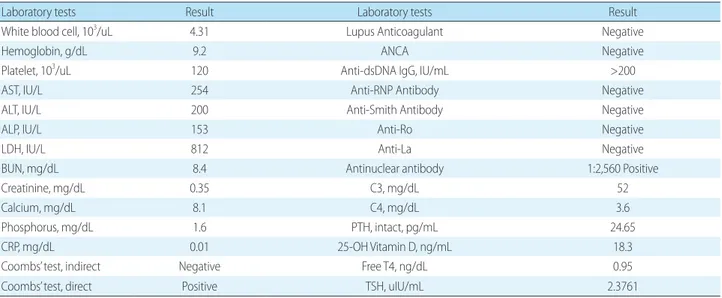
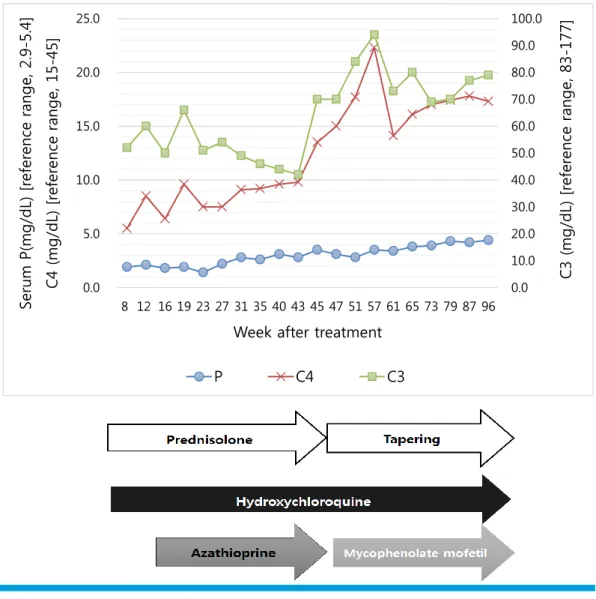
Severe Hypophosphatemia in a Girl with Systemic Lupus Erythematosus
전체 글
수치
관련 문서
Design and synthesis of novel threosyl-5_-deoxyphosphonic acid purine analogues as potent anti-HIV agents. Synthesis of novel 3'-hydroxymethyl
relationship and and and and school school school school mark, mark, mark, mark, shows shows shows shows positive positive positive result positive result
tricuspidata on the production of proinflammatory cytokines in TNFα+IFNγ-stimulated HaCaT cells ...15 Fig.5: The cell viability of sub-fractions from 70% EtOH
As a result of pyrG N-PCR sequence analysis, the positive rate of Borrelia in 27 wild mice captured in October and November was 29.63% (8 positive / 27 total) and only
It is the purpose of this study to investigate the anti microbacterial Activity and anti inflammatory Effects of water extract Rhododendron brachycarpum
A stress component is positive when it acts in the positive direction of the coordinate axes, and on a plane whose outer normal points in one of the positive coordinate
A positive-feedback loop is formed by an amplifier and a frequency-selective network Figure 13.1 The basic structure of a sinusoidal oscillator.. A positive feedback loop is
Competence had a positive (+) influence on satisfaction with human relationships and self-confidence also had the positive (+) influence. However, in attractiveness,