관련 문서
Specifically, Mises showed that the effect of inflation was not the same in the different periods since German people at that time had changed the expectation of
웹 표준을 지원하는 플랫폼에서 큰 수정없이 실행 가능함 패키징을 통해 다양한 기기를 위한 앱을 작성할 수 있음 네이티브 앱과
⑥ We regret (to say that) we are unable to accept your order at the last(previous) price(s) because of a rise in (the) price of the. materials.(재료가격의
The index is calculated with the latest 5-year auction data of 400 selected Classic, Modern, and Contemporary Chinese painting artists from major auction houses..
The “Asset Allocation” portfolio assumes the following weights: 25% in the S&P 500, 10% in the Russell 2000, 15% in the MSCI EAFE, 5% in the MSCI EME, 25% in the
1 John Owen, Justification by Faith Alone, in The Works of John Owen, ed. John Bolt, trans. Scott Clark, "Do This and Live: Christ's Active Obedience as the
The concentration of the dissolved metal as well as that of the adsorbed to solid particles depends mainly on the concentration of the metal in the inflow water
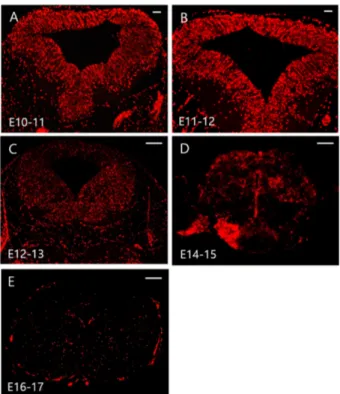
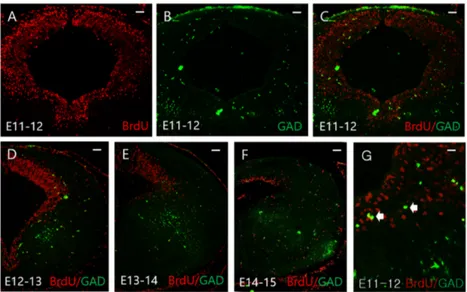
For the study of inhibitory synapse development, we analyzed the intensity, area, and density of Gephyrin or VGAT clusters in primary hippocamapal