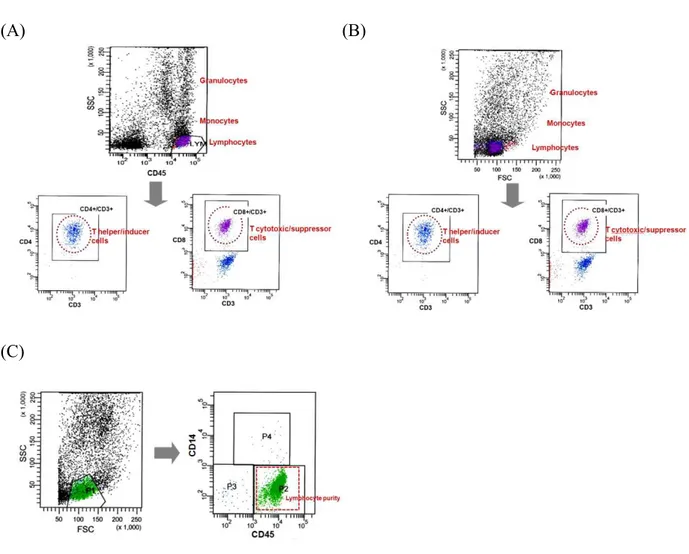
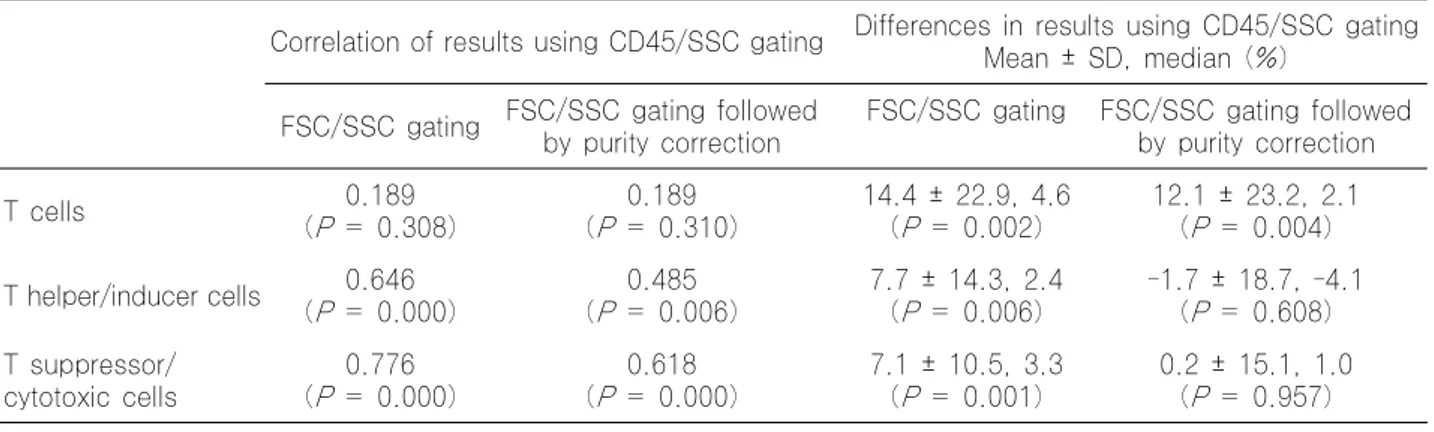
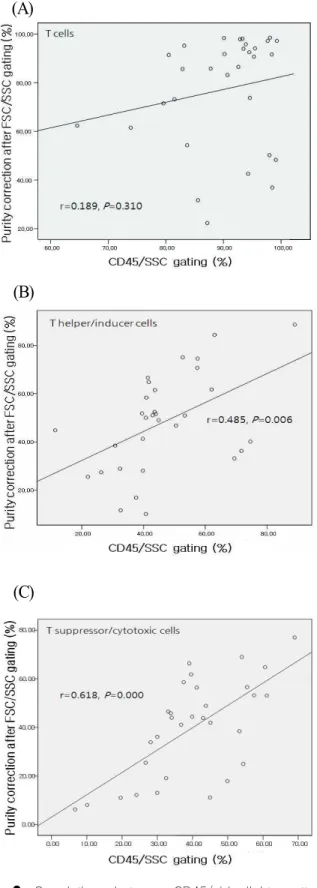
CD45 is Essential for Lymphocyte Gating in a T-lymphocyte Subset Assay of Bronchoalveolar Lavage Fluid by Flow Cytometry
전체 글
수치
관련 문서
The purpose of this study is to evaluate the marginal and internal fit of coping made by CAD/CAM using different scanning methods.. Zirconia coping was made
The fibrinolytic enzyme was purified by using DEAE sepharose CL-6B fast flow chromatography followed by Sephadex G-75 gel filtration and POROS 20
→ For the turbulent jet motion and heat and mass transport, simulation of turbulence is important.. in the mean-flow equations in a way which close these equations by
n The magnitude of the buoyant force acting on a floating body in the fluid is equal to the weight of the fluid which is displaced by
Values of Glucose, T-protein, T-cholesterol and Triglyceride of Blistered fluid caused by Buhang blistering --- 19..
1) If the fluid is real (viscous fluid) and if no energy is being added, then the energy line may never be horizontal or slope upward in the direction of flow.. 2) Vertical
plasma is treated as a single hydrodynamic fluid acted upon by electric and
• Diffusion Equation for fluid flow in porous media... Fluid flow