의학 석사학위 논문
Characterization of Neural Stem Cells Using
the Nestin Enhancer
아 주 대 학 교 대 학 원
의 학 과
Characterization of Neural Stem Cells
Using the Nestin Enhancer
by
Soon-tae You
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
MASTER OF MEDICAL SCIENCES
Supervised by
Haeyoung Suh-Kim, Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
유순태의 의학 석사학위 논문을 인준함.
심사위원장 김 승 업 인
심 사 위 원 이 영 돈 인
심 사 위 원 서 해 영 인
아 주 대 학 교 대 학 원
2006 년 12 월 21 일
CONTENTS
CONTENTS ··· ⅰ LIST OF FIGURES ··· ⅲ LIST OF TABLES ··· ⅴ ABSTRACT ··· ⅵ . Ⅰ INTRODUCTION ··· 1 . Ⅱ MATERIALS AND METHODS ··· 5A. MATERIAL ··· 5
B. METHODS ··· 7
1. Neural stem cell cluture ··· 7
2. Reporter gene constructs ··· 7
3. Reporter gene assay ··· 9
4. Luciferase assay ··· 9
5. β-gal assay and X-gal stain ··· 9
6. N2E(+4)/ N2Em(+4)-LacZ transgenic mice ··· 11
7. Immunocyto(histo)chemistry ··· 11
8. Sorting GFP(+) cells from nestin-GFP transgenic mice NSC ··· 13
9. Microarray analysis of GFP(+) and GFP(-) cells ··· 14
. Ⅲ RESULTS ··· 15
1. Nestin-GFP transgenic mice neural stem cell culture ··· 15 2. Regulation of nestin gene by the nestin enhancer in the second intron 17
3. E-boxes on Nestin Enhancer ··· 19 4. Regulation of nestin expression by E-box on Nestin Enhancer ··· 19 5. bHLH transcription factors the effects on nestin gene expression ··· 21 6. Nestin Enhancer (N2E) / Nestin Enhancer mutant (N2Em) – LacZ
transgenic mice ··· 23 7. ngn1 expression on GFP(+) cell of Nestin-GFP transgenic mouse NSC 26 8. Characterization of GFP(+) cells using microarray analysis ··· 27 . Ⅳ DISCUSSION ··· 32 . Ⅴ CONCLUSION ··· 38 REFERANCE ··· 39 국문요약 ··· 43
LIST OF FIGURES
Fig. 1. Conserved sequences in the 3’ half of the 2nd intron of rat,
mouse, and human nestin genes··· 4
Fig. 2. Construction of reporter vector··· 10
Fig. 3. GFP+ cells express neural precursor cell markers in neurospheres
derived from nestin-GFP transgenic mice··· 16
Fig. 4. ngn1 activated the nestin enhancer in P19 cells··· 18
Fig 5. ngn1 specifically activated the E-box mediated reporter gene
activity in P19 cells ··· 20
Fig 6. Transactivation of nestin enhancer by ngn1 ··· 22
Fig 7. Specific induction of the 2nd intron by early proneural bHLH proteins 23
Fig. 8. Transgenic mice expressing LacZ driven by nestin enhancer with
Fig. 9. Colocalization of LacZ and neural stem cell markers in E11.5
transgenic mouse brain carrying the wild type E-box ··· 26
Fig. 10. Coexpression of nestin, GFP and ngn1 in neurospheres derived
from nestin-GFP transgenic mice ··· 27
Fig. 11. Percentage of GFP-positive cells at different passages··· 28
Fig. 12. Sorting out GFP positive and negative cells from nestin-GFP
transgenic mice neurosphere using FACS ··· 30
LIST OF TABLES
Table 1. Primary antibody specification··· 13
Table 2. The gene expression increased in GFP(+) cells of
nestin-GFP transgenic mouse neurosphere ··· 32
Table 3. The gene expression increased in GFP (-) cells of
nestin-GFP transgenic mouse neurosphere ··· 33
Table 4. neurogenin 1 expression level in GFP (+) and (-) cells of
- ABSTRACT -
Characterization of Neural Stem Cells Using the Nestin Enhancer
Nestin, one of the class VI intermediate filament, is known as a specific marker for neural stem cells and regulated by the enhancer located on the second intron of the gene. The second intron is highly conserved among species and crucial for the nestin expression in central nerve system (CNS) progenitor cells. The transgenic mice that constructed to express green fluorescence protein (GFP) with nestin enhancer have been developed and have great advantage to represent live neural stem cells during research in vivo and vitro.
In this study, we characterized GFP expressing the neural stem cells or progenitors
in vitro culture using various antibodies. We also investigated that ngn1, which is a
basic helix-loop-helix (bHLH) transcription factor that is expressed in neuronal precursors during development of the nervous system, can promote nestin expression by nestin enhancer located on nestin 2nd intron. Two E-boxes (CANNTG) in 637bp of the nestin 2nd intron (nestin enhancer) were thought to be strong candidates for bHLH factors during the early neurogenesis. ngn1 increased the second E-box mediated reporter gene activity. To evaluate the functional mechanism of E-box mediated nestin gene expression in vivo, we generated transgenic (Tg) mice with the 637bp fragment of nestin second intron containing the wild type or a mutated
E-boxes. Wild type tg mice successfully exhibited CNS-specific LacZ expression. In contrast, tg mice containing the mutant sequence exhibited very few lacZ-stained cells or a few positive cells in ectopic sites. These results indicate that proneural ngn1 promotes the nestin expression through a E-box located in the nestin second intron. Finally, we also performed microarray to characterize the GFP positive and negative cells in nestin-GFP tg mice neurosphere. The microarray profile may help to understand the meaning of GFP in the tg neural stem cells and may give cue to isolate neural stem cell from progenitors.
Key words: Nestin, GFP, transgenic mouse, basic Helix-Loop-Helix, neurogenin1,
I. INTRODUCTION
Neural stem cells in the developing and adult mammalian nervous system proliferate, self-renew and migrate to become functional neurons, astrocytes and oligodendrocytes in right position by various signals from environment. NSCs can also be generated form more primitive embryonic stem cells (reviewed by Gage, 2000). One of the most well defined characteristic of NSC is that express one of intermediate filament protein, nestin (Lendahl et al., 1990; Dahlstrand et al., 1995). During differentiation of NSC, the nestin protein is replaced to other intermediate protein that expressed in specific cell lineage such as Glial Fibrillary Acidic Protein(GFAP) and neurofliaments(NF) (Zimmerman et al., 1994; Dahlstrand et al., 1995; Lothian and Lendahl, 1997).
Nestin is one of class VI intermediate filament that is expressed in muscle precursors and neural precursors during development. Its gene expression is regulated by the enhancers located on the introns. Nestin gene is composed of four exons and three introns. The first intorn is important for expression of nestin in mesoderm originated cells, and the second intron contains the enhancer sequence for expressing in neural precursor (stem) cells (Zimmerman et al., 1994). The nestin second intron is evolutionary conserved; especially the 637base pair sequence of 1.8kbp rat nestin second intron (from 1162bp to 1798bp) shows high homology among rat, mouse and human. This 637 base pair of nestin second intron is known
that suitable to regulate nestin expression in central nerves system (Lothian et al., 1997). To visualize the nestin expression of neural stem cells, many groups constructed transgenic mice that contain nestin promoter, the enhancer and reporter gene inside mouse genome (Yamaguchi et al., 2000; Kawaguchi et al., 2001).
During development of central nerves system, the expression of crucial proteins is regulated by complex signaling pathways. One of the regulate molecule called basic Helix-Loop-Helix (bHLH) group is a transcription factor that regulate gene expression. It binds to DNA domain called E-box (CANNTG) as hetero- or homodimer, and stimulates or inhibits the gene transcription (Gradwohl et al., 1996; Massari and Murre, 2000). Especially, in central nerves system the bHLH transcription factors is known that have crucial roles in differentiation of glia and neurons from neural progenitor(stem) cells (Sun et al., 2001; Zhou and Anderson., 2002).
One of bHLH transcription factor, neurogenin1 (ngn1) is known that regulate neuronal cell fate. Expression of ngn1 suppresses JaK-STAT pathway, which is important for glial differentiation and induces neurogenesis by functioning as a transcriptional activatior. Janus kinase (JaK) is one of receptor-associated tyrosine kinase, which activated by leukemia inhibitory factor (LIF) or ciliary neurotrophic factor (CNTF). When LIF or CNTF binds to receptors, Signal transducers and activators of transcription 1 and 3 (STAT1 and STAT3) is phopholyated by JaK, and phosholyated STAT makes complex with smad and CBP/P300 to activate transcription of glial genes (i.e. GFAP) to induce glial differentiation. However,
ngn1 blocks phospholyation of STAT and STAT/CBP/p300/smad complex conformation to inhibit glial gene expression, and stimulates neuronal differentiation genes (i.e. NeuroD) expression with CBP/p300/smad complex (Sun et al., 2001).
The second intron of nestin gene contains 14 putative bHLH transcription factor binding site (E-box), and the evolutionary conserved 637bp of the second intorn has 6 of E-box (Fig. 1). In mice development, the neurogenesis is the most prosperous during embryonic day (E) 8 to 16 and the gliogenesis is started after E16. The bHLH transcription factor, neurogenin1 is shortly expressed during E 8.5 to 10.5 in neural precursor cells (Ma et al., 1998). Nestin expression begins at E 7.75 and is thriving at E 10.5 (Dahlstrand et al., 1995). With those time scale, we can predict the nestin expression is somewhat related and regulated by ngn1.
In this study, we examined the activity of the 637bp nestin enhancer (N2E) of nestin second intron in neural progenitor cell, and roles of bHLH transcription factor as the regulator of nestin expression. Also, we constructed N2E-LacZ and nestin enhancer mutant (N2Em)-lacZ transgenic mouse to visualize nestin enhancer activity and see the roles of bHLH transcription factor in vivo. Finally, we characterized the GFP (+) cells in nestin-GFP transgenic mouse using microarry analysis.
E1 E2 E4 E3 E5 E6
Fig. 1. Conserved sequences in the 3’ half of the 2nd intron of rat, mouse, and Human nestin genes. Sequence alignment of nestin 2nd intron from 1047bp to 1738bp. gi 2209201 :
Rattus norvegicus, gi 38098855 : Rus musculus, gi 2209203 : Homo sapiens. It also shows the putative six E-boxes on the 2nd intron.
II. MATERIALS AND METHODS
A. Materials
Nestin-GFP transgenic mice were donated from Dr. Yamaguchi M (Tokyo University, Japan). All culture media and trypsin-EDTA were purchased from Gibco (Carlsbad, CA, USA), Accutase was purchased from Innovative Cell Technologies (San Diego, CA, USA), and Hanks’ Balanced Salt Solution (HBSS) and fetal bovine serum (FBS) were from HyClone Inc. (Logan, UT, USA). Epidermal growth factor (EGF) was from Daewoong Pharmaceutical Research Lab (Hyangnam, Korea), and basic fibroblast growth factor (bFGF) was supplied from Dong-a Phamaceutical Research Lab. (Yongin, Korea). pGL3 promoter vector, pGEM® T easy vector system I and Dual luciferase® reporter assay kit was purchased from Promega (Madison, WI, USA), and βgnlacZ vector was donated from Dr. Park S (Sookmyung women’s University, Korea). LipofectAmine® was purchased from Invitrogen (Carlsbad, CA, USA). Tissue Freezing Medium® was purchased from Leica Microsystems (Jung) (Heidelberger, Germany). Manufactures of primary antibodies and dilution factors is listed on table 1. Secondary antibodies Alexa 488/568 conjugated goat/donkey anti-mouse / rabbit / goat IgG were purchased form molecular probe (Carlsbad, CA, USA). Anti-goat IgG antibody and ABC Kit were purchased form Vector Laboratory (Polo Alto, CA, USA). RNAzloTM B reagent and
Qiagen (Hilden, Germany). GeneChips for microarray were purchased from Affymetrix (Santa Clara, CA, USA). Fluorescence microscope was manufactured by Olympus (Tokyo, Japan), and confocal laser scanning microscope was from Zeiss (Oberkochen, Germany). Fluorescence Activated Cell Sorter (FACS) was from Becton Dickinson (Franklin Lakes, NJ, USA). And other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
B. METHODS
1. Neural stem cell culture
Embryonic day 12.5 nestin-GFP transgenic mice forebrain was dissected and the cerebral meninges were completely removed. The tissue was washed with HBSS and treated with Accutase in five volume of the packed tissue for 3 to 5 minutes at room temperature. Using 200μl pipette, the tissue was dissociated toward single cell and wished again with HBSS. The dissociated cells were plated on culture dish with culture media (DMEM:F12(1:1) with N2 supplement, 20ng/ml bFGF, EGF, 2μg/ml heparin sulfate) in 1X105 cell/ml concentration. Primary cultured neural stem cells
are incubated on 37 with 5% CO℃ 2, and every other day half of the media was
replaced to fresh media. After 5-7 days, the cultured neural stem cell formed ~100μm diameter sphere (neurosphere). The neurospheres were dissociated with accutase to passage the cells.
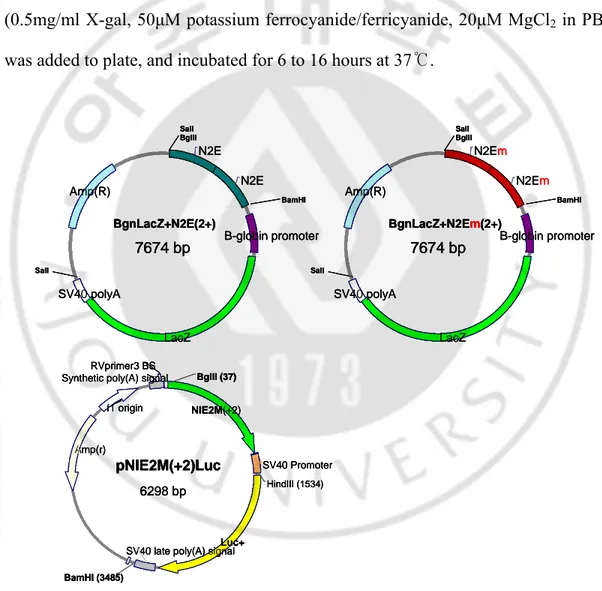
2. Reporter gene constructs (1) βgnlacZ-N2E(+n)
637 base pair (from 1162bp to 1798bp; nestin enhancer:N2E) of the nestin 2nd
intron was obtained form pNPEeGFP, a gift of Dr. Masahiro Yamaguchi (Tokyo University), using following primer with polymerase chain reaction (PCR).
forward primer ; 5'-agatctccctgaagagtttgtgat-3' reverse primer ; 5'-ggatccaagcctgggaacctcgtc-3'
The PCR product was inserted toward pGEM® easy vector system I, and nestin enhancer (the PCR product) was cut with restriction enzyme BglII and BamHI. βgnlacZ reporter vector, which has a β-globin promoter and lacZ gene was digested with BglII and the nestin enhancer was inserted in the digested site to form βgnlacZ-N2E(+1). To make multicopy nestin enhancer in reporter gene, βgnlacZ-N2E(+n-1) was digested with BglII and inserted the N2E in digested site. The nestin enhancers were serially inserted uitill 4 copies.
(2) βgnlacZ-N2Em(+n) and pNEm0.7luc(+2)
To make mutation on second E-box in the nestin enhancer, I used point mutagenesis method described in Molecular Cloning (Sambrook and Russell, 2001). Briefly, I performed two PCR with mutated sequence containing primer as following and primer described in construction of βgnlacZ-N2E(+n) for the nestin enhancer of pNPEeGFP vector.
mutant forward ; 5'-tcttaggccctctggatggtagtgtggacaaa-3' mutant reverse ; 5'-tttgtccacactaccatccagagggcctaaga-3'
With two pair of primer, forward/ mutant reverse and mutant forward/ reverse , I got two fragment of DNA with mutated sequence, then performed the third PCR with primer in (1) and two fragment as template to form full sequence of nestin enhancer with mutated E-box. The construction of βgnlacZ-N2Em(+n) was same as described in construction of βgnlacZ-N2E(+n) without N2Em instead of N2E. For pNEm0.7luc(+2), the pGL3 promoter vector was cut with BglII and inserted two
copies of 2nd E-box mutated nestin enhancer that cut with BglII and BamHI.
Schematic diagram of all constructed reporter vectors are shown in fig. 2.
3. Reporter gene assay
P19 mouse embryonic carcinoma cell line was maintained with 10% FBS in DMEM and incubated in 37 with 5% CO℃ 2. 1.5x105cells were plated in each well of 12 well
plate and incubated for 16 hours. Then, the reporter gene and bHLH expression vector was transfected using LipofectAmineTM reagent following manufacture’s instruction. 2 days after transfection, cells were lysised with passive lysis buffer in dual luciferase assay system kit, and Bradford assay was performed. To examine the reporter gene activity, 20-30μg of cell lysate were tested with luciferase assay or β-gal assay. For lacZ gene, X-β-gal stain was also performed.
4. Luciferase Assay
Transfected cell were lysised using passive lysis buffer, and 20μg of proteins were assayed using dual lucifease reporter assay system kit following manufacture’s instruction. Transfection efficiency was compensated using RL-TK value.
5. β-gal assay and X-gal stain
In case of β-gal assay, 30μg proteins from cell lysates 30μl were mixed with 270μl of β-gal assay solution (264μg/ml ONPG, 1mM MgCl2, 45mM
on 37 until the mixture turned to faint yellowish color. The reaction was stopped ℃ by adding 500μl 1M Na2CO3, and 200μl of the solution were placed on 96well plate
and read optic density at 420nm with Emax spectrophotometer.
For X-gal stain, transfected p19 cells were fixed with 2.5% glutaraldehyde in PBS for 10 minutes and washed with PBS three times. 200μl of staining solution (0.5mg/ml X-gal, 50μM potassium ferrocyanide/ferricyanide, 20μM MgCl2 in PBS)
was added to plate, and incubated for 6 to 16 hours at 37 .℃
N2Em N2Em B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E +) 7674 bp m(2 SalI BglII SalI BamHI N2E N2E B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E(2+) 7674 bp SalI BglII SalI BamHI SV40 Promoter Luc+
SV40 late poly(A) signal Amp(r)
f1 origin Synthetic poly(A) signal
RVprimer3 BS HindIII (1534) NIE2M(+2) BamHI (3485) BglII (37) pNIE2M(+2)Luc 6298 bp N2Em N2Em B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E +) 7674 bp m(2 SalI BglII SalI BamHI N2Em N2Em B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E +) 7674 bp m(2 SalI BglII SalI BamHI SalI BglII N2E N2E B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E(2+) 7674 bp SalI BamHI SalI BglII N2E N2E B-globin promoter LacZ SV40 polyA Amp(R) BgnLacZ+N2E(2+) 7674 bp BamHI SalI
Synthetic poly(A) signal RVprimer3 BS
SV40 Promoter
Luc+
SV40 late poly(A) signal Amp(r) f1 origin HindIII (1534) NIE2M(+2) BamHI (3485) BglII (37) pNIE2M(+2)Luc 6298 bp
Synthetic poly(A) signal RVprimer3 BS
SV40 Promoter
Luc+
SV40 late poly(A) signal Amp(r) f1 origin HindIII (1534) NIE2M(+2) BamHI (3485) BglII (37) pNIE2M(+2)Luc 6298 bp
Fig. 2. Construction of reporter vector. Shows BgnlacZ-N2E(+2), BgnlacZ-N2Em(+2) and pNEm0.7luc+2
6. N2E(+4)/ N2Em(+4)-LacZ transgenic mice
βgnlacZ-N2E(+4) and βgnlacZ-N2Em(+4) were used to product N2E(+4)/ N2Em(+4)-LacZ transgenic mice. The injection of transgene to C57BL/6 was performed in Sookmyung University (Dr. Park S). Briefly, superovulated C57BL/6 female mice were mated with stud male mice to generate fertilized eggs. The Fertilized eggs were collected in 1 cell stage, and the transgene were injected to the fertilized eggs with microinjection method. The 1-cell eggs were incubated overnight in 37 5% CO℃ 2. Finally, two cell stage fertilized eggs were transplanted to the
oviduct of surrogate mother ICR female. Integration of the transgenes to mice was determined by PCR analysis of yolk sack DNA with following primers, and X-gal stain with whole embryos.
forward primer ; 5’-attaccagttggtctggtgtc-3' reverse primer ; 5'-ggatccaagcctgggaacctcgtc-3'
7. Immunocyto(histo)chemistry
Cultured neural stem cells (neurosphere) were collected and fixed with 4% paraformaldehyde for 10 minutes, and washed with PBS for three times. Fixed neurospheres were frozen with Tissue Freezing Medium® and sliced in 7μm thick with Lieca cryostat. The slices were attached to slide glasses and stored at -20℃ until stain the sample.
X-gal stained E11.5 N2E/ N2Em-LacZ transgenic mice embryos were stored in 70% EtOH, the embryos were dehydration with serially changed to 100% EtOH,
then the embryos were placed in Xylene to remove EtOH. Finally, embryos were placed on paraffin to make paraffin block. The paraffin blocks were sliced in 5 μm thick with Lieca microtome. The slices were attached to slide glasses and re-hydrated rolling back the dehydration steps.
Sample on slide glasses are treated with blocking solution (0.1% Triton X-100, 0.1% bovine serum albumin (BSA), 10% normal goat (donkey) serum in PBS) for 1 hour at room temperature, then primary antibodies diluted on blocking solution were applied to sample and incubated at 4℃ overnight; the dilution factors and manufactures are in table 1. Following several washing with washing solution (0.1% Triton X-100, 0.1% BSA in PBS), samples were incubated in Alexa conjugated anti-mouse/rabbit/goat IgG (1:500) secondary antibody for 1 hour in dark chamber RT. After sufficient washing with washing solution, samples were mounted with Vectashield (mounting medium for fluorescence, Vector) with Hoechst 33258 (Molecular probe) as counter stain.
For DAB staining, sample slide were immersed in 0.3% H2O2 to blocking endogenous
peroxidase for 30 minutes. After three washes with PBS, non-specific binding were blocked with blocking solution for 1 hour at room temperature. The primary antibodies diluted on blocking solution were applied to sample and incubated at 4℃ overnight. Next day, the slide were washed with washing solution and incubated with horse anti-mouse/rabbit biotinylated antibody (1:200) for 1 hour, washed again with washing solution and incubated with vectastain ABC Kit (1:50) for 30 minuets. Finally, signals were
visualized with DAB. After washes in PBS, the sample were dehydrated and mounted with coverslip.
8. Sorting GFP(+) cells from nestin-GFP transgenic mice NSC
Nestin-GFP transgenic mice neurospheres were cultured as described method 1. Neurospheres were dissociated with 200μl accutase and suspended in 400μl growth media, then re-suspended in PBS with 1mM EDTA in 1X106 cell/ml concentration.
Using BD FACSVantage system, GFP(+) and GFP(-) cells were separately collected in tubes.
1˚ ANTIBODY SOURCE DILUTION MANUFACTURE
α-nestin(Rat401) α-GFP α-GFP α-Ki67 α-musashi α-Sox2 α-ngn1 α-βtubulinIII(Tuj1) α-GFAP Mouse Mouse Rabbit Rabbit Rabbit Rabbit Goat Mouse Rabbit 1:500 1:500 1:500 1:500 1:250 1:1000 1:50 1:500 1:500
Developmental Study of Hybridoma Bank Molecular probe Molecular probe Abcam Chemicon Abcam Santacruze biotechnology Covance Sigma
9. Microarray analysis of GFP(+) and GFP(-) cells
RNA of GFP(+) and (-) cells were prepared using RNAsol™ B, then cleaned with RNeasy mini kit following manufacture’s instruction. The prepared RNA were sent to Center for NeuroGenomics in Korea University to perform microarray. The detailed protocol of microarry can get from Affymetric website (www.affymetric.com) or Seoulin bioscience (www.seoulin.co.kr). Briefly, cDNA were synthesized using cleared RNA sample mouse, and then Biotin-labeled cRNA were synthesized with cDNA. The cRNA were treated with fragmentation buffer to make fragmentation of cRNA. Fragmentation of cRNA were hybridized in Genome 430A 2.0 array chip using hybridization oven 640, stained and scanned with fluidic station 450, and scanner 3000. The arrays were analyzed with GeneChip operating software ver. 1.2.0.037.
III. RESULTS
1. Nestin-GFP transgenic mice neural stem cell culture
The E12.5 transgenic mouse embryos, which have transgene containing eGFP with nestin promoter and the nestin second intron (Fig. 3A) expressed bright GFP in whole central nervous system. The GFP expression means that nestin gene is expressed by regulation of nestin second intron. Also, it means that the neural stem cells expressing nestin protein exist all around CNS.
To obtain neural stem cells from nestin-GFP transgenic mice, the forebrain of the embryonic day 12.5 embryo was dissected and treated with Accutase to acquire single cell. The primary neural stem cells were cultured in DMEM:F12(1:1) with N2 supplement and supplied EGF and bFGF 20ng/ml. The media also contained 2μg/ml heparin sulfate to stabilize bFGF in the media (Caldwell et al, 2004). After 6-7days from primary culture, I could observe 100-200μm in diameter sphere shaped cell aggregation (neurosphere), and about 70% of the cells in the spheres were expressing GFP (Fig. 3B).
Neurosphere were sliced with 5μm thick and stained with various stem cell markers (Fig. 3C). Cells in neurospheres were expressing nestin and other stem cell markers such as musashi, sox2. The proliferating cell marker Ki67 showed in small population of sphere (~1%), but it can not be said that the neurosphere does not proliferate. The Ki67 antibody detects proliferating cell just before cells were fixed. Also, it can be possible the size of neurosphere inhibit to proliferation of neurosphere
more. I also stained with differentiated cell marker Tuj1 (neuron) and GFAP (astrocyte), but I could not detect any Tuj1 or GFAP positive cell in the neurosphere. With these results, I could conclude that the GFP(+) and GFP(-) cells in neurosphere are proliferating and not differentiated neural stem (progenitor) cells in our culture condition.
Fig. 3. GFP+ cells express neural precursor cell markers in neurospheres derived from nestin-GFP transgenic mice. (A) Schematic diagram on nestin reporter gene in the transgenic mouse. (B) primery neurosphere population has GFP(+) and (-) cells in neurosphere (C) Various neural stem cell / progenitor markers (nestin, Ki67, musashi and sox2) were coexpressed with GFP, but differentiated cell markers (GFAP and Tuj1) were not.
2. Regulation of nestin gene by the nestin enhancer
in the second intron
Nestin gene is composed with four exons and three introns. The second intron of the gene is known that regulate gene expression in central nervous system. Also, the nestin second intron has evolutionary conserved sequence in posterior 3’ half of the sequence - more than 70% of sequence homology among mouse, rat and human: for 1.8kbp rat nestin second intron, it is located sequence from 1162bp to 1798bp (Fig. 1). For convenience, I named this 637base pair sequence as “Nestin Enhancer” (N2E).
To test nestin gene regulation by nestin sequence and nestin enhancer, I performed luciferase assay in P19 cell line. The reporter gene used in this experiment was pNE1.7luc and pNE0.7Luc+2, which contains whole nestin second intron or two copies of nestin enhancer in pGL3 promoter vector. As transfected amount of bHLH transcription factor neurogenin1 increased, the luciferase activity was increased in both reporter gene (pNE1.7Luc and pNE0.7Luc+2) in dose dependent matter (Fig. 4). However, the nestin enhancer contained reporter gene has more sensitive activity on ngn1. It can be possible the activity is higher in pNE0.7Luc+2 caused by the more binding ngn1 on enhancer due to double copies of nestin enhancer on reporter gene, but the anterior part of the intron might acts as the repressor of nestin expression in neural stem cells as a report previous (Zhang et al., 2005). At least, I could conclude that the nestin enhancer is regulating nestin expression by proneural gene neurogenin.
2ndIntron
Fig. 4. ngn1 activated the nestin enhancer in P19 cells. (A) Nestin 2nd intron and enhancer containing luciferase reporter constructs (B) Reporter gene activity of pNE0.7luc+2 was highly increased by ngn1 in a dose dependent manner.
0 1 2 3 4 Ngn1
-
-pNE0.7luc+2 pNE1.7luc Relativ e luciferase activ ity (F. I.)
pNE0.7luc+2
pNE1.7luc
TATAA
B
1kb 0.7kb TATALuciferase
1kb 0.7kbLuciferase
0.7kb 0.7kb TATALuciferase
0.7kb 0.7kb TATALuciferase
3. E-boxes on Nestin Enhancer
” to regulate gene expressi
gene by ngn1. However, the first and m
4. Regulation of nestin expression by E-box on Nestin Enhancer
y the second E-box or not, I constructed
activate nestin gene expression by second E-box on nestin enhancer.
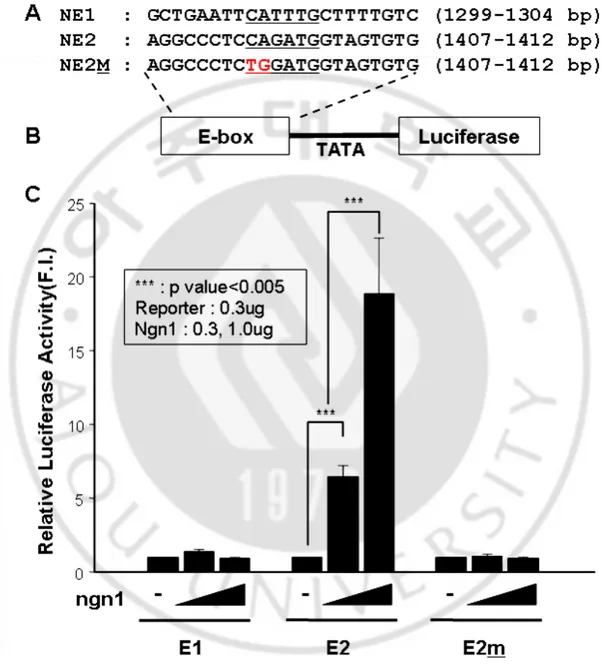
bHLH transcription factors binds to DNA sequence called “E-box (CANNTG) on. The second intron of nestin gene has 14 putative E-boxes, and 6 of the E-box are located on the nestin enhancer (Fig. 1). Especially, the first and second E-box of the nestin enhancer are evolutionary conserved among mouse, rat and human. So, we constructed luciferase reporter gene with those two E-box or mutant E-E-box to exam the binding affinity of bHLH transcription factor, ngn1.
The luciferase activity was increased in the second E-box containing reporter utated second E-box didn’t show activity increase by ngn1 (Fig. 5). With this data, we predicted that ngn1 mediated nestin gene expression is regulated by the second E-box on nestin enhancer.
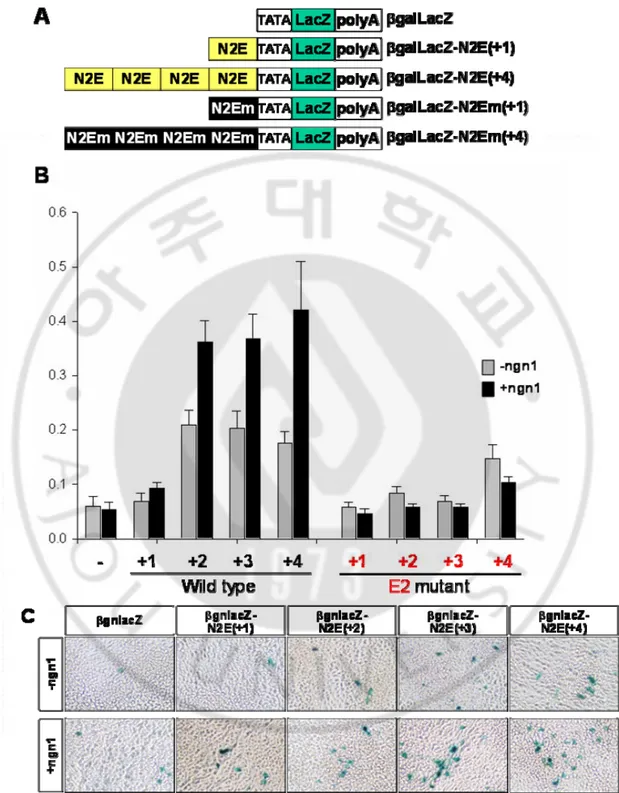
To exam whether bHLH transcription factor activates nestin enhancer b βgnlacZ expression vector containing nestin enhancer or second E-box mutated nestin enhancer. I transfected the reporter gene with ngn1 in P19 cell line and perform β-gal assay and X-gal stain (Fig. 6). By increasing the copies of nestin enhancers, the transactivity of β-gal was increased by ngn1. However, the second E-box mutated nestin enhancer didn’t show any increase by ngn1. With this result, we could conclude that the bHLH transcription factor may
Fig 5. ngn1 specifically activated the E-box mediated reporter gene activity in P19 cells. (A), (B) E-box sequences and their mutant forms-containing luciferase reporter constructs (C) E2-mediated reporter gene activity was increased by ngn1 in a dose dependent manner.
5. bHLH transcription factors the effects on nestin gene expression
Development of central nervous system is known to be regulated by many signaling molecules including bHLH transcription factors. We were targeting on
bHLH transcription factor on nestin enhanc
bHLH transcription factor on nestin expression, we examined the effects of many er. To exam the effect of various bHLH transcription factors on nestin gene expression, nestin enhancer containing pNE0.7luc+2 reporter vector and bHLH were transfected on P19 cell line and evaluated luciferase activity (Fig. 7). The early proneural genes- ngn1, ngn2 and mash1- during development of CNS induced luciferase activity, but relatively late proneural genes –math1, math3 and neuroD- didn’t induce luciferase activity on pNE0.7luc+2. Because nestin and proneural genes expression are overlapped in some period during CNS development, this presents the proneural bHLH transcription factors may be enhancing nestin expression by regulating nestin enhancer.
Fig 6. Transactivation of nestin enhancer by ngn1. (A) construction of βgnlacZ-N2E(+n) and N2EM(+n). (B) β-gal assay of reporter gene that contains wild type nestin enhancer and 2nd E-box mutated enhancer. (C) X-gal staining of βgnlacZ-N2E(+n) in P19 cell line
Fig 7. Specific induction of the 2nd intron by early proneural bHLH proteins. (A) A reporter gene containing the 3’ half of the 2nd intron. (B) Early proneural bHLH proteins prominently promoted luciferase activity whereas the effects of late proneural bHLH proteins such as math1, math3, and neuroD were minor.
6. Nestin Ehnahcer (N2E) / Nestin Enhancr mutant (N2Em) -
LacZ transgenic mice
To examine the effect of nestin enhancer and second E-box of nestin enhancer in
vivo, we construct transgenic mice with βgnlacZ-N2E(+4) and βgnlacZ-N2Em(+4).
university. Integration of transgene was checked with PCR, and then the transgene integration was confirmed with X-gal stain of E11.5 embryos. The LacZ gene was highly expressed in entire CNS including dosal root ganglion, trigeminal nerve in the N2E-lacZ embryo (Fig. 8A, D and G). However, the lacZ expression of N2Em-lacZ is various in each embryo (Fig. 8B, C, E, H, F and I). Some of embryo showed that is normal expression pattern on surface, and others showed ectopic expression. When we section the embryos, the expression patterns of LacZ were quite different between wildtype and E-box mutated nestin enhancer (Fig. 8J and K). The LacZ expression showed radial formation from ventricle to marginal layer in wildtype nestin enhancer, but the LacZ expression was obserbed only in marginal layer in second E-box mutaed transgenic mice. Because nestin gene expression begins at E7.75 and neurogenin1 expression started at E 8.5 in development mouse embryo, the lacZ on marginal layer can be explained as the nestin gene expression is regulated by factors rather than ngn1 with nestin enhancer before E8.5 and enhancing the expression by ngn1 after E8.5.
X-gal stained embryos were sectioned and stained with stem cell marker anti-nestin and anti-sox2. Also, they were stained with bHLH transcription factor neurogenin1. As a result, the dark blue x-gal stained cells were also stained with stem cell markers, anti-nestin and sox2. Furthermore, the dark blue lacZ expressing cells also expressed neurogenin1 (Fig. 9).
Fig. 8. Transgenic mice expressing LacZ driven by nestin enhancer with wild type and mutated E-box (A~C) X-gal staining of whole embryos at E11.5 (D~F) Dorsal view of LacZ expression in the brain (G~I) Dorsal view of LacZ expression in the spinal cord (J~L) Transverse sections of the telencephalic vesicles. Note high levels of LacZ expression in the ventricular zone of the wild type transgenic embryos. (M) Summary of LacZ expression in the transgenic mice
Fig. 9. Colocalization of LacZ and neural stem cell markers in E11.5 transgenic mouse brain carrying the wild type E-box. (A), (D), (G) Telencephalone, (B),(E), (H) midbrain (C), (F), (I) hindbrain
7. ngn1 expression on GFP(+) cell of Nestin-GFP transgenic
mouse NSC
We observed the LacZ expression that expressed by regulation of nestin enhancer was colocalized with ngn1 in N2E-LacZ transgenic mice. To observe colocalization of neurogenin and GFP in nestin-GFP transgenic mice, we cultured neural stem cells from transgenic mice to form neurosphere. The neurosphere were sectioned and
stained with anti-GFP and anti-ngn1 and observed with confocal laser scanning microscope. The cells in neurosphere expressed nestin. Noticeable observation is that the GFP positive cells are all expressing neurogenin1 in the neurosphere (Fig. 10).
Hoechst
Nestin
GFP Hoechst
ngn1
GFP
Fig. 10. Coexpression of nestin, GFP and ngn1 in neurospheres derived from nestin-GFP transgenic mice. Expression of nestin-GFP and nestin (A) and nestin-GFP and ngn1 (B) in the neurosphere
8. Characterization of GFP(+) cells using microarray analysis
The GFP expression of nestin-GFP transgenic mice is regulated by nestin second intron. However, not all cells in nestin-GFP transgenic mice neurosphere expressing GFP, even though they are expressing nestin protein (Fig. 3 and 10). The GFP positive cells in nestin-GFP transgenic mice neurosphere were dissociated and counted on slide glass with cover glass using the fluorescence microscope. As a
result, the GFP positive populations were decreased by each passage (Fig. 11), even though the total cells generated were increased in each passage. This might caused by the proliferation rate of GFP positive cells was much smaller than the GFP negative cells. Also, it is possible that GFP positive cells were differentiated to progenitors that still expressing nestin gene but not regulated by nestin enhancer.
0 10 20 30 40 50 60 70 80 90 PC p1 p2 p3 Pe rc e n ta g e %GFP(+)
Fig. 11. Percentage of positive cells at different passages. The percentage of GFP-positive cells decreased in each passage. PC : primary culture, p1-3 : passage 1 to 3.
The neurosphere expressing nestin but not GFP shows that the expression of nestin gene is regulated by other elements rather than the nestin second intron in CNS. This means the cells in neurosphere might have various staged cells. To investigate the difference between GFP positive and GFP negative cells in nestin-GFP transgenic mice NSC, we performed microarray after sorting nestin-GFP positive and negative cells with FACS. Because the GFP positive cell population was gradually
decrease by the passage, I used only primary sphere to sort using FACS analyzer to maximize the GFP positive cell yield (Fig. 12). The intermediate region of GFP expression were not collected to minimize the cross contamination. Also, I assumed that weak GFP expression means that the GFP transcription was already turned off and remaining GFP detected. When the GFP positive cells after sorting were re-analyzed with FACS, about 98.5% of cells were detected as strong GFP expressing cells (data not shown). It means the FACS analyzer separate GFP positive and negative cells not perfectly. This might caused by the GFP positive and negative cells in same drop are detected as GFP positive by the FACS. Even though the separation was not perfect, it was enough to see the difference of gene expression between GFP positive and negative.
Sorted GFP positive and negative cells were collected in 5ml round bottom tube and the total RNA was prepared with RNA sol. Bee™. Then, the total RNA was cleaned up using Qiagen RNeasy mini kit. Because the purity of input RNA is important for successful analysis, the total RNA were cleaned up using RNeasy mini kit and the quality of RNA in each step were checked with gel image (Fig. 13). It showed that the quality of input RNA and final fragmented cRNA were good to perform microarray. Also, I could conclude the array were performed well with the report file: the value of background & noise, housekeeping control probe set and spike control were meet with the acceptable range.
Fig. 12. Sorting out GFP positive and negative cells from nestin-GFP transgenic mice neurosphere using FACS. GFP negative cells were in R2 and GFP positive cells were in R3. The intermediate regions were not collected.
Gene expression profile from microarray showed that 93 annotated genes were decreased and 104 genes were increased relatively in GFP positive cells. When the genes with normalized expression level increased or decreased by two-fold or more are selected, 30(decreased) and 31(increased) were remained. Then, the genes with
“absent” expression in both samples were eliminated, and finally 16 genes in GFP positive (Table 2) and 8 genes in GFP negative (Table 3) were shown the increased expression. For neurogenin1 (neurod3), the expression level were ranked as “absent” in the gene profile, but the expression level were shown to be slightly increased in GFP positive cells (Table 4).
Fig. 13. Total RNA and cRNA preparation for microarray. the gel image for quality control in each step were shown. The purified total RNA (left), purifed cRNA (middle), and fragmented cRNA(right)
Table 2. The gene expression increased in GFP(+) cells of nestin-GFP transgenic mouse neurosphere. The gene expression increased more than two folds in GFP positive cells were described with brief gene function
Table 3. The gene expression increased in GFP (-) cells of nestin-GFP transgenic mouse neurosphere. The gene expression increased more than two folds in GFP negative cells were described with brief gene function
Table 4. neurogenin 1 expression level in GFP (+) and (-) cells of nestin-GFP transgenic mice neurosphere. 1438441_at and 1450836_at are the probe ID of Affimetrix Gene chip representing neurogenin1 (neuroD3). Both in GFP(+) and (-) gene expression were ranked as “Absent”, but the expression were slightly decreased in GFP(-) cells comparing to input.
IV. DISCUSSION
Intermediate filament is characterized that expressed during specific period during development. The class VI intermediate filament, nestin protein is expressed in neural stem (precursor) cells in CNS, and it is replaced by other cell type specific intermediate filament such as GFAP, NF4 during differentiation of NSC (Dahlstrand et al,. 1995). The nestin expression is known that one of the characteristic for the neural stem cells. If the live neural stem cells can be isolated efficiently, the stem cells can be easily used in cell therapy for neurodegenerative diseases. Therefore, many researchers have been tried to identify the neural stem cells using physical properties or non-fixed stain (i.e. surface marker) (Murayama et al,. 2002).
In this study, the neural stem cells were isolated and cultured from E12.5 nestin-GFP transgenic mice embryo, and the cultured neural stem cells were formed neurosphere. The cells in neurosphere were expressed neural stem cell markers such as nestin, sox 2 and musashi, but not differentiated cell markers (Tuj1 and GFAP). Therefore, the neurosphere in our culture method maintained the undifferentiated condition. Even though, the Ki67 positive cells in neurosphere were around 1%, the total cell number always increased after a week culture from passage. Ki67 is known as a nuclear antigene associated with cell proliferation and is present throughout the active cell cycle (G1,S, G2 and M phases) but absent in resting cells (Lalor et al., 1987). The percentage of Ki67 positive cells in the neurospheres in figure 3 may be
relatively small because the Ki67 antibody only detected the proliferating cells when the neurospheres were fixed. Also, it is possible that the cells in neurosphere proliferate faster when the size of neurosphere is smaller and getting slower after the size is bigger (over 50μm). Because smaller sized neurospheres were more easily stuck in plastic wears during preparing neurosphere sample, I could collect neurosphere at least 50μm in diameter.
I constructed various reporter genes that regulate reporter gene expression by nestin enhancer of nestin second intron, and evaluated that the effect of bHLH transcription factor ngn1 on nestin enhancer activation. Even though the nestin expression begins before ngn1 expression during neurodevelopment, the nestin enhancer was activated by ngn1 (also activated by early proneural gene ngn2 and mash1) in reporter gene assay. It could be possible that proneural bHLH groups are enhancing the nestin expression in specific period when the bHLH transcription factors binds to nestin enhancer. By evaluate reporter gene assays using E-boxes and E-box mutant of nestin enhancer and E-box mutation on nestin enhancer, I could conclude that the second E-box of nestin enhancer is crucial for activation of nestin enhancer by bHLH transcription factors.
The transgenic mice in this study have great advantage to investigate nestin gene regulation. Because the N2E-LacZ transgenic mice contained nestin enhancer, β-globin promoter and LacZ gene in the transgene, the lacZ gene expression is only regulated by only nestin enhancer; we could observe the effect of nestin enhancer rather nestin promoter or fore part of nestin second intron. Also, multicopies of
nestin enhancer in the transgene help to enhance sensitively the lacZ gene expression by the regulation of nestin enhancer (Fig. 6). This may overcome the limitation of sensitivity to β-galactosidase activity detection in the comparison with GFP (Chiocchetti et al., 1997). Therefore, the comparison with nestin-GFP transgenic mice may give a cue to explain the regulation of nestin gene. Also, if the nestin-GFP and N2E-lacZ transgenic mice are hybridized by crossing each other, it will help to visualize the difference of nestin gene regulation by nestin 2nd intron and nestin
enhancer.
When the E12.5 nestin-GFP transgenic mice forebrain were primary cultured, sphere shaped cell aggregation, called “neurosphere” were formed. The neurosphere forming is known as one of the characteristic of neural stem cells (Gage, 2000; Bottai et al., 2003). I could observe that not all nestin expressing cells in neurosphere were expressing GFP; nestin was expressed even in GFP negative cells. It could be explained that the nestin second intron is crucial for expression of nestin gene (Dahlstrand et al., 1995; Lendahl et al., 1997) but many other factors and pathways (ie. Notch signaling) also can regulate the nestin expression in neural stem cells (Mellodew et al., 2004). The enhancer in eukaryotic cells is important to regulating gene expression, but the promoter of one gene has a role in actual transcription of the gene. I could find a report that the ubiquitous transcription factor that related transcription of house keeping gene, SP1 and SP3 are important to nestin gene expression (Cheng et al., 2004).
The GFP expression in nestin-GFP transgenic mice neurosphere was decreased when they were cultured in growth medium in vitro. It could be possible that the GFP expressing cells are differentiated further linage and turned off the GFP, but the nestin expression and sphere formation ability were not vanished in our neurosphere. The stem cells in CNS are limited in number and suppressed the proliferation in adult (Mazurova et al., 2006), and the GFP expression cells has slow proliferation rate (Ma et al., 2006). With those report, I could conclude that the GFP positive cells in neurosphere are real stem cells and the negative cells are progenitors. To investigate the difference between GFP positive and negative cells, I performed gene array with RNA of cells isolated by GFP. With the array profile, we can check the different gene expression and investigate the relationship of nestin enhancer with the gene expression variation. The 16 genes in GFP positive cells and 8 genes in GFP negative cells of nestin-GFP transgenic neurosphere were shown the increased level of the expression more than two fold. The difference of gene expression between GFP positive and negative cells represents that the GFP expression could be one of criteria for neural stem cell categorization. With those gene expression profiles, we can find the visible maker for more early staged stem cells.
V. CONCLUSION
In this study, I showed that nestin gene expression is regulated by nestin enhancer, especially second E-box. The microarry data will help to find the new neural stem cell specific markers. If we find the neural stem cell specific surface markers, we could isolate stem cells without any treatment for transplantation or cell therapy.
REFERANCE
1. Bottai D, Fiocco R, Gelain F, Defilippis L, Galli R, Gritti A, Vescovi LA: Neural stem cells in the adult nervous system. J Hematother Stem Cell Res 12(6):655-70, 2003
2. Caldwell MA, Garcion E, terBorg MG, He X, Svendsen CN: Heparin
stabilizes FGF-2 and modulates striatal precursor cell behavior in response to EGF. Exp Neurol 188(2):408-20, 2004
3. Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X, Jing N: Characterization and
promoter analysis of the mouse nestin gene. FEBS Letters 565:195-202, 2004
4. Chiocchetti A, Tolosano E, Hirsch E, Silengo L, Altruda F: Green fluorescent
protein as a reporter of gene expression in transgenic mice. iochim Biophys Acta 1352(2):193-202, 1997
5. Dahlstrand J, Lardelli M, Lendahl U: Nestin mRNA expression correlates
with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 84(1):109-29, 1995
7. Gradwohl G, Fode C, Guillemot F: Restricted expression of a novel murine
atonal-related bHLH protein in undifferentiated neural precursors. Dev Biol 180(1):227-41, 1996
8. Kawaguchi A, Miyata T, Sawamoto K, Takeshita N, Murayama A, Akamatsu M, Ogawa M, Okabe M, Tano Y, Goldman S, Okano H:
Nestin-EGFP transgenic mice : visualization of the selfrenewal and multipotency of CNS stem cell. Mol Cell Neurosci 17:259-273, 2001
9. Lalor PA, Mapp PI, Hall PA, Revell PA: Proliferative activity of cells in the
synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int 7(5):183-6, 1997
10. Lendahl U, Zimmerman L, McKay R: CNS stem cells express a new class of
intermediate filament protein. Cell 585-595, 1990
11. Lothian C, Lendahl U: An evolutionarily conserved region in ther second intron
of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci 9:452-462, 1997
12. Ma BF, Liu XM, Xie XM, Zhang AX, Zhang JQ, Yu WH, Zhang XM, Li SN, Lahn BT, Xiang AP: Slower cycling of nestin-positive cells in neurosphere
13. Ma Q, Fode C, Guillemot F, Anderson DJ: Neurogenin1 and neurogenin2
control two distinct waves of neurogenesis in developing dorsal root ganglia.
Genes Dev 13(13):1717-28, 1999
14. Massari ME, Murre C: Helix-loop-helix proteins: regulators of transcription in
eucaryotic organisms. Mol Cell Biol 20(2):429-40,2000
15. Mazurova Y, Rudolf E, Latr I, Osterreicher J: Proliferation and
differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum. Neurodegener Dis 3(1-2):12-8, 2006
16. Mellodew K, Suhr R, Uwanogho D, Reuter I, Lendahl U, Hodges H, Price J:
Nestin expression is lost in a neural stem cell line through a mechanism involving the proteasome and Notch signaling. Dev Brain Res 151:13-23, 2004
17. Murayama A, Matsuzaki Y, Kawaguchi A, Shimazaki T, Okano H: Flow
cytometric analysis of neural stem cells in the developing and adult mouse brain.
J Neurosci Res 69(6):837-47,2002
18. Sambrook J and Russell D: Molecular Cloning, a Laboratory manual. 3rd ed.
New York, Cold Spring Harbor Laboratory Press, pp. 13.36-13.39, 2001
19. Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME: Neurogenin promotes neurogenesis and inhibits glial
20. Yamaguchi M, Saito H, Suzuki M, Mori K: Visualization of neurogenesis in the
central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11:1991-1996, 2000
21. Zhang Q, Qin H, Lang B, Liu H, Han H, Ju G: Different regions of the mouse
nestin enhancer may function differentially in nestin expression in an NSC-like cell line and astrocyte. Neurosci lett 379:90-95, 2005
22. Zhou Q, Anderson DJ: The bHLH transcription factors OLIG2 and OLIG1
couple neuronal and glial subtype specification. Cell 109(1):61-73, 2002
23. Zimmerman L, Parr B, Lendahl U, Cunningham M, Mckay R, Gavin V, Mann J, Vassileva G, McMahon A: Independent regulatory elements in the
nestin gene direct transgene expression to neural stem cells or muscle precursors.
국문요약
-Nestein Enhancer 를 이용한 신경줄기세포의 특성에 관한연구
아주대학교 대학원의학과 유 순 태
(지도교수 : 서 해 영)
신경계의 신경줄기세포(Neural Stem Cell; NSC)는 발생 과정 중 다양한 내부 혹은 외부적인 요인으로부터 영향을 받아 신경계를 구성하는 신경세포 혹은 교세포등 적절한 세포로 분화하여 자신이 맡은 역할을 하게 된다. 이 때 basic helix-loop-helix(bHLH) 전사인자들은 신경줄기세포가 신경세포 (Neuron), 혹은 교세포(Glia)로 분화하는데 결정적인 역할을 하게 된다. 특히 Neurogenins(ngns)은 신경줄기세포가 각각 neuron 로 분화하도록 결정짓는 역할을 하는 중요한 인자로 생각되고 있다. 신경줄기세포의 표지인자로 잘 알려져 있는 nestin 은 신경줄기세포에서 특이적으로 세포내에서 발현하는 중간형 세사(intermediate filament protein) 으로 알려져 있다. 이 nestin 은 신경줄기세포뿐 아니라 심장근세포, 성상세포(astrocyte)등의 세포에서도 발현일 되는 것으로 알려졌다 이는 nestin 의 발현에서 세포의 성격에 따라 작용하는 enhancer 가 다르고, 이중 nestin 의 두번째 intron 은 중추신경계에서 nestin 단백질 발현을 조절하는
enhancer 로 작용한다. 또한 nestin 이 중추신경계의 신경전구세포(neural progenitor cell)에서 발현할 때, nestin 의 두 번째 intron 중 종간 매우 비슷하게 유지되어있는 637bp 으로도 nestin 의 발현을 조절할 수 있다.
살아있는 신경줄기세포에서 nestin 의 발현을 표시하는 방법은 reporter gene 의 도입이외에는 특별한 방법이 없다. 세포에는 세포막에 여러 가지 단백질과 당 등으로 구성되는 여러 종류의 receptor 및 구조가 존재하며, 이는 surface marker 로 쓰일 수 있다. 따라서 nestin 이외의 다른 surface marker 의 발현을 확인한다면 다른 표지인자를 이용하여 살아있는 순수 줄기세포의 분리가 용이할 것이다.
본 연구에서는 신경전구세포(neural progenitor cell)에서 nestin enhancer 로 알려진 두 번째 intron 의 637bp(NIN)의 활성을 조사하고, 그러한 활성 조절인자로서 bHLH 의 역할을 조사하며, NIN-LacZ 로 형질 전환된 마우스를 이용하여 bHLH 전사인자의 역할을 규정하는데 있다. 또한 Nestin-GFP 형질전환 생쥐의 Nestin-GFP 발현 세포의 성격을 파악하고, microaray 로 Nestin-GFP 를 발현하지 않는 세포와의 차이를 확인하였다.
핵심어 : Nestin, GFP, transgenic mouse, basic Helix-Loop-Helix, neurogenin, microarray