PREVENTION RESEARCH □ ORIGINAL ARTICLE □
141 책임저자:윤정현, 609-735, 부산시 금정구 장전동 산30
부산대학교 약학대학 약학부 Tel: 051-510-2804, Fax: 051-513-6754 E-mail: jyoon@pusan.ac.kr
접수일:2011년 5월 31일, 1차 수정일:2011년 6월 7일, 2차 수정일:2011년 6월 10일, 게재승인일:2011년 6월 13일
Correspondence to:Jeong-Hyun Yoon
Department of Pharmacy, College of Pharmacy, Pusan National University, San 30, Jangjeon-dong, Geumjeon-gu, Busan 609-735, Korea Tel: +82-51-510-2804, Fax: +82-51-513-6754
E-mail: jyoon@pusan.ac.kr
Montiporyne A Induces Apoptosis in SK-MEL-2 Human Melanoma Cells
Nam Deuk Kim, Kyoung-Mi Lee, Hye Joung Choi, Mohammad Akbar Hossain, Dong Hwan Kim, Jung Yoon Jang, Jee H. Jung and Jeong-Hyun Yoon
Department of Pharmacy (BK21 Program), College of Pharmacy, Molecular Inflammation Research Center for Aging Intervention (MRCA), Pusan National University, Busan 609-735, Korea
In this study, we investigated the effects of montiporyne A which was isolated from the stony coral
Montipora species, on the proliferation of SK-MEL-2 human skin melanoma cells (mutant p53).Twenty-four hour treatment of montiporyne A inhibited the growth of melanoma cells and induced apoptotic cell death. This montiporyne A-induced apoptosis in the SK-MEL-2 cells was closely linked with the down-regulation of Bcl-2 protein expression and the cleavage of poly(ADP-ribose) polymerase.
Montiporyne A treatment also caused a marked increase in the level of p21
WAF1/CIP1protein in a p53-independent manner. Based on our data, montiporyne A can be considered as a good candidate for an effective chemotherapeutic agent inducing apoptosis of cancer cells, although further study will be needed to be confirm. (Cancer Prev Res 16, 141-146, 2011)
Key Words: Montiporyne A, Human skin cancer cells, Apoptosis
INTRODUCTION
Montiporyne A is a novel acetylenic compound extracted from the stony coral Montipora species. The molecular formula of montiporyne A was established as C
15H
20O.
1,2)A preliminary experiment using montiporyne A showed cytotoxic effects on a small panel of human solid tumor cells lines, such as A549 human lung cancer, SK-OV-3 human ovarian cancer, SK-MEL-2 human skin cancer, XF498 human CNS cancer, HCT15 hu- man colon cancer. However, only cytotoxic sulforhodamine B assay was conducted and, therefore, the underlying molecular mechanism of the cytotoxicity of montiporyne A was not fully elucidated.
Human malignant melanoma is characterized as a cancer by both rapidly rising incidence and growing lifetime risk and now is responsible for over 7,000 deaths in the United States
annually. Furthermore, although early stage melanoma in the epidermis or superficial dermis is curable, the prognosis for the patients with deep invasion of the dermis and metastases is dismal. This poor prognosis is due to the lack of effective treatments.
3)Therefore, developments of new chemotherapeutic strategies, which can facilitate the death of cancer cells, are an urgent objective. One of the strategies for the therapy involves the induction of programmed cell death (apoptosis) of cancer cells. Apoptosis may be induced in some cancer cells by dereg- ulation of cell cycle control genes.
4∼6)The majority of human solid tumors is genetically unstable and has defects in cell cycle checkpoint control mechanism.
7,8)Such tumors frequently con- tain mutations that disrupt the G1 components of cell cycle (e.g., p53 and Rb) and this affects the ability of chemo- therapeutic drugs to inhibit cell proliferation and to induce apoptosis.
Historically, one abundant source of novel therapeutic agents
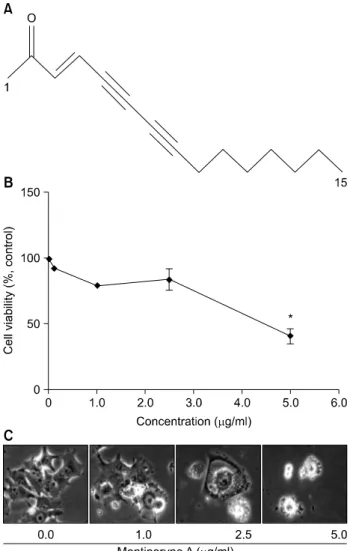
Fig. 1. Chemical structure of montiporyne A (A). Concentration- dependent effects of montiporyne A on the proliferation and morphology of SK-MEL-2 cells. (B) Cells were grown at 70%
confluence and were treated with variable concentrations of montiporyne A for 24 h and the viability was measured by the metabolic-dye-based MTT assay. Viability of control cells was set at 100% and viability relative to the control is presented.
Results are expressed as percentage of the vehicle treated control±SD of three separate experiments. The significance was determined by Student’s t-test (*p<0.05). (C) Morpho- logies of cells treated with variable concentrations of montiporyne A for 24 h were then observed under a light microscopy. Magnification, ×200.
has been natural products.
9,10)Many naturally derived therapies have come from terrestrial sources; however, over the last thirty years scientists have started exploring one of the greatest sour- ces of biodiversity on the planet, the oceans. Approximately 2,500 marine natural products were identified, many belonging to new chemical classes.
11)Hence, the exploration of natural products is still critical to the identification of novel chemical structures, which will lead to effective new treatments for cancers. In this study, we examined whether montiporyne A isolated from the stony coral Montipora species inhibits cell growth and induces apoptosis in SK-MEL-2 human melanoma cells.
MATERIALS AND METHODS
1. Preparation of motiporyne AMontiporyne A was isolated from Montipora sp. and kindly provided by Dr. Jee H. Jung (Department of Pharmacy, Pusan National University, Busan, South Korea). The isolation meth- od of montiporyne A has been reported previously.
1,2)Montiporyne A was dissolved in DMSO (Sigma-Aldrich Co., St. Louis, MO, USA) to make 24μg/ml stock solution and stored at -20
oC until use. The stock solution of montiporyne A was diluted with medium to the desired concentration prior to use. The maximal concentration of DMSO did not exceed 0.1% (v/v) in the treatment range (1.0∼5.0μg/ml), where there was no influence on the cell growth (data not shown).
The structure of montiporyne A is shown in Fig. 1A.
2. Cell culture and montiporyne A treatment
SK-MEL-2 human melanoma cell lines (mutant p53) were purchased from American Type Culture Collection (Manassa, VA, USA). They were routinely maintained in RPMI 1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (Gibco BRL) and streptomycin-penicillin (100 units/ml; Gibco BRL) as previously reported.
12,13)Cells were cultured in a 5% CO
2incubator at 37
oC, and the medium was changed 3 times per week. The cells at 60∼80% con- fluence were treated with concentrations of montiporyne A at 0∼5.0μg/ml for 3, 6, 15, or 24 h.
3. Assessment of cell proliferation by MTT assay
The proliferation of cells was assessed by using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide]
assay, which is based on the conversion of MTT to MTT-for- mazan by mitochondrial enzymes as previously described.
14)At least three independent experiments were performed. The effect of montiporyne A on growth inhibition was assessed as percent cell viability where vehicle-treated cells were taken as 100%
viable. Cell number was counted as another measure for cell
growth. Briefly, after incubation with or without ircinin-1, cells
were detached by 0.05% trypsin-EDTA and counted with a hematocytometer.
4. Morphological changes under microscope
SK-MEL-2 cells were seeded (2×10
5cells/well) into a 6-well plate and allowed to adhere overnight. The cells were incubated with 0, 1.0, 2.5, and 5.0μg/ml of montiporyne A for 24 hr.
Cells in control wells were treated with an equivalent volume of ethanol. Optic phase-contrast photographs were taken with Zeiss Axiovert 100 inverted microscope (Göttingen, Germany).
5. Gel electrophoresis and Western blotting
The cells were harvested, lysed, and protein concentrations were quantified using the Bio-Rad protein assay (Bio-Rad Lab., Hercules, CA, USA) by the procedure described by the manufacturer. For the Western blot analysis, an equal amount of protein was subjected to electrophoresis on SDS-poly- acrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA) by electroblotting.
Blots were probed with the desired antibodies for 1 h, in- cubated with diluted enzyme-linked secondary antibodies and then visualized by the enhanced chemiluminescence (ECL) ac- cording to the recommended procedure (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). The primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and Calbiochem (Cambridge, MA, USA).
Peroxidase-labeled secondary antibodies were purchased from Amersham Biosciences Corp., Little Chalfont, Bucks, UK).
6. Statistics
Data were expressed as the mean±SD of three separate ex- periments and analyzed by Student’s t-test. The means were considered significantly different at *p<0.05.
RESULTS
1. Montiporyne A induces cell growth inhibition in SK-MEL-2 cells
The effect of montiporyne A on the viability of SK-MEL-2 cells was assessed via MTT dye assay at various concentrations.
As shown in Fig. 1B, the cell viability was decreased in a con- centration-dependent manner when the cells were exposed to the concentrations of montiporyne A greater than 2.5μg/ml for 24 h. The dose required for half-maximal inhibition (IC
50)
of SK-MEL-2 cell growth was approximately 4.5μg/ml.
Microphotographs of montiporyne A-treated SK-MEL-2 cells also showed distinct morphological changes and cell viability (Fig. 1C). There were cytoplasmic bubbles and some kinds of segment of cell bodies, and cell number was also decreased.
2. Montiporyne A induces apoptosis in SK- MEL-2 cells
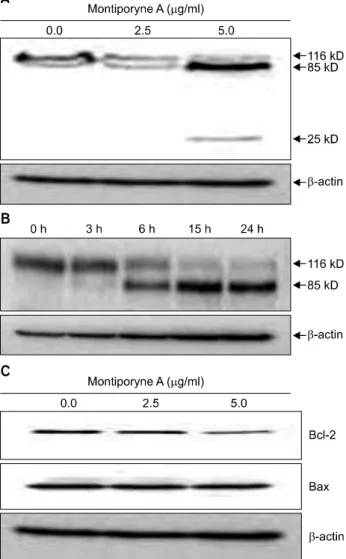
Degradation of polypeptide including poly (ADP-ribose) pol- ymerase (PARP), was examined to see the possible involvement of apoptosis-associated protease during the growth inhibition of the SK-MEL-2 cells. PARP cleavage was evident by the appear- ance of the p85 and p25 PARP cleavage fragments in the 5.0 μg/ml of montiporyne A-treated cells which underwent apop- tosis (Fig. 2A). Moreover, SK-MEL-2 cells treated with 5.0μg/
ml of montiporyne A for 6, 15, and 24 h clearly demonstrated the induction of PARP cleavage in time-dependent manner (Fig. 2B). These results demonstrated that montiporyne A in- duced apoptotic cell death.
We next examined whether montiporyne A induces cell death by modulating the expression of Bcl-2 family members, such as Bcl-2 and Bax, which ultimately determine a cell’s re- sponse to apoptotic stimuli. The Western blotting results showed that the significant down-regulation of Bcl-2 ex- pression, but the level of Bax protein was not changed in the montiporyne A-treated SK-MEL-2 cells for 24 h (Fig. 2C).
3. Montiporyne A induces cleavage of cas- pase-3 in SK-MEL-2 cells
Our previous work have suggested that downregulation of
Bcl-2 protein may potentiate apoptosis by controlling mi-
tochondrial cytochrome c release into the cytosol and sub-
sequent activation of the caspase cascade.
13)To determine
whether mitochondrial cytochrome c release and caspase activa-
tion are involved as downstream effectors in death-signaling
pathway-mediated montiporyne A-triggered apoptosis, cytosolic
extract of untreated or montiporyne A-treated cells were ana-
lyzed for accumulation of cytosolic cytochrome c and the enzy-
matic activity of caspase-3. The results from Western blotting
demonstrated that cytosolic cytochrome c gradually accumu-
lated in response to montiporyne A (Fig. 3A). In addition, cas-
pase-3 was proteolytically processed into small active fragments
(Fig. 3A). Moreover, 5.0μg/ml of montiporyne A treatment
on SK-MEL-2 cells for 3, 6, 15, and 24 h clearly demonstrated
Fig. 3. Effects of montiporyne A on the protein expression of cytochrome c, and proteolytic activation of caspase-3 in SK-MEL-2 cells. (A) The cells were treated with variable concentrations of montiporyne A for 24 h. The cytosolic fraction for cytochrome c and total cell lysates for pro-caspase-3 were processed for Western blot analysis as described in “Materials and Methods.” (B) Time-dependent proteolytic activation of pro-caspase-3 in SK-MEL-2 cells treated with 5.0μg/ml montiporyne A for 3, 6, 15, and 24 h.
Representative results from three independent experiments are shown. β-actin was used as a loading control.
Fig. 2. Apoptosis inducing effect of montiporyne A on SK-MEL-2 cells. (A) The cells were treated with variable concentrations of montiporyne A for 24 h. Total cell lysates were processed for Western blot analysis as described in
“Materials and Methods.” The membrane was probed with the antibodies against PARP and visualized using an ECL detection system. (B) Time-dependent cleavage of PARP protein in SK-MEL-2 cells treated with 5.0μg/ml montiporyne A for 3, 6, 15, and 24 h. (C) Effect of montiporyne A on Bcl-2 family proteins in SK-MEL-2 cells. The cells were treated with variable concentrations of montiporyne A for 24 h. Total cell lysates were processed for Western blot analysis as described in “Materials and Methods.” The membrane was probed with the antibodies against Bcl-2 and Bax and visualized using an ECL detection system. Representative results from three independent experiments are shown. β-actin was used as a loading control.
the time-dependent activation of pro-caspase-3 (Fig. 3B).
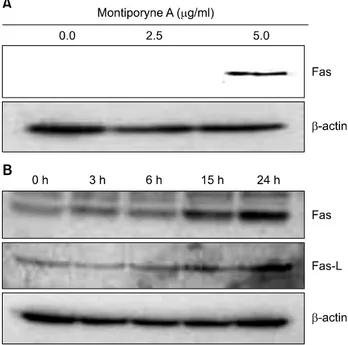
4. Montiporyne A upregulates the expression of Fas and Fas-L in SK-MEL-2 cells
The apoptosis can be also induced through the Fas and Fas-L pathway that activates FADD and caspase-8, which in turn ac- tivate caspase-3.
15,16)We further demonstrated that montipor- yne A-induced apoptosis through a membrane-mediated mech- anism was supported by up-regulated expression of Fas with a variable concentrations of montiporyne A (Fig. 4A) and time-dependent up-regulation Fas and Fas-L with 5.0μg/ml of montiporyne A treatment on SK-MEL-2 cells for 3, 6, 15, and 24 h (Fig. 4B).
5. p53-independent up-regulation of p21WAF1/CIP1 protein in SK-MEL-2 cells treated with monti- poryne A
The tumor suppressor gene, p53, has been shown to regulate
a DNA damage-triggered G1 checkpoint by up-regulating the
Fig. 4. Effects of montiporyne A on the expression of Fas and Fas-L in SK-MEL-2 cells. (A) The cells were treated with variable concentrations of montiporyne A for 24 h. Total cell lysates were processed for Western blot analysis with anti-Fas or -Fas-L antibody as described in “Materials and Methods.”
(B) Time-dependent induction of the expression levels of Fas and Fas-L in SK-MEL-2 cells treated with 5.0μg/ml montiporyne A for 3, 6, 15, and 24 h. Representative results from three independent experiments are shown. β-actin was used as a loading control.
Fig. 5. Concentration-dependent effects of montiporyne A on the protein levels of p53 and p21 in SK-MEL-2 cells. To study concentration-dependent effects of montiporyne A, the cells were treated with variable concentrations of montiporyne A for 24 h. Total cell lysates were processed for Western blot analysis with antibodies against p53, p21, and β-actin, and ECL detection. A representative blot is shown from three independent experiments. β-actin was used as a loading control.
expression of p21
WAF1/C1P1, a cyclin-dependent kinase (Cdk) inhibitor.
17,18)Our results showed that montiporyne A treat- ment resulted in a concentration-dependent increase in the p21
WAF1/CIP1protein level via p53-independent manner in SK-MEL-2 cells which have mutant type p53 (Fig. 5).
DISCUSSION AND CONCLUSION
Montiporyne A seems to have potential cytotoxic effects on the proliferation of human skin melanoma cells via apoptosis.
Montiporyne A reduced the viability of SK-MEL-2 cells through the induction of apoptosis in concentration- and time-dependent manners. By observing the cleavage of PARP, we could confirm that montiporyne A induced apoptosis in SK-MEL-2 cells.
Apoptosis is important regulatory mechanisms that removes unwanted cells during the development and thereby maintain the cell homeostasis. The molecular mechanisms by which anti-
cancer agents induce apoptosis involve the activation of proa- poptotic signaling or the inhibition of survival signaling.
Therefore, the balance between the survival and death signal transduction pathways is important in controlling apopto- sis.
13,19)It has been shown that the Bcl-2 family plays an important regulatory role in apoptosis, either as an inhibitor or as an activator. In particular, Bcl-2 has been reported to directly in- hibit members of the caspase family, including caspases-3 and -9.
20)Thus, it has been suggested that the ratio between the level of pro-apoptotic Bax protein and that of the anti-apop- totic factor Bcl-2 protein determines how a cell responds to an apoptotic signal.
19)In this study, montiporyne A altered the levels of Bax and Bcl-2 expression in SK-MEL-2 cells, resulting in an increase in the ratio of Bax/Bcl-2 (Fig. 2C).
p53 plays a key role in mediating the cell response to various
stressors, mainly by inducing or repressing a number of genes
involved in cell cycle arrest, senescence, apoptosis, DNA repair,
and angiogenesis. p53 mutations are observed in a significant
minority of tumors. In the remaining cases, the alteration of
interactive components or target genes could contribute, to
some extent, to the reduced ability of p53 efficiently managing
stressful events. While the prognostic and predictive value of
p53 is still in debate, there is an increasing interest for
p53-based therapies.
21)Furthermore, our study found that
montiporyne A increased the expression of p21
WAF1/CIP1in a
p53-independent manner as previously reported.
13,22)As a conclusion, montiporyne A demonstrated cytotoxicity and induced apoptosis in SK-MEL-2 cells. Our data also sug- gest that the anti-proliferative effects of montiporyne A on SK-MEL-2 cells appear to be mediated, at least partially, through the p53-independent regulation of p21
WAF1/CIP1ex- pression, and by a reduction of Bcl-2. Therefore, the develop- ment of modified synthetic montiporyne A derivatives, pro- vided by increased its antitumor activity, would represent a po- tential new class of chemotherapeutic agents. Although our studies have commenced to elucidate the antitumor activities of montiporyne A, further study of montiporyne A would be needed to confirm its potential as a candidate for chemo- therapeutic agent.
ACKNOWLEDGEMENT
This work was supported by Pusan National University Research Grant, 2008.
REFERENCES
1) Bae BH, IM KS, Choi WC, Hong J, Lee CO, Choi JS, Son BW, Son JI, Jung JH. New acetylenic compounds from the stony coral Montipora sp. J Nat Prod 63, 1511-1514, 2000.
2) Alam AAlam N, Bae BH, Hong J, Lee CO, Im KS, Jung JH. Cytotoxic diaetylenes from the stony coral Montipora species. J Nat Prod 64, 1059-1063, 2001.
3) Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma:
systematic review and meta-analysis of clinical trials. Melanoma Res 21, 165-174, 2011.
4) Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5, 876-885, 2005.
5) Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH.
Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res 65, 6934-6942, 2005.
6) Hsu YL, Kuo PL, Lin LT, Lin CC. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen- activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther 313, 333-344, 2005.
7) Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited.
Cancer 60, 3689-3695, 2000.
8) Nagle DG, Zhou YD, Mora FD, Mohammed KA, Kim YP.
Mechanism targeted discovery of antitumor marine natural products. Curr Med Chem 11, 1725-1756, 2004.
9) Jimeno JM. A clinical armamentarium of marine-derived anti-cancer compounds. Anticancer Drugs 13(1 Suppl), S15-19, 2002. Review
10) Kim MN, Ahn EY, Park SE, Hossain MA, Kim MY, Moon JO, Kim ND, Yoon JY. Morin inhibits the growth of murine hepatoma cells via cell cycle arrest and induction of apoptosis.
Cancer Prev Res 15, 190-197, 2010.
11) Carte BK. Marine natural products as a source of novel pharmacological agents. Curr Opin Biotechnol 4, 275-279, 1993.
12) Choi HJ, Bae SJ, Kim ND, Jung JH, Choi YH. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells. Int J Mol Med 14, 1091-1096, 2004.
13) Choi HJ, Choi YH, Yee SB, Im E, Jung JH, Kim ND.
Ircinin-1 induces cell cycle arrest and apoptosis in SK-MEL-2 human melanoma cells. Mol Carcinogenesis 44, 162-173, 2005.
14) Tada H, Shiho O, Kuroshima K, Koyoma M, Tsukamoto K.
An improved colorimetric assay for interleukin-2. J Immunol Methods 93, 157-165, 1986.
15) Nagata S, Golstein P. The Fas death factor. Science 267, 1449-1456, 1995.
16) Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 281, 1305-1308, 1998.
17) Dermers GW, Foster SA, Halbert CL, Galloway DA. Growth arrest by induction of p53 in DNA. Proc Natl Acad Sci USA 91, 4382-4386, 1994.
18) Hartwell LH, Kastan MB. Cell cycle control and cancer.
Science 266, 1821-1828, 1994.
19) Salomons GS, Brady HJ, Verwijs-Janssen M, van den Berg JD, Hart AA, van den Berg H, Behrendt H, Hahlen K, Smets LA. The Bax alpha:Bcl-2 ratio modulates the response to dexamethasone in leukaemic cells and is highly variable in childhood acute leukaemia. Int J Cancer 71, 959-965, 1997.
20) Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC.
The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 16, 6914-6925, 1997.
21) Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer 13, 293-325, 2006.
22) Im EO, Choi YH, Paik KJ, Suh H, Jin Y, Kim KW, Yoo YH, Kim ND. Novel bile acid derivatives induce apoptosis via a p53-independent pathway in human breast carcinoma cells. Cancer Lett 163, 83-93, 2001.