ABSTRACT
Purpose: The aim of this study was to evaluate the enhancement of osteogenic potential of biphasic calcium phosphate (BCP) bone substitute coated with Escherichia coli-derived recombinant human bone morphogenetic protein-2 (ErhBMP-2) and epigallocatechin-3- gallate (EGCG).
Methods: The cell viability, differentiation, and mineralization of osteoblasts was tested with ErhBMP-2-/EGCG solution. Coated BCP surfaces were also investigated. Standardized, 6-mm diameter defects were created bilaterally on the maxillary sinus of 10 male New Zealand white rabbits. After removal of the bony windows and elevation of sinus membranes, ErhBMP-2-/EGCG- coated BCP was applied on one defect in the test group. BCP was applied on the other defect to form the control group. The animals were sacrificed at 4 or 8 weeks after surgery. Histologic and histometric analyses of the augmented graft and surrounding tissue were performed.
Results: The 4-week and 8-week test groups showed more new bone (%) than the
corresponding control groups (P<0.05). The 8-week test group showed more new bone (%) than the 4-week test group (P<0.05).
Conclusions: ErhBMP-2-/EGCG-coated BCP was effective as a bone graft material, showing enhanced osteogenic potential and minimal side effects in a rabbit sinus augmentation model.
Keywords: Biphasic calcium phosphate; Bone morphogenetic protein 2; Bone substitute;
epigallocatechin-3-gallate
INTRODUCTION
Escherichia coli-derived recombinant human bone morphogenetic protein-2 (ErhBMP-2) has been used as a substitute for rhBMP-2, which is derived from BMP gene—transfected Chinese hamster ovary cells [1-4]. ErhBMP-2 could overcome the limitations (high cost and low yield) of rhBMP-2, which is produced in mammalian cells. ErhBMP-2 has shown compatible biologic activity and enhancement of bone formation in a dose-dependent manner [2,5].
However, cyst-like bone void formation and soft tissue swelling have been reported as side effects [6]. Therefore, it is recommended to use BMP-2 only for limited clinical cases, and at the lowest possible concentration [6-9].
Research Article
Received: Feb 6, 2019 Accepted: Mar 27, 2019
*Correspondence:
Sungtae Kim
Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea.
E-mail: kst72@snu.ac.kr Tel: +82-2-2072-4712 Fax: +82-2-744-0051
†Jae-ho Hwang and Seung-Han Oh contributed equally to this study.
Copyright © 2019. Korean Academy of Periodontology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://
creativecommons.org/licenses/by-nc/4.0/).
ORCID iDs Jae-ho Hwang
https://orcid.org/0000-0001-8349-316X Seunghan Oh
https://orcid.org/0000-0002-7250-721X Sungtae Kim
https://orcid.org/0000-0001-6361-4104 Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1535).
Jae-ho Hwang 1,†, Seunghan Oh 2,†, Sungtae Kim 1,*
1 Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea
2 Department of Dental Biomaterials, Institute of Biomaterials-Implant, Wonkwang University School of Dentistry, Iksan, Korea
Improvement of the osteogenic
potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone
substitute: in vitro and in vivo activity
Implant Science
Author Contributions
Conceptualization: Sungtae Kim, Jae- ho Hwang; Formal analysis: Seunghan Oh; Funding acquisition: Sungtae Kim;
Investigation: Jae-ho Hwang, Seunghan Oh;
Methodology: Jae-ho Hwang, Seunghan Oh; Software: Jae-ho Hwang, Seunghan Oh;
Validation: Seunghan Oh; Writing - original draft: Jae-ho Hwang; Writing - review &
editing: Seunghan Oh, Sungtae Kim.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea, which is produced by drying the plant Camellia sinensis [10]. This biologically active catechin is considered to have many beneficial effects including anticancer and anti-inflammatory properties, as well as decreasing blood pressure and serum lipid levels without any significant side effects [11,12]. EGCG has also been found to be effective for periodontal regeneration by inducing apoptotic cell death in osteoclasts [13,14]. In addition, EGCG also has direct effects on osteoblasts [15]. EGCG enhanced bone formation by inhibiting the synthesis of interleukin-6 and osteocalcin and by upregulating the synthesis of vascular endothelial growth factor [16-18]. No serious side effects of EGCG application have been found.
Biphasic calcium phosphate (BCP) bone substitute, a synthetic bone substitute, is composed of hydroxyapatite (HA) and β-tricalcium phosphate (TCP). BCP has a similar structure to that of human bone, and its resorption can be controlled by changing the ratio of HA to TCP [19,20]. BCP mainly has osteoconductive properties, but it does not have osteoinductive properties. Therefore, various efforts have been made to improve the osteogenic potential of BCP. Enhancement of osteogenic potential was confirmed when a biologically active peptide or ErhBMP-2 was coated on BCP using a specific coating procedure [21,22]. Even a low dose of ErhBMP-2 (0.05 mg/mL) led to improvements in osteogenic potential similar to those observed with a high dose (0.5 mg/mL) when a specific coating procedure was applied [9].
Based on the results of these studies, coating BCP with a combination of EGCG and ErhBMP-2 (0.05 mg/mL) could be considered as a way to improve osteogenic potential and minimize possible side effects. In a previous study in dogs, ErhBMP-2-/EGCG-coated BCP was confirmed to lead to enhanced osteogenic potential [22]. However, the effect of this coated BCP has not been evaluated in other animal models. Therefore, the purpose of this study was to evaluate the enhancement of osteogenic potential by ErhBMP-2-/EGCG-coated BCP in a standardized rabbit sinus augmentation model.
MATERIALS AND METHODS
In vitro test
Alkaline phosphatase (ALP) activity assay
After 3, 7, and 14 days of incubation, osteoblasts cultured in a 24-well plate were rinsed with 500 μL of phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA) solution, lysed by 500 μL of lysis buffer solution (25 mM Tris [pH 7.6], 150 mM NaCl, and 1% NP-40), and stored in an ice bath for 30 minutes. To measure ALP activity, 50 μL of cell lysate was mixed with 200 μL of para-nitrophenylphosphate (Sigma-Aldrich, St. Louis, MO, USA), and the mixed solution was stored at 37°C for 30 minutes to activate the reaction. After 30 minutes, 50 μL of 3 N NaOH (Sigma-Aldrich) was added to stop the reaction. The absorbance of each solution was measured at 405 nm using an enzyme-linked immunosorbent assay (ELISA) reader (SpectraMax 250, Thermo Electron Co., Waltham, MA, USA). The rest of the cell lysate was used for measurements of the total protein content (Bradford protein assay kit, Sigma-Aldrich).
Alizarin red assay
Extracellular matrix mineralization of osteoblasts was tested with alizarin red staining. After 14, 21, and 28 days of incubation, osteoblasts cultured in a 24-well plate were rinsed with 500 μL of PBS (Invitrogen) solution and fixed with 500 μL of 4% paraformaldehyde (Sigma-
Aldrich) solution at room temperature for 20 min. After fixation, the cells were stained with 500 μL of 2% alizarin red (pH 4.2, Sigma-Aldrich) solution for 20 minutes and then washed twice with 500 μL of double-distilled water. The stained sample was reacted with 500 μL of 10% acetic acid (JT Baker, Center Valley, PA, USA) for 30 minutes and then stored at 85°C for 10 minutes. The supernatant was collected from the reacted solution after high-speed centrifugation (11,000 rpm for 15 minutes). The same volume of 10% ammonium hydroxide (Sigma-Aldrich) was added to the supernatant in order to neutralize it. The absorbance of each solution was measured at 405 nm using a microplate ELISA reader (SpectraMax 250, Thermo Electron Co.).
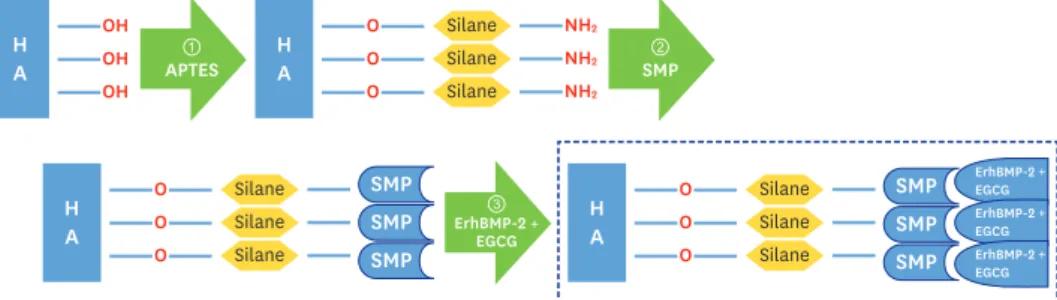
Preparation of ErhBMP-2-/EGCG-coated BCP
ErhBMP-2 (Cowellmedi, Busan, Korea) and EGCG (Sigma-Aldrich) immobilized grafting materials were stored at 70°C in a deep-freezer. When required, the frozen solution was placed in a lyophilizer (Ilshin Laboratory, Seoul, Korea) for 1 day to sublimate the liquid phase in a vial without denaturation of ErhBMP-2 and EGCG. The BCP surface (OSTEON, Dentium, Seoul, Korea) was coated with a combination of ErhBMP-2 and EGCG using a 3-step technique [23,24]. The first step was 3-aminopropyltriethoxysilane (Sigma-Aldrich), in which the OH− of HA was reacted with a silane coupling agent. The second step was to combine N-succinimidyl-3-maleimidopropionate (SMP; Sigma-Aldrich) with amino radicals.
The last step was to combine EGCG and ErhBMP-2 with SMP. The success of the surface treatment was confirmed through field-emission scanning electron microscopy (FE-SEM;
S4800, Hitachi/Horiba, Japan) (Figure 1).
Cell viability test and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay Human mesenchymal stem cells (hMSCs) were plated on each specimen (0.25 mL of BCP and 0.25 mL of ErhBMP-2-/EGCG-coated BCP), which had been previously prepared in a 24-well plate at a density of 1×104 cells/mL. The fluorescein diacetate (FDA; Sigma-Aldrich) staining technique was applied to count viable hMSCs that adhered to the specimen. At 2 and 24 hours after plating, hMSCs on the substrates were rinsed with 500 μL of PBS solution (Invitrogen) and incubated with 500 μL of FDA working solution (50 μg of FDA dissolved in 10 mL of PBS solution) for 30 seconds, and then washed 3 times using 500 μL of PBS solution. The washed specimens were imaged with the aid of an inverted fluorescence microscope (CKX41, Olympus, Tokyo, Japan). An MTT assay was also applied. After the selected incubation periods, the samples were washed with 500 μL of PBS solution and transferred to a new 24-well plate. MTT dye agent (1 mL, Sigma-Aldrich) was added to each well. After 3 hours of incubation in a 5% CO2 incubator, 1 mL of isopropanol was added to each well and the
HA H
A
H A
Silane Silane Silane
Silane Silane Silane
Silane Silane Silane
APTES① ②
SMP
ErhBMP-2 +③ EGCG
NH2
NH2
NH2
O O O
O O O OH OH OH
SMP SMP SMP
H A
O O O
ErhBMP-2 + EGCG ErhBMP-2 + EGCG ErhBMP-2 + EGCG
SMP SMP SMP
Figure 1. Schematic diagram of preparation of BCP coated with low-concentration ErhBMP-2 and EGCG 1. APTES treatment; 2. Bifunctional cross-linker (SMP) connection; 3. Immobilized ErhBMP-2 and EGCG grafting.
HA: hydroxyapatite, BCP: biphasic calcium phosphate, ErhBMP-2: Escherichia coli-expressed recombinant human bone morphogenetic protein 2, EGCG: epigallocatechin-3-gallate, APTES: 3-aminopropyltriethoxysilane, SMP:
succinimidyl-3-maleimidopropionate.
plate was shaken for 30 minutes. The absorbance of each solution was measured using a microplate ELISA reader (SpectraMax 250, Thermo Electron Co.).
Surface analysis
The elemental composition, atomic concentrations, and chemical states of the elements present on each sample's surface were measured quantitatively using X-ray photoelectron spectroscopy (XPS; K-Alpha ESKA, Thermo Electron Co.).
In vivo test
Study design and animals
Ten male New Zealand white rabbits (with 20 maxillary sinuses) weighing 2.5–3.0 kg were divided into 2 groups according to the type of bone graft material used: ErhBMP-2-/EGCG- coated BCP (study group) on one side and BCP (control group) on the other side. Each group was then subdivided into 4- and 8-week healing groups before being euthanized. The selection process, management, surgical protocol, and preparation applied to the animals were reviewed and approved by the Institutional Animal Care and Use Committee of Yonsei Medical Center, Seoul, Korea (2011-0056-4) (Table 1).
Surgical procedure
All surgical procedures were performed under general anesthesia. The animals were first anesthetized intramuscularly with a mixture of ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea) and xylazine (Rompun, Bayer Korea, Seoul, Korea). The surgical site was isolated by shaving and sterilizing with povidone-iodine solution, and the animals were further anesthetized using 2% lidocaine with 1:100,000 epinephrine by infiltration. A straight incision was made in the sagittal plane, and a full-thickness flap was then reflected laterally to expose the maxillary sinus. Standardized and circular windows with a diameter of 6 mm were prepared bilaterally on the maxillary sinus using a saline-cooled trephine bur (3i Implant Innovation, Palm Beach Gardens, FL, USA) [25]. The reference point for micro-computed tomography (micro-CT) analysis and specimen preparation was indicated by a pin inserted at the point where the sagittal midline met the imaginary line connecting the right and left windows. Following careful removal of the trephined bony disks, ErhBMP-2-/EGCG-coated BCP on one side (study group) and BCP on the other side (control group) were applied to the windows. The skin/periosteum flap was sutured using 4-0 Monosyn (glyconate absorbable monofilament, B-Braun, Aesculap, Center Valley, PA, USA), with periosteum repositioned over the windows. Each animal was allowed a healing period of either 4 or 8 weeks postoperatively and then was euthanized.
Radiographic analysis: micro-CT
The block sections, including the augmented sinus and the surrounding bone, were removed and then fixed in 10% neutral-buffered formalin for 10 days. X-ray imaging was then performed using micro-CT (SkyScan 1076, SkyScan, Aartselaar, Belgium) at a resolution of 35 μm (100 kV and 100 μA). OnDemand3D software (Cybermed, Seoul,
Table 1. Study design
Groups Materials Healing period Number (%)
4-week control BCP 4 weeks 5
8-week control BCP 8 weeks 5
4-week study ErhBMP-2-/EGCG-coated BCP 4 weeks 5
8-week study ErhBMP-2-/EGCG-coated BCP 8 weeks 5
BCP: biphasic calcium phosphate, ErhBMP-2: Escherichia coli-expressed recombinant human bone morphogenetic protein 2, EGCG: epigallocatechin-3-gallate.
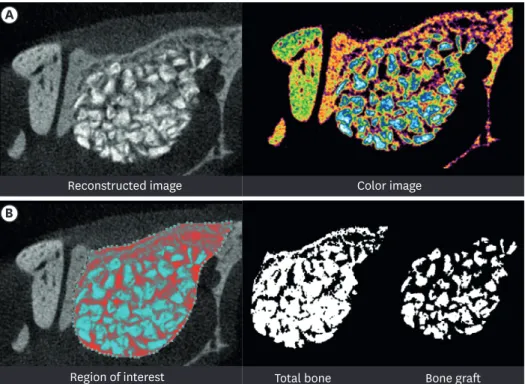
Korea) was used to reconstruct the area of interest. The total augmented volume was measured volumetrically using the CT analyzer program supplied with the micro-CT system (SkyScan), as shown in Figure 2 [25]. The total augmented volume and new bone volume were measured at the area of interest where the greatest volume was present in the reconstructed image.
Histologic analysis
After rinsing with water, all specimens were decalcified in 5% formic acid for 14 days and embedded in paraffin, followed by slicing into sections that were approximately 5 μm thick coronally along the center of the augmented sinus. The 2 central-most sections were selected from each block, stained with hematoxylin and eosin, and examined under a light microscope (BX40, Olympus). Three regions of interest were selected from each slide: the defect margin area, the area near the Schneiderian membrane, and the center of the augmented area.
Histometric analysis
The following 2 parameters were measured using an automated image-analysis system (Image-Pro Plus, The Proven Solution, Chicago, IL, USA) [9,21].
New bone (%) = 100×newly formed area/total augmented bone area Remaining graft (%) = 100×remaining graft area/total augmented bone area Statistical analysis
Data from the ALP activity assay and alizarin red assay were analyzed statistically using 1-way analysis of variance, followed by the Tukey honest significant difference multiple-range test
A
Color image Reconstructed image
Bone graft Total bone
Region of interest B
Figure 2. Radiographic analysis: micro-CT. (A) Reconstructed image of micro-CT. (B) Region of interest: new bone volume was calculated as the remaining graft volume subtracted from the total augmented volume.
CT: computed tomography.
as a post hoc test (α=0.05). The micro-CT data and histomorphometric measurements of the samples were analyzed statistically using standard statistical software (SPSS version 15.0, SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used to compare differences between the control and study groups, as well as differences within each group according to the healing period. The cutoff for statistical significance was set at P<0.05, and the data are presented as mean and standard deviation.
RESULTS
In vitro test ALP activity assay
ALP activity increased in all groups during the experimental period, and it was greater in the combination solution (ErhBMP-2/EGCG) than in the EGCG or control solution at 3, 7, and 14 days.
Alizarin red assay
The concentration of alizarin red was higher in the ErhBMP-2 solution than in the other solutions after 14 days of incubation. However, at 21 and 28 days there was not any significant difference between the ErhBMP-2 and combination (ErhBMP-2/EGCG) solutions, and the values found in the ErhBMP-2 and combination (ErhBMP-2/EGCG) solutions were higher than those in the EGCG and control solutions (P<0.05).
Cell viability test: FDA staining and MTT assay
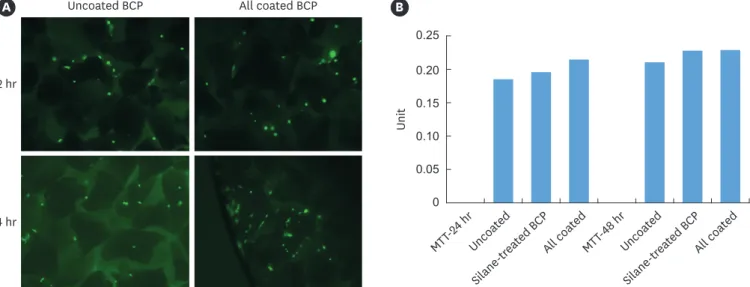
Adherent hMSCs became prominent in the ErhBMP-2-/EGCG-coated BCP at an early stage (within the initial 24 hours of incubation). The cell viability was not different between the BCP and ErhBMP-2-/EGCG-coated BCP. The results obtained in the MTT assay were consistent with FDA staining, with the level of cell proliferation being higher in the ErhBMP-2-/EGCG-coated BCP than in the control BCP (Figure 3).
0 0.25
0.15 0.20
0.05
Unit 0.10
MTT-24 hr
MTT-48 hr Silane-
treated BCPAll coat ed Uncoated BCP
2 hr
24 hr
All coated BCP
A B
Uncoat ed
Silane-
treated BCPAll coat ed Uncoat
ed
Figure 3. (A) Results of fluorescein diacetate staining at 2 and 24 hours. (B) Results of the MTT assay at 24 and 48 hours.
BCP: biphasic calcium phosphate, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Surface analysis
The binding energies were compared with those in the literature [23,24] to determine whether each reaction step had been successfully completed. The Si2p spectrum near 100 eV that was evident in the XPS scan of BCP coated with ErhBMP-2 and EGCG indicated that the silanization of BCP was successful, since this spectrum was also found in the XPS scan of silane-treated BCP. Additionally, the presence of the N1s and C1s spectrums formed due to amide species indicated that the ErhBMP-2 and EGCG immobilization process was successful. Additional spectra, such as those of Na1s and Cl2p, obtained in the XPS scan of ErhBMP-2-/EGCG-coated BCP were due to the presence of PBS, which was used to preserve the coated specimens. In the FE-SEM analysis, the BCP and ErhBMP-2-/EGCG-coated BCP surfaces showed different surface characteristics (Table 2).
In vivo test
Radiographic and histometric analyses
Volumetric parameters were measured using the micro-CT data. The study and control groups exhibited similar mean new bone volume and total augmented volume after 4 and 8 weeks of healing. New bone volume and total augmented volume were greater in the study group than in the control group after 4 and 8 weeks of healing. The values after 8 weeks of healing were also slightly greater than those observed after 4 weeks of healing (Table 3).
New bone (%) in the 4-week and 8-week study groups was greater than in the corresponding control groups (P<0.05). New bone (%) in the 8-week study group was also greater than in the 4-week study group (P<0.05) (Table 4).
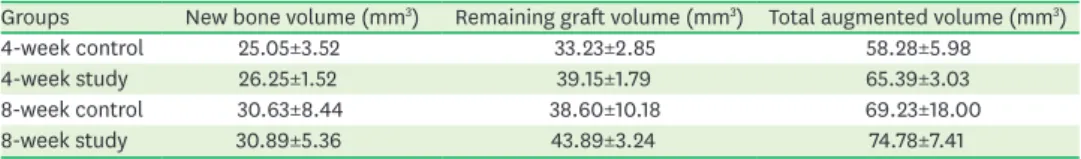
Histologic analysis
The amount of vascularization and newly formed blood vessels in the 4-week control group (Figure 4D) was less than the 4-week study group (Figure 5D). The amount of immature woven bone, which was undergoing mineralization, was greater in the 4-week control group (Figure 4B) than in the 4-week study group (Figure 5B). Newly formed bone was sporadically Table 2. Percentages of element content (%)
Element Silane-treated All coated
Nitrogen 1.14 1.44
Silicon 2.03 1.69
Carbon 21.76 23.07
Table 3. Radiographic analysis in the standardized rabbit sinus model
Groups New bone volume (mm3) Remaining graft volume (mm3) Total augmented volume (mm3)
4-week control 25.05±3.52 33.23±2.85 58.28±5.98
4-week study 26.25±1.52 39.15±1.79 65.39±3.03
8-week control 30.63±8.44 38.60±10.18 69.23±18.00
8-week study 30.89±5.36 43.89±3.24 74.78±7.41
Table 4. Histometric analysis in the standardized rabbit sinus model
Groups New bone Remaining graft
4-week control 14.30±3.33 31.94±3.65
4-week study 23.34±4.81a) 32.50±9.00
8-week control 19.71±10.54 27.00±5.87
8-week study 31.60±4.94a),b) 29.59±2.63
Data are shown as mean±standard deviation or number (%).
a)Statistically significant difference from the control group in the same week (P<0.05); b)Statistically significant difference between 4 and 8 weeks in the same study group (P<0.05).
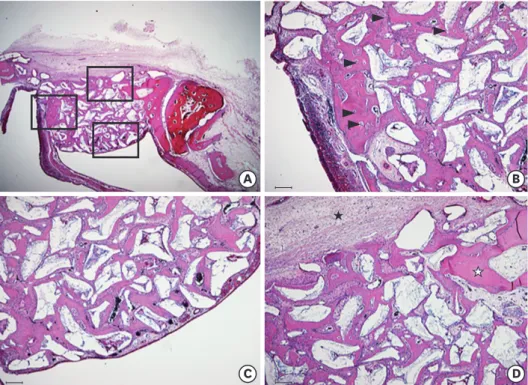
found between the graft materials. Knife-edged bone graft materials were also identified in the 4-week study group (Figure 5B). However, a significant difference was not observed in this regard with the 4-week control group (Figure 4B).
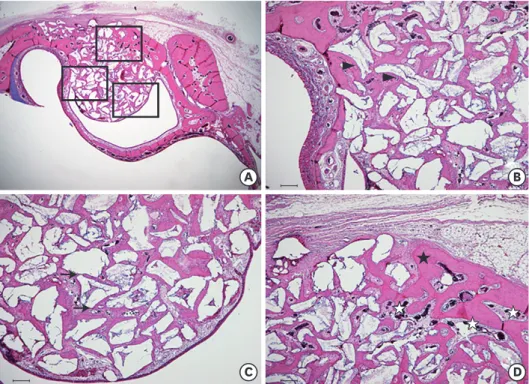
In contrast, after 8 weeks of healing, the Schneiderian membrane had slightly thickened.
The shape of the graft material had also become smoother, indicating the resorption of graft-material particles (Figure 6C and D). In addition, more stretching of the Schneiderian membrane, indicating that more new bone had formed toward the membrane, was found in this group (Figure 6C). In particular, a newly formed bony bridge was found after 8 weeks of healing between the graft material particles close to the Schneiderian membrane, showing very similar characteristics to the preexisting bone: an intensely stained, well- lined pattern of compact lamellar bone with a few osteocytes and marrow spaces (Figure 6C and D). The connective tissue seemed to have been replaced by mineralized tissue between 4 and 8 weeks.
DISCUSSION
In the present study, a standardized rabbit sinus model with the same shape and size of the window was used [25]. The rationale behind this model was to provide the same conditions for the control and the study groups, thereby minimizing procedural errors. Additionally, the degree of anatomical variation between individuals is smaller in the rabbit sinus model than
A B
D C
★
★
Figure 4. Histological findings of the 4-week control group. (A) Area of interest (H&E). (B) Defect margin area (H&E, scale bar: 100 μm): immature osteocytes in the lacunae (arrowhead) were observed, which was a distinctive feature of the control group. (C) Near the Schneiderian membrane (H&E, scale bar: 100 μm).
(D) Center of the augmented area (H&E, scale bar: 100 μm): the original curvature of the nasal bone was not restored, and loose connective tissue and adipose tissue were seen (black asterisk). Matured lamellar bone (white asterisk) can be hardly seen, either at the defect margin or in between graft materials.
H&E: hematoxylin and eosin.
A B
D C
★
★ ★
★
Figure 5. Histological findings of the 4-week study group (A) Area of interest (H&E). (B) Defect margin area (H&E, scale bar: 100 μm): the number of immature osteocytes (arrowhead) was dramatically reduced. (C) Near the Schneiderian membrane (H&E, scale bar: 100 μm): black arrows indicate the shape of sharp, knife-edged bone graft materials, which were not greatly different from what was observed in the control group. (D) Center of the augmented area (H&E, scale bar: 100 μm): more vascularization and the presence of newly formed blood vessels (white asterisk) were identified. Black asterisks indicate that the majority of new bone formation occurred in a confined area, especially at the lateral side of the surgically created window.
H&E: hematoxylin and eosin.
A B
D C
★
Figure 6. Histological findings of the 8-week study group (A) Area of interest (H&E). (B) Defect margin area (H&E, scale bar: 100 μm). (C) Area near the Schneiderian membrane (H&E, scale bar: 100 μm): shallow, round, and ovoid-shaped graft materials were identified, indicating resorption of graft material particles. Intensely stained, well-lined patterns of compact lamellar bone (black asterisk) were prominent between graft material particles close to the Schneiderian membrane, showing very similar characteristics to the pre-existing bone. (D) Middle area (H&E, scale bar: 100 μm): surgically created window closure was markedly advanced and mature lamellar bone seemed to be predominant.
H&E: hematoxylin and eosin.
in larger animals, which allows a consistent reproduction of defects in different individuals.
Preservation of the Schneiderian membrane was also possible with the delicate use of a trephine bur for osteotomy [25,26], as evidenced by the present histologic findings. In terms of group design, the 4-week healing group and 8-week healing group were selected to correspond to healing periods of 3–4 months and 6–8 months in human [27-30], since the metabolic rate of rabbits is roughly 3–4 times greater than that of humans.
The combination solution (ErhBMP-2/EGCG) and the ErhBMP-2 solution were equivalently effective in increasing ALP activity until 14 days. The combination solution (ErhBMP-2/
EGCG) and the ErhBMP-2 solution were equivalently effective at increasing mineral nodule formation at 14, 21, and 28 days. As shown by the MTT assay and FDA staining, ErhBMP-2-/
EGCG-coated BCP demonstrated a greater level of cell proliferation than BCP in the early stage. The cell viability observed with ErhBMP-2-/EGCG-coated BCP was equivalent to that observed with BCP. In summary, enhanced proliferation and differentiation of osteoblasts in response to ErhBMP-2/EGCG solution was confirmed. Cell viability was not influenced significantly by ErhBMP-2-/EGCG-coating. Based on the results of these in vitro studies, which showed improved osteogenic potential and unchanged cell viability, ErhBMP-2-/EGCG- coated BCP can be considered as a possible alternative to BCP.
Surface analysis characterized the structural composition of ErhBMP-2-/EGCG-coated BCP via XPS. The presence of each element, distribution modality, and spectrum found in the XPS scan provided information on whether each reaction step had been successfully completed.
By evaluating the spectra found on the XPS scan, it was confirmed that silanization and the ErhBMP-2 and EGCG immobilization process were successfully achieved. All specimens of ErhBMP-2-/EGCG-coated BCP were evaluated with this surface analysis before application in subsequent in vivo studies.
In the histomorphometric analysis, the 4-week and 8-week test groups showed more new bone (%) than the corresponding control groups (P<0.05), indicating that the osteogenic potential of BCP was improved by ErhBMP-2/EGCG coating. The 8-week test group showed more new bone (%) than the 4-week test group (P<0.05), confirming both the early and delayed effects of ErhBMP-2/EGCG coating. The overall enhancement of osteogenic potential in ErhBMP-2-/EGCG-coated BCP could support the possibility of replacing regular BCP with this coated BCP for clinical applications. Serious side effects of ErhBMP-2 were not found on the histologic evaluations. It seems that there was more soft tissue swelling in the study group, since the distance between particles in the study group was greater than in the control group in the histologic evaluation, and the total augmented volume was also slightly greater in the study group than in the control group in the radiographic evaluation. However, this minor soft tissue swelling during the early healing period might have positively affected new bone formation in the test group; the space between particles in the 4-week study group was mainly composed of connective tissue, but it had changed into new bone in the 8-week study group. In addition, no serious inflammatory reaction was found in the study group.
Therefore, the initial swelling in response to ErhBMP-2 could be considered as preparation for more new bone formation, rather than as a serious side effect.
In addition, delayed resorption of particles in response to the ErhBMP-2/EGCG coating was also confirmed; more remaining graft (%) in the histometric analysis and more remaining graft volume in the radiographic analysis were found in the study group than in the control group. Based on these results, it can be inferred that the EGCG coating may have induced
apoptotic cell death of osteoclasts and inhibited their formation, as suggested by previous studies [14,27], thereby slowing the rate of bone resorption by osteoclasts. It is also anticipated that this less-resorptive property is particularly advantageous for the long-term stability of the newly formed bone against the air pressure within the sinus cavity, which may interfere with new bone formation through the activation of osteoclasts and bone resorption [27]. Consequently, it could affect the rate of bone formation and resorption during the healing phase. The replacement of bone graft material with newly formed bone does not necessarily occur in a 1:1 ratio, as new bone formation was clearly evident in both study groups [28-30]. Furthermore, when analyzing the correlation between the reduced amount of graft and the increased amount of new bone formation, both study groups showed an increased amount of new bone formation that was relatively greater than the reduced amount of graft, suggesting that ErhBMP-2-/EGCG-coated BCP as a grafting material was more effective for new bone formation than BCP.
Our findings clearly suggest that ErhBMP-2-/EGCG-coated BCP could be a promising bone graft material, maximizing the osteogenic potential of ErhBMP-2, while simultaneously compensating for its side effects. In addition, owing to its less resorptive properties, it is especially advantageous in a situation where long-term new bone formation activity with enhanced osteogenic potential is required.
In conclusion, ErhBMP-2-/EGCG-coated BCP was effective as a bone graft material with enhanced osteogenic potential and minimal side effects in a rabbit sinus augmentation model.
REFERENCES
1. Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg 2000;38:645-9.
PUBMED | CROSSREF
2. Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli.
Biomaterials 2010;31:3512-9.
PUBMED | CROSSREF
3. Long S, Truong L, Bennett K, Phillips A, Wong-Staal F, Ma H. Expression, purification, and renaturation of bone morphogenetic protein-2 from Escherichia coli. Protein Expr Purif 2006;46:374-8.
PUBMED | CROSSREF
4. Vallejo LF, Brokelmann M, Marten S, Trappe S, Cabrera-Crespo J, Hoffmann A, et al. Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J Biotechnol 2002;94:185-94.
PUBMED | CROSSREF
5. Choi KH, Moon K, Kim SH, Yun JH, Jang KL, Cho KS. Purification and biological activity of recombinant human bone morphogenetic protein-2 produced by E. coli expression system. J Korean Acad Periodontol 2008;38:41-50.
CROSSREF
6. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP- 2). Spine J 2008;8:1011-8.
PUBMED | CROSSREF
7. Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000;27:479-86.
PUBMED | CROSSREF
8. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off- label usage of rhBMP-2 in the anterior cervical spine. Spine(Phila Pa 1976) 2006;31:2813-9.
PUBMED | CROSSREF
9. Choi H, Park NJ, Jamiyandorj O, Choi KH, Hong MH, Oh S, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2. J Periodontal Implant Sci 2012;42:119-26.
PUBMED | CROSSREF
10. Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr 2000;130:2409-12.
PUBMED | CROSSREF
11. Tosetti F, Noonan DM, Albini A. Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer 2009;125:1997-2003.
PUBMED | CROSSREF
12. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 2006;72:1439-52.
PUBMED | CROSSREF
13. Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, et al. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun 2002;292:94-101.
PUBMED | CROSSREF
14. Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res 2004;39:300-7.
PUBMED | CROSSREF
15. Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem 2007;18:341-7.
PUBMED | CROSSREF
16. Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Harada A, et al. (-)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm Metab Res 2008;40:674-8.
PUBMED | CROSSREF
17. Tokuda H, Takai S, Matsushima-Nishiwaki R, Akamatsu S, Hanai Y, Hosoi T, et al. (--)-Epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J Cell Biochem 2007;100:1146-53.
PUBMED | CROSSREF
18. Kato K, Otsuka T, Adachi S, Matsushima-Nishiwaki R, Natsume H, Kozawa O, et al. (-)-Epigallocatechin gallate inhibits thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Med Rep 2011;4:297-300.
PUBMED
19. Kim S, Jung UW, Lee YK, Choi SH. Effects of biphasic calcium phosphate bone substitute on circumferential bone defects around dental implants in dogs. Int J Oral Maxillofac Implants 2011;26:265-73.
PUBMED
20. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med 2003;14:195-200.
PUBMED | CROSSREF
21. Choi H, Park NJ, Jamiyandorj O, Hong MH, Oh S, Park YB, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences. J Periodontal Implant Sci 2012;42:166-72.
PUBMED | CROSSREF
22. Shin YS, Seo JY, Oh SH, Kim JH, Kim ST, Park YB, et al. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis 2014;20:281-7.
PUBMED | CROSSREF
23. Xiao SJ, Textor M, Spencer ND. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir 1998;14:5507-16.
CROSSREF
24. Durrieu MC, Pallu S, Guillemot F, Bareille R, Amédée J, Baquey CH, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med 2004;15:779-86.
PUBMED | CROSSREF
25. Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res 2012;23:682-9.
PUBMED | CROSSREF
26. Misch CE. Contemporary implant dentistry. St. Louis (MO): Mosby; 1999.
27. Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. (-)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res 2007;42:212-8.
PUBMED | CROSSREF
28. Xu H, Shimizu Y, Ooya K. Histomorphometric study of the stability of newly formed bone after elevation of the floor of the maxillary sinus. Br J Oral Maxillofac Surg 2005;43:493-9.
PUBMED | CROSSREF
29. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res 2002;13:405-9.
PUBMED | CROSSREF
30. Frenken JW, Bouwman WF, Bravenboer N, Zijderveld SA, Schulten EA, ten Bruggenkate CM. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: a clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin Oral Implants Res 2010;21:201-8.
PUBMED | CROSSREF