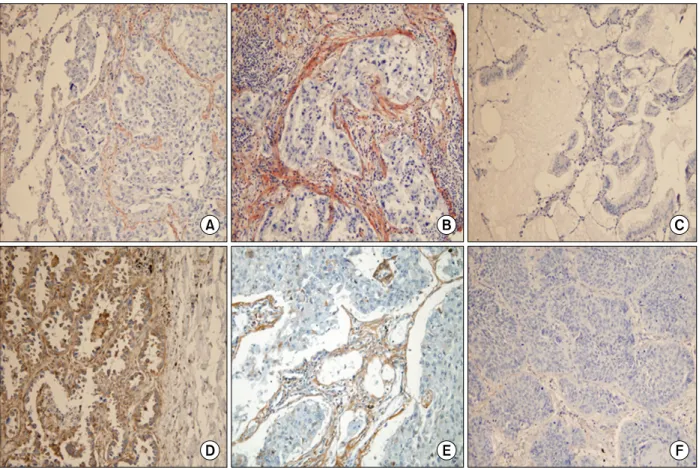
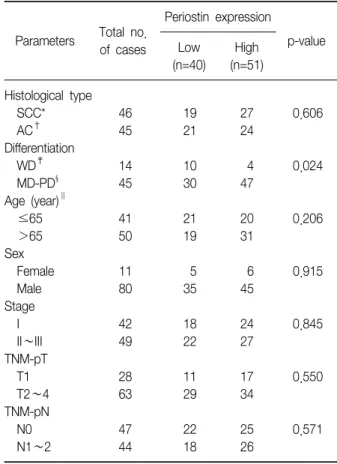
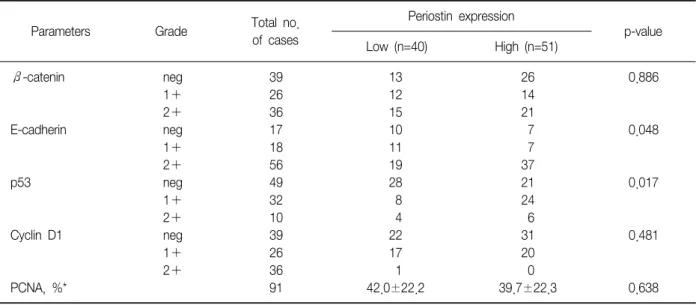
Overexpression of Periostin Protein in Non-Small Cell Lung Carcinoma is Not Related with Clinical Prognostic Significance
전체 글
수치
관련 문서
Compromised CD4+ CD25(high) regulatory T-cell function in patients with relap sing-remitting multiple sclerosis is correlated with a reduced frequency of
Percentage of patients with portal systemic encephalopathy according to presence of small intestine
• Phase II clinical trials of PI3Kδ inhibitor (idelalisib) showed dramatic response in patients with previously treated indolent non-hodgkin’s
Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable
Patients with breast cancer; ovarian cancer; renal cell carcinoma; pancreatic neuroendocrine cancer; colorectal cancer; head and neck cancer; non-small cell lung
In addition, the expression of REDD1 was increased when Huh7 cells were treated with PA, and overexpression of REDD1 affected cell survival and lipid
Differential Expression of Desmoglein1, Desmoglein3, Epithelial Membrane Antigen, Ber-EP4 and CD10 in Basal Cell Carcinoma and Squamous Cell Carcinoma
Objective: The purpose of this study was to analyze recent trend in incidence of basal cell carcinoma and squamous cell carcinoma in patients from the Gwangju City