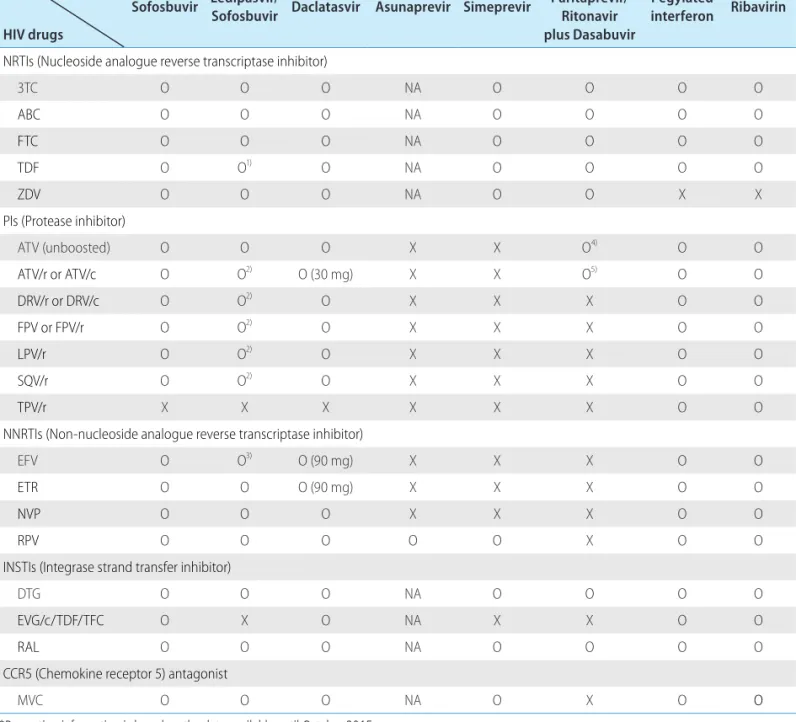
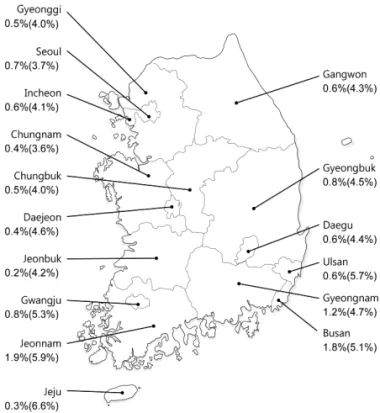
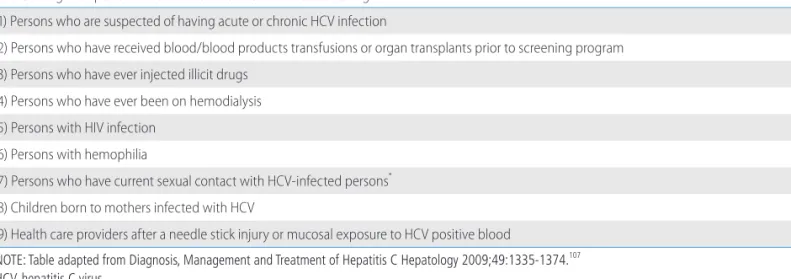
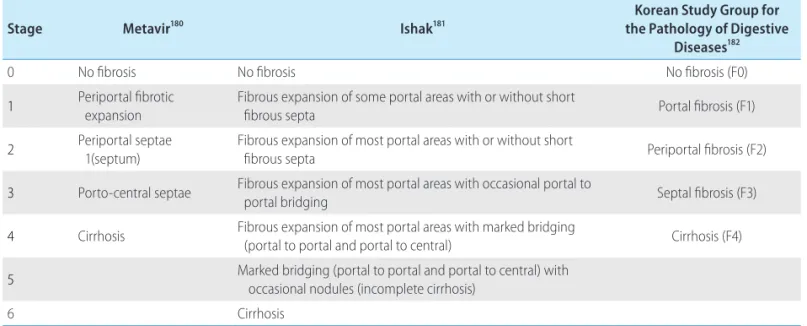
KASL clinical practice guidelines : management of hepatitis C
전체 글
수치

관련 문서
Prioritize and invest in inclusive programs that promote a culture of peace, social cohesion, global citizenship principles, environmental sustainability,
3) ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart
< 0.1 mg/L Penicillin G or ampicillin Cefotaxime, ceftriaxone, or chloramphenicol 0.1-1.0 mg/L Cefotaxime or ceftriaxone Cefepime, meropenem.. ≥ 2.0
Severe disease activity and cy- tomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Guidelines for the management of
Especially, Mg-Al system alloys are inexpensive and have standard mechanical properties microstructure and deformation behaviorsat high temperature were investigated..
– Land: Food, Urbanization, Fibre, Water, Conservation – Energy: Utility and cost as a delivered energy form.. • US has land
Consequently, the present study was designed to investigate the effect of adding 60 mg of raloxifene hydrochloride daily to women treated with GnRH-a for severe endometriosis
Harmonics generated by Control Element Drive Mechanism Control System(CEDMCS) affected the MG Set operation such as increased voltage waveform distortion and... MG Set