329 책임저자:정익주, 519-809, 전남 화순군 화순읍 일심리 160번지
화순전남대학교병원 혈액종양내과 Tel: 061-379-7632, Fax: 061-379-7628 E-mail: ijchung@chonnam.ac.kr
접수일:2009년 11월 6일, 게재승인일:2009년 11월 20일
Correspondence to:Ik Joo Chung
Department of Internal Medicine, Chonnam National University Hwasun Hospital, 160, Ilsim-ri, Hwasun-eup, Hwasun-gun 519-809, Korea Tel: +82-61-379-7632, Fax: +82-61-379-7628
E-mail: ijchung@chonnam.ac.kr
19- nor -1α-25-Dihydroxyvitamin D
2(Paricalcitol) Induces Apoptosis in Gastric Cancer Cells
Woo Kyun Bae, Ji Hee Lee, Myung Suk Park, Jae Sook Ahn, Jun Eul Hwang, Hyun Jeong Shim, Sang Hee Cho and Ik-Joo Chung
Department of Hematology-Oncology, Chonnam National University Medical School, Gwangju 501-757, Korea The active metabolite of vitamin D3 (1,25-dihydroxyvitamin D3, calcitriol) has shown potent antitumor activity for multiple cancers in vitro and in vivo models. Concerns about causing hypercalcemia by calcitriol and the desire for more potent agents have prompted the development of low-calcemic vitamin D analogs.
The compound 19-nor-1α-25-dihydroxyvitamin D2 (paricalcitol) demonstrated anticancer effects on gastric cancer cell lines expressing vitamin D receptor (VDR). The expression of the VDR protein was increased by paricalcitol in a dose-dependent manner. Paricalcitol inhibited gastric cell growth and induced apoptosis in AGS, SNU719 and MKN45 cell lines. Paricalcitol increased the expression of the caspase-3 cleaved protein and decreased the expression of the anti-apoptotic Bcl-2 protein. Furthermore, paricalcitol promoted early and late apoptosis. This low-calcemic vitamin D analog was effective in vitro and is a promising agent for the prevention and treatment of gastric cancer. (Cancer Prev Res 14, 329-334, 2009) Key Words: Paricalcitol (19-nor-1α-25-dihydroxyvitamin D2), Gastric cancer, 1,25(OH)2D3, Apoptosis
INTRODUCTION
Vitamin D is a steroidal hormone that is primarily made available in the body through endogenous synthesis in the skin in response to ultraviolet light, and then is metabolized in the liver and kidney.1) The effects of vitamin D are mediated by the binding of 1 α,25-dihydroxyvitamin D3 [1,25(OH)2D3] to a vitamin D receptor (VDR), a member of the steroid hormone receptor superfamily.2) The VDR is not only located in the tissues responsible for its metabolism and calcium homeostasis (kidney, intestine, and bone), it is also located in more than 30 different tissues and cell lines.
Some of these tissues include brain, breast, colon, heart, liver, prostate, pancreas, skin, stomach and cells such as activated B- and T-lymphocytes and macrophages.3) Once 1,25(OH)2D3 binds to the VDR in the nucleus it forms a heterodimeric complex with the retinoid x receptor (RXR).4) The VDR-RXR complex binds to a hexameric binding motif in the promoter region of the vitamin D
response element (VDRE).5) The active metabolite of vitamin D3 (1 α, 25-dihydroxyvitamin D3, calcitriol) binds to VDR and promotes interaction with VDREs in the promoter regions of target genes,6) resulting in transactivation of genes that promote cell cycle arrest, apoptosis, differentiation, antiangiogenesis and inhibition of pro-growth/ pro-survival signaling pathways.7)
Studies using model systems of prostate adenocarcinoma,8) breast cancer,9) colon carcinoma10) and pancreatic cancer11) have shown that the administration of 1,25(OH)2D3 or vitamin D analogues had significant anticancer effects. The effects of 1,25(OH)2D3 and its derivatives have been shown to function through the VDR to regulate proliferation, apoptosis and angiogenesis.12∼14)
These data demonstrate the importance of 1,25(OH)2D3 as an anti-tumor agent. Unfortunately, 1,25(OH)2D3 is limited in its therapeutic application due to the associated hypercalcemia.15) Thus, there is great interest in the development of a compound that mimics the chemical structure of the potent hormone in such a way that its anti-tumor effects are enhanced, while the calcemic
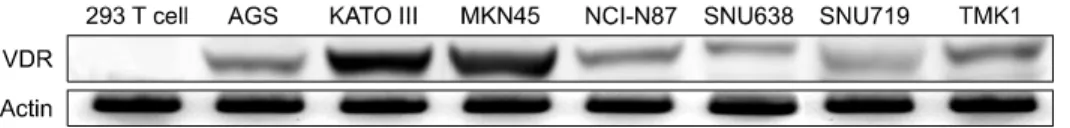
Fig. 1. The various gastric cancer cell lines expressed VDR: Protein expression was determined by Western blot analysis with anti-VDR antibodies; Actin was used as loading control.
activity is reduced.
A novel class of vitamin D analogues has been developed. The calcitriol analogue, 19-nor-1α-25-dihydroxyvitamin D2 (parical- citol, Zemplar), was approved by the Food and Drug Admi- nistration in 1998 for the prevention and treatment of secondary hyperparathyroidism associated with chronic renal failure. In pa- tients with chronic renal failure, paricalcitol is three to four times less calcemic than 1,25-dihydroxyvitamin D.16)
We designed this study to assess the significance of vitamin D receptors in gastric cancer cells and to evaluate the potential role of vitamin D in the treatment of gastric cancer. Gastric cancer cells were treated with paricalctiol to elucidate its mole- cular effects on apoptosis.
MATERIALS AND METHODS 1. Chemicals and reagents
Paricalcitol (PAR, Abbott Laboratories, Chicago, IL., USA) was purchased as a 5μg/ml stock dissolved in 30% propylene glycol and 20% alcohol. Calcitriol was diluted in RPMI 1,640 medium (Invitrogen, Frederick, Md., USA) before use.
2. Cell culture conditions
Human gastric cancer cell lines (AGS, MKN45, SNU719, 293Tcell, KATO III, NCI-N87, SNU638, TMK1) were obtained from the American Type Culture Collection (Manassas, VA.,USA), and were grown in RPMI supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2.
3. MTT assay
For the Cell Proliferation assays, the cells were seeded in 24-well plates (5×104 cells/well), and treated with vehicle control or various concentrations of paricalcitol. The cells were pulsed with EZ-Cytox (Daeil Lab Service, Seoul, Korea) for 2 hours. The absorption by the cells was then read at 450 nm with an ELISA reader (infinite M200 TECAN, Tecan Austria GmbH, Austria). The results are
representative of at least three independent experiments.
4. Western blot analysis
Anti-rabbit VDR (Santa Cruz Biotech), cleaved capase-3 (Cell Signaling), p21 (Santa Cruz Biotech) and actin (H-196, Santa Cruz Biotech) and anti-mouse Bcl-2 (Santa Cruz Biotech), actin (C-2, Santa Cruz Biotech) antibodies were used with the appropriate secondary antibodies (Amersham). The cells were treated with various concentrations of paricalcitol and were harvested for im- munoblot analysis 48 hours after inhibitor treatment. The cellular proteins were separated, transferred and immunoblotted. Immu- noreactive bands were visualized using a secondary horseradish- peroxidase-conjugated anti-rabbit or anti-mouse antibody provide- din the Western blot Detection Kit, Chemioluminescent system (Millipore, USA). The blot was reprobed with anti-actin antibodies (Santa Cruz Biotech) as loading control.
5. Flow cytometric analysis
The cells were plated on 12-well plates at a density of 1×105 cells/well overnight, followed by treatment with the vehicle control or various concentrations of paricalcitol for 48 hours. The cells were trypsinized, washed with ice-cold PBS and fixed with ice-cold 70% ethanol. Approximately 105 cells were resuspended in PBS containing Annexin V-APC and 7-AAD (Annexin V-APC and 7-Aminoactinomycin D Apoptosis assay kit, BD Pharmingen) for 30 min in the dark and were then sorted by FACS analysis.
6. Statistical analysis
The results are expressed as mean±standard deviation (SD).
The Student’s t-test was used to determine significance. A p
<0.05 was considered as significant.
RESULTS
1. Expression of VDR in gastric cancer cell lines The 293 T cell, AGS, KATO III, MKN45, NCI-N87, SNU-
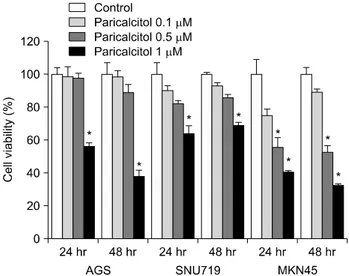
Fig. 2. Effect of paricalcitol on cell viability in gastric cancer cells: AGS, SNU719 and MKN45 were seeded in 24-well plates at a density of 5×104 cells/well and treated with paricalcitol for 24 h and 48 h. Values are means±SD. *p<0.05.
Fig. 3. Paricalctiol regulated ex- pression of VDR and apoptotic related proteins in gastric can- cer cells: The cells were treated with vehicle (control), 0.5μM or 1μM of paricalcitol for 48 h.
(A) Western blot analysis with anti-VDR, anti-Bcl-2, anti- Cas- pase 3 and anti- cleaved cas- pase3 antibodies; (B) Bcl-2 and Cleaved- Caspase3 data expres- sed as a percentage of the con- trol level. Values are means±SD.
*p<0.05.
638, SNU719 and TMKI cell lines were examined for VDR expression (Fig. 1). It is well known that the VDR is necessary, but not sufficient, to ensure that cancer cells will be sensitive to paricalcitol. The AGS, MKN45 and SNU719 cell lines were
chosen for the present studies because they represented moderate and high expression of vitamin D receptor.
2. Effect of paricalcitol on gastric cancer cells
The inhibition of proliferation by paricalcitol in the AGS, SNU719 and MKN45 gastric cell lines was assessed. As shown in Fig. 2, after 24 and 48 hours of treatment, growth of the gastric cancer cells was significantly inhibited in a dose- dependent manner in vitro. Growth inhibition with over 1μM paricalcito was statistically significant in the MKN45, AGS and SNU719 (p<0.05).
3. Effect of paricalcitol on expression of VDR and apoptosis regulators
The effect of paricalcitol on VDR protein expression in gastric cancer cells was analyzed. VDR protein expression was increased after treatment with paricalcitol in dose dependant manner in AGS, MKN45 and SUN719 (Fig. 3A).
Activation of VDR results in several downstream effects such as cell cycle arrest and apoptosis. To investigate the involve- ment of Bcl-2 and Caspase-3 in apoptosis, protein expression levels of both Bcl-2 and Caspase-3 were studied after
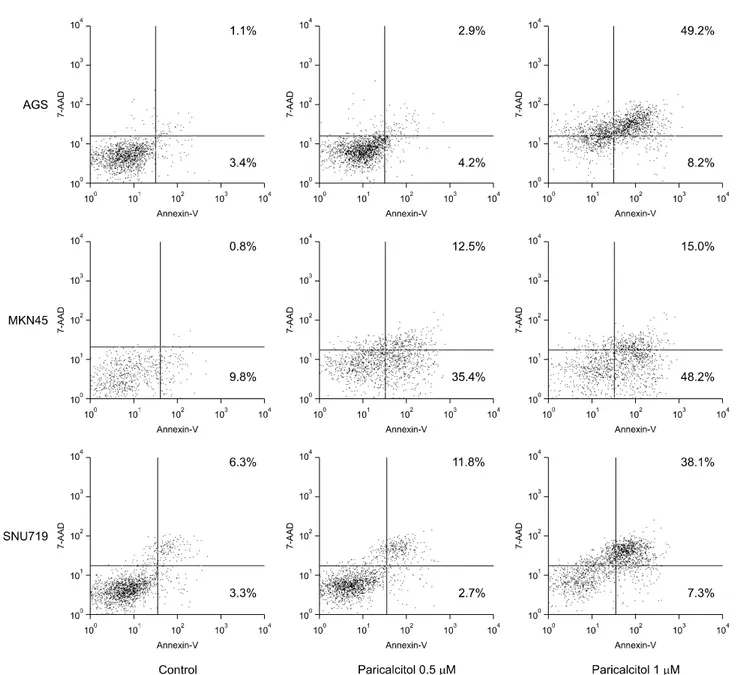
Fig. 4. Apoptotic effects of paricalcitol on gastric cancer cells: AGS, MKN45 and SNU719 cells were treated with vehicle (control), 0.5μM or 1μM paricalcitol for 48 h. Cells were collected, stained with Annexin V-APC and 7-AAD, and analyzed by flow cytometry.
paricalcitol treatment in cultured gastric cells. As shown in Fig.
3A, the expression levels of pro-apoptotic Caspase 3 (17 KDa) were significantly increased, whereas anti-apoptotic Bcl-2 was decreased with paricalcitol treatment. Paricalcitol significantly downregulated Bcl-2 protein expression in the MKN45 cells at all concentrations. Paricalcitol significantly increased expre- ssion of the cleaved-caspase3 protein in all three cell lines (Fig.
3B).
To determine the effect of paricalcitol on apoptosis, AGS, MKN45 and SNU719 were treated with 0.5μM and 1μM
of paricalcitol for 48 hours, as this compound significantly increased gastric cancer cell apoptosis under these conditions.
Paricalcitol promoted the accumulation of cells in the early/late apoptotic phase (Fig. 4). The percentage of cells in the late phase increased in AGS and SNU719, respectively. However, paricalcitol increased the early apoptotic cells in MKN45.
DISCUSSION
We observed that the vitamin D analog paricalcitol inhibited
proliferation of gastric cancer cell lines in vitro by modulating apoptosis. Paricalcitol-induced apoptosis in gastric cells was accompanied by the decreased expression of Bcl-2 protein with an alteration in the level of caspase 3 activity. In another study,17) treatment with the vitamin D3 analog EB1089 inhibited the proliferation of NCI-H929 cells, decreased the expression of Bcl-2, increased caspase 3 acitivity and p38 kinase acitivy, and suppressed p44 extracellular signal-related kinase acitivity during apoptosis. The effect of paricalcitol on p38 and extra- cellular signal-related kinase in gastric cancer cells was not in- vestigated in this study.
Calcitriol exerts anti-tumor effects by regulating key medi- ators of apoptosis, such as representing the expression of the anti-apoptotic, pro-survival proteins Bcl-2 and Bcl-XL, or in- ducing the expression of pro-apoptotic proteins (such as BAX, BAK and BAD). It has been reported that calcitriol down- regulates Bcl-2 expression in MCF-7 breast tumor and HL-60 leukemia cells and upregulates BAX and BAK expression in prostate cancer, colorectal adenoma and carcinoma cells.18) In addition to regulating the expression of the Bcl-2 family, calcit- riol might also directly activetes caspase effector molecules, al- though it is unclear whether calcitriol-induced apoptosis is caspase-dependent.18) In support of this idea, the treatment of mouse SCC tumor cells with calcitriol increased VDR ex- pression and concomitantly inhibited the phosphorylation of ERK.19) Upstream of ERK, the growth-promoting and pro- survival signaling molecule MEK is cleaved and inactivated in a caspase dependent manner in cells that undergo apoptosis after treatment with calcitriol.
In the current study, the downstream molecular effects of paricalcitol on gastric cells were evaluated. This is the first in-depth study of the molecular mechanisms associated with the action of paricalcitol on gastric cells. The molecular response of paricalcitol involves regulation of the cell cycle and apoptosis pathways. Paricalcitol had a modest pro-apoptotic effect on gastric cells.
The anti-cancer effect of paricalcitol was demonstrated on gastric cells and the molecular response of gastric cells was similar to other cancer cell types. As a low-calcemic vitamin D analog, paricalcitol retained the anticancer activity of cal- citriol and might be a potentially useful alternative to calcitriol for the prevention and treatment of cancer. The ease of ad- ministration and broad biological effects of paricalcitol, and potentiation of several anticancer drugs, strongly support to
continue the development of paricalcitol as an anticancer drug.
CONCLUSION
Calcitriol induces antiproliferative effects in tumor cells characterized by induction of cell cycle arrest and apoptosis as well as perturbations of cell survival signals. Paricalcitol is less calcemic but retains yet retain the antitumor activity of calcitriol. The results observed in this study presents evidence that paricalcitol could provide an effective treatment for gastric cancer in humans. Therefore, we believe that these data have the potential to provide a novel therapeutic approach for the treatment of gastric cancer.
REFERENCES
1) Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 320, 980-991, 1989.
2) Evans RM. The steroid and thyroid hormone receptor super- family. Science 240, 889-895, 1988.
3) Holick MF, Vitamin D. A millenium perspective. J Cell Bio- chem 88, 296-307, 2003.
4) Carlberg C. Current understanding of the function of the nuclear vitamin D receptor in response to its natural and synthetic ligands. Recent Results Cancer Res 164, 29-42, 2003.
5) Issa LL, Leong GM, Eisman JA. Molecular mechanism of vitamin D receptor action. Inflamm Res 47, 451-475, 1998.
6) Christakos S, Raval-Pandya M, Wernyj RP, Yang W.
Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem J 316, 361-371, 1996.
7) Johnson CS, Hershberger PA, Trump DL. Vitamin D-related therapies in prostate cancer. Cancer Metastasis Rev 21, 147-158, 2002.
8) Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, Dhir R, Shurin Z, Day RS, Trump DL, Johnson CS. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology 50, 999-1006, 1997.
9) Colston KW, Chander SK, Mackay AG, Coombes RC. Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem Pharmacol 44, 693- 702, 1992.
10) Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res 53, 3712-3718, 1993.
11) Colston KW, James SY, Ofori-Kuragu EA, Binderup L, Grant AG. Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in
vivo and in vitro. Br J Cancer 76, 1017-1020, 1997.
12) Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvala H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm 64, 357-406, 2002.
13) Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE.
1 alpha,25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res 87, 214-220, 2000.
14) Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J.
1,25-Dihydroxyvitamin D3 induces morphological and bioche- mical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol 58, 367-376, 1996.
15) Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3
(calcitriol). J Urol 159, 2035-2039.
16) Robinson DM, Scott LJ. Paricalcitol: a review of its use in the
management of secondary hyperparathyroidism. Drugs 65, 559-576, 2005.
17) Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol 109, 576-583, 2000.
18) Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvälä H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm 64, 357-406, 2002.
19) McGuire TF, Trump DL, Johnson CS. Vitamin D3-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-re- gulation of MEKK-1. J Biol Chem 276, 26365-26373, 2001.