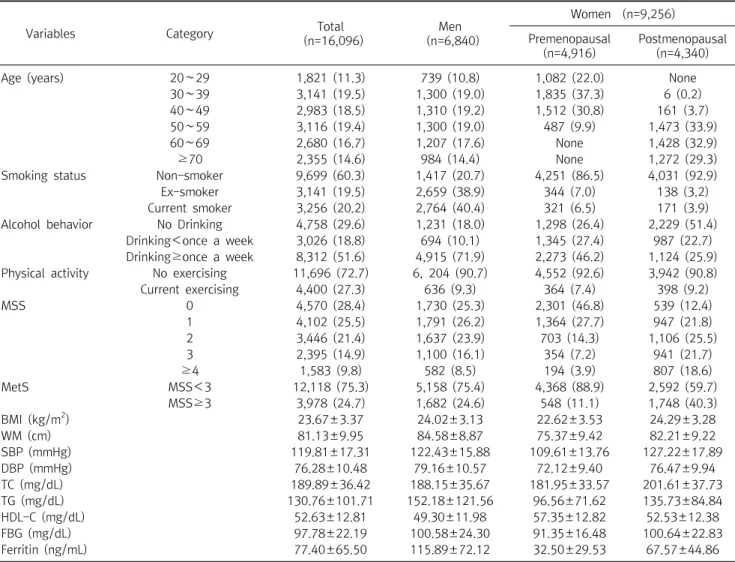
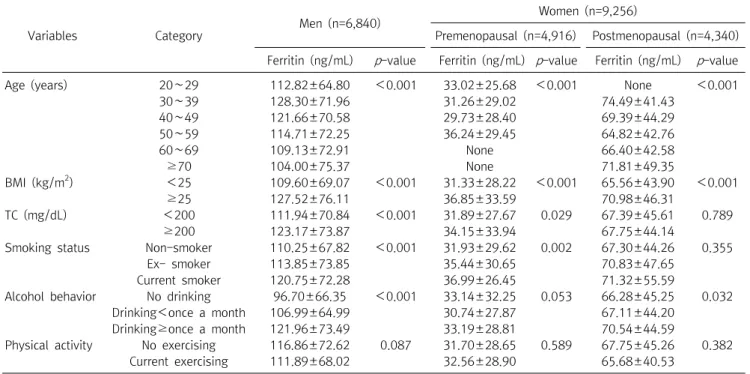
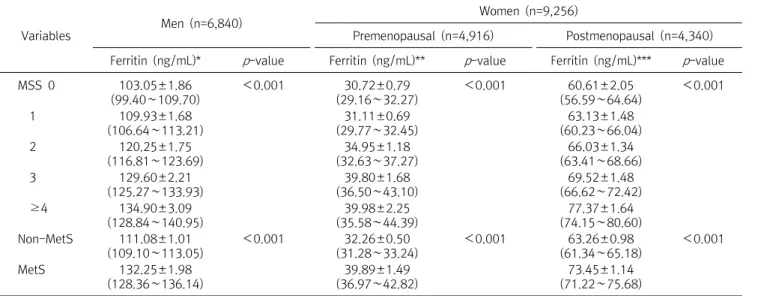
In this study, it can be seen that sex and metabolic syndrome are important for biological factors, and occupation, smoking, and exercise are important
This study was conducted to analyze the effects of obesity on the core stability and metabolic syndrome risk factors of middle - aged women.. The effect
An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences.. The facilitation of social-emotional understanding
In addition, measurements at 0 and 12 weeks (body composition, α-amylase, metabolic syndrome related factors) were performed twice. For 12 weeks, the
The median concentrations of FDP were significantly elevated ( p= 0.02) and the median concentrations of fibronogen were decreased ( p= 0.021) in abnormal bleeding patients
To quantify ignitability as ignition characteristics, the minimum ignition energy (MIE) for liquid fuel was defined and measured under elevated