Regulatory Factors of Cellular Senescence
in Human Diploid Fibroblast :
Regulatory Factors of Cellular Senescence
in Human Diploid Fibroblast
: SA-p-Erk1/2, PP1/2A, PKCα
by
Hong Seok Kim
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the
Degree of
DOCTOR OF PHILOSOPHY
Supervised by
In Kyoung Lim, M.D., Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
-ABSTRACT-
Regulatory Factors of Cellular Senescence
in Human Diploid Fibroblast : SA-p-Erk1/2, PP1/2A, PKC
a
Proliferation of normal diploid cells is arrested irreversibly after a certain number of divisions, called replicative senescence, which is paragon of the general process of cellular senescence. Once senescent, cells continue to function metabolically, but will not respond to mitogens. Decreased growth rate, limited cell division, flat and large cell shapes, and increase of cell cycle regulator proteins are well-known characteristics of cells entered into senescence. Extracellular signal-regulated kinase 1/2 (Erk1/2) is one of the mitogen-activated protein kinase (MAPK). Erk1/2 signaling promotes cell proliferation and cell surnival. Ironically, p-Erk1/2 level is up-regulated in senescent cells. In the present study, the mechanism of senescence-associcated cytoplasmic induction of p-Erk1/2 (SA-p-Erk1/2) proteins in human diploid fibroblasts was investigated. Erk1/2 proteins were efficiently dephosphorylated in vitro by protein phosphatases 1 and 2A (PP1/2A). Specific activity of PP1/2A activity significantly decreased during cellular senescence, whereas their protein expression levels did not. SA-p-Erk1/2 was most likely due to the oxidation of PP1/2A, which resulted from continuous exposure of the cells to vast amounts of reactive oxygen species (ROS) generated during cellular senescence. Treatment of senescent human diploid fibroblast (HDF) cells with 12-O-tetradecanoylphorbol-13-acetate (TPA), a well known protein kinase C (PKC) activator, induced morphological change and thymidine
incorporation. PKC is a serine/threonine kinase and regulates signal transduction pathways involved in gene expression, cell proliferation, and differentiation. We also investigated activation and down-regulation of PKCa by TPA treatment, which reverses senescent HDF cells to young cell like shapes. Within 30min after TPA treatment, binding of PKCa and p-Erk1/2 was significantly increased, and both of them translocated to nucleus with progression of the cell cycle. When PKCa was down-regulated with siRNA treatment, the generation of ROS in mid-old cells was decreased accompanied with a typical change of the senescent HDF to young-cell like morphology. PKCa down-regulation induced the decrease of p53, p21WAF1, and SA-p-Erk1/2 in old cells. In addition, PKCa down regulated
mid-old cells regained its proliferation capacity. Therefore, these results suggest that PKCa works not only for maintenance of senescence phenotypes, but also for regulation of proliferation ability in cellular senescence.
TABLE OF CONTENTS
ABSTRACT ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙i TABLE OF CONTENTS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙iii LIST OF FIGURES ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙v Ⅰ. INTRODUCTION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙1 A. Cellular Senescence ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 1 B. Extracellular Signal-regulated Protein Kinase 1/2 (Erk1/2) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 2 C. Protein Phosphatase 1 and 2A (PP1/2A) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 4 D. Protein Kinase C (PKC) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙5 E. Purposes of This Study ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙7 Ⅱ. MATERIALS AND METHODS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙8
A. Materials ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 8 B. HDF Cell Culture ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 8
C. Assay of Senescence Associated β-galactosidase ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 9 D. Subcellular Fractionation ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 10 E. Immunoprecopitation ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙11 F. Immunoblot Analysis ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 12 G. PKCa Activity Assay ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙12 H. Immunocytochemistry ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙13 I. Measurement of ROS by FACS Analysis ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙13 J. Protein Phosphatases 1 and 2A Assay ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 14
K. Restoration of Protein Phosphatases 1 and 2A Activity by Thiol specificReagents ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙15 L. [3H]-thymidine Incorporation Assay ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙16
M. Statistics ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙16 Ⅲ. RESULTS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 17 A. P-Erk1/2 Proteins were Markedly Induced in Senescent Cells ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙17 B. Various Phosphatase Can Dephosphorylate p-Erk1/2.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙17
C. Activity of PP1/2A were Significantly Reduced duringCellular Senescence. ∙∙∙∙∙∙∙∙24 D. ROS was the Mechanism of Reduction of PP1/2A Activities during Cellular
Senescence. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙29 E. TPA Treatment Induces Reversal of Senescence Phenotype ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙34
F. TPA Activates and Induces Translocation of PKC ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 35 G. Activated PKC a Interacts with p-Erk1/2 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 41 H. Increased ROS Level in Old Cells Activates PKC ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙41 I. Senescence Phenotype is Able to be Reversed by PKCa Down-regulation ∙∙∙∙∙∙∙∙∙∙∙∙ 42 J. PKCa Down-regulated Old cells Regained Proliferation Ability ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 48 Ⅳ. DISUSSION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙53 Ⅴ. CONCLUSION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙62 REFERENCE∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙64 국문요약 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙80
LIST OF FIGURES
Fig. 1. P-Erk1/2 Proteins were Markedly Induced, but they were not Nuclear Translocated by EGF Treatment in Old HDF Cells.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙20
Fig. 2. SA-p-Erk1/2 is Competent Substrate for Alkaline Phosphatase, Protein Phosphatases 1/2A, Protein-tyrosine Phosphatase, and Dual-phosphatase rVHR.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙21
Fig. 3. Anti-p-Erk1/2 Antibody can not Detect SA-p-Erk1/2 after PP1 and PP2A Treatment. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 23
Fig. 4. Activities of Protein phosphatases 1 and 2A were Decreased, but their Protein Expression Levels were not changed During Cellular Senescence. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙26
Fig. 5. Reactive Oxygen Species Generated During Cellular Senescence, Regulated PP1/2A Activities and p-Erk1/2 Levels. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙31
Fig. 6. Protein Phosphatase 1 and 2A Activities were Restored by Thiol-specific Reducing Agents in Mid-old HDF Cells. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙33
Fig. 8. TPA Induced DNA Synthesis Only in the Old, but not in the Young HDF Cells.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙37
Fig. 9. Biochemical Evidence of Cellular Senescence were Reversed by TPA Treatment.∙∙∙∙38
Fig. 10. TPA Activated and Induced Nuclear Translocation of PKCa. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙39
Fig. 11. Activated PKCa interacts with p-Erk1/2.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 44
Fig. 12. Persistent treatment of TPA down-regulated PKCa.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 45
Fig. 13. Increased ROS Level Activated PKCa but did not Induce Intracellular Translocation.∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙46
Fig. 14. Senescence Phenotype was able to be Reversed by PKCa Down-regulation. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙49
Fig. 15. PKCa Down-regulated Old Cells Regained Proliferation Ability ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙52
Ⅰ
. INTRODUCTION
A. Cellular senescence
The term ‘cellular senescence' is currently used to identify the progressive decline in proliferative capacity that cells of higher eukaryotes undergo in culture, when they are allowed to propagate in a continuous series of population doublings. This phenomenon occurs in normal cell cultures from many animal species, but it is particularly stringent in human cells (Peacocke and Campisi, 1991). The senescence phenomenon was first formally described as the process that limits the proliferation of normal human cells in culture (Hayflick, 1965). Senescent cells acquire multiple phenotypic changes, in additionto an irreversible growth arrest, some of which can compromisetissue structure and function. These findings have led to the hypothesis that the senescencephenomenon is an example of evolutionary antagonistic pleiotropy. Thus, this may benefit organisms early in life by preventingcancer, but be detrimental later in life as senescent cellsaccumulate (DePinho, 2000; Repetto and Balducci, 2002). A diversity of stresses can induce cellular senescence. These include the dysfunctional telomeres that resultfrom repeated cell division (replicative senescence), severeor irreparable DNA damage (genotoxic stress), the expressionof certain oncogenes (oncogene-induced senescence), and agentsor conditions that disrupt chromatin organization. Despite thedisparate mechanisms by which these stresses act, senescentcells share numerous features, including an arrest of cell proliferationthat cannot be reversed by
physiological signals, an enlarged flattened morphology, expression of a neutral b-galactosidase (senescence-associated b-galactosidase), and an altered pattern of gene expression. The senescence phenomenon depends on the activities of two major tumor suppressor pathways, one controlled by p53and another controlled by p16INK4a and pRB
(Serrano and Blasco, 2001).
B. Extracellular signal-regulated protein kinase 1/2 (Erk1/2)
ERK1 and ERK2 are proteins of 43 and 41 kDa, respectively, that are nearly85% overall identical, with much greater identity in the core regions involved in binding substrates (Boulton et al., 1990; Boulton et al., 1991). The two phosphoacceptor sites, tyrosine and threonine, which are phosphorylated to activatethe kinases, are separated by a glutamate residue in both ERK1and ERK2 to give the motif TEY in the activation loop (Payne et al., 1991). Both are ubiquitously expressed, although their relative abundancein tissues is variable. For example, ERK2is the predominant species in many immune cells, while they may be equally expressed in several cells of neuroendocrineorigin. They are stimulated tosome extent by a vast number of ligands and cellular perturbations,with some cell type specificity (Lewis et al., 1998). In fibroblasts (the cell type in which the generalizations about their behavior and functionshave been developed), they are activated by serum, growth factors,cytokines, certain stresses, ligands for G protein-coupled receptors (GPCRs), and transforming agents, to name a few. They are highlyexpressed in postmitotic neurons and other highly differentiatedcells (Boulton et al., 1991). Raf acting through MEK
and ERK has beenfound to play a critical role in the control of cell cycle progression (Marshall, 1999). The majority of ERK substrates are nuclear proteins, but others are found in the cytoplasm and other organelles. Activated ERKs can translocate to the nucleus, where they phosphorylate and regulate various transcription factors, such as Ets family transcription factors (e.g., Elk-1), ultimately leading to changes in gene expression (Zuber et al., 2000; Schulze et al., 2004). MAPK activity is tightly regulated by phosphorylation and dephosphorylation.The activation of the MAPK activity requires the dual phosphorylationof the Ser/Thr and Tyr residues in the TXY kinase activation motif (Ahn et al., 1991; Canagarajah et al., 1997), and deactivation occurs through the action of either Ser/Thr protein phosphatase (Alessi et al., 1995), protein-tyrosinephosphatase (PTP) (Alessi et al., 1995; Pulido et al., 1998), or dual specificity phosphatases (Sun et al., 1993; Zheng and Guan, 1993). The dual phosphatases are capable of catalyzing theremoval of the phosphoryl group from Tyr(P) as well as Ser(P)/Thr(P)residues. The dual specificity phosphatases that specificallydephosphorylate and inactivate the p-Erk1/2 are called MAP kinasephosphatases (MKPs), and at least 9 mammalian MKPs have beenidentified so far (Keyse, 1998; Tanoue et al., 1999). At first, Erk pathway (Raf → MEK → Erk1/2) has long been associated with cell growth, cell proliferation, and cell survival (Ballif and Blenis, 2001; Rubinfeld and Seger, 2005; Meloche and Pouyssegur, 2007). However, despite well-recognized positive effect of Erk activation on G1 to S progression, there are also examples where Erk mediates a G1 phase arrest via induction of p21WAF1 (Pumiglia and Decker, 1997; Bosch et al., 1998;
C. Protein phosphatase 1 and 2A (PP1/2A)
There is increasing evidence that relatively few Ser- or Thr-specific protein phosphatases with pleiotropic action participate incellular regulation. Four major classes of protein phosphatase,type 1 (PP1), type 2A (PP2A), type 2B (PP2B or calcineurin),and type 2C (PP2C), have been identified in mammalian cells (Cohen, 1989; Mumby and Walter, 1993). PP1 occurs in three isoforms (a, b/d, and g1) and containsa catalytic subunit (PP1C), regulatory subunit (inhibitor 2),a G subunit, and an M subunit. PP1C binds to two cytosolic thermostable proteins, termed inhibitor-1 (I-1) and inhibitor-2 (I-2), which completely abolish its activity at nanomolar concentrations (Nimmo and Cohen, 1978). The I-1 tightly binds to PP1 both in vitro and in vivoand requires phosphorylation by cAMP-dependent protein kinase to inhibit a number of phosphoproteins (Huang and Glinsmann, 1976; Vulsteke et al., 1997; Trinkle-Mulcahy et al., 1999), whereas phosphorylation of I-2 at Thr72
by glycogen synthasekinase leads to activation of the enzyme (Hemmings et al., 1982; DePaoli-Roach, 1984). PP2A is aheterotrimeric enzyme consisting of a 36-kDa catalytic subunit(C), 65-kDa structural subunit (A), and a variable regulatorysubunit (B). PP2A is unaffected by the above inhibitors, but highly effectively inhibited by low concentrations of okadaicacid (Takai, 1988; Cohen, 1989; Wera and Hemmings, 1995). PP2A is active toward most substrates inthe absence of divalent cation, whereas PP2B and PP2C have absolute requirements for Ca2+ and Mg2+, respectively (Cohen, 1989).
D. Protein kinase C (PKC)
Protein kinase C (PKC) is composed of a family of serine/threonine kinase that modulate the function of variety of signal transduction pathways, leading to gene expression, cell proliferation, and differentiation. PKC isoforms can be classified into three subgroups. The conventional PKC isozymes, namely a, bI, bII, and g, are activated by Ca2+,
phosphatidylserine (PS) and diacylglycerol (DAG), or phorbol esters [phorbol 12-myristate 13-acetate (PMA)]. The novel PKC isozymes, consisting of PKC-d, -e, -h, -q, and –m, are activated by PS and DAG or PMA but are insensitive to Ca2+. The atypical isoforms, z and
i/l are not affected to Ca2+, DAG, or PMA but are dependent on PS for activation (Clemens
et al., 1992; Dekker and Parker, 1994; Nishizuka, 1995). In addition, oxidative stress has been reported to induce prolonged activation of PKC within the cells (Gopalakrishna and Anderson, 1989; Palumbo et al., 1992; Brawn et al., 1995; Whisler et al., 1995), Avtivated PKC translocates from the cytosol to membranses and/or to the nucleus (Vijayan et al., 2004; Kenessey et al., 2006). The growth–regulatory consequences of PKC activation suggest a link between PKC signaling and control of the cell cycle machinery. Activation of PKC has been shown to result in alterations of the cell cycle progression in either stimulatory or inhibitory directions in several systems (Coppock et al., 1992; Gruber et al., 1992; Sasaguri et al., 1993; Zhou et al., 1993; Hamada et al., 1996; Thompson and Fields, 1996). PKCa has been implicated in control of the G1/S transition (Sasaguri et al., 1993; Frey et al., 1997;
Besson and Yong, 2000; Frey et al., 2000), while PKCbII has been shown to play a role in progression from G2 into M phase (Thompson and Fields, 1996) and PKCd has been
associated with control of M phase (Watanabe et al., 1992).
E. Purposes of this study
In this study, we investigated mechanism of induction of cytoplasmic p-Erk1/2 and role of PKC in senescent cells.
- First, to investigate whether any change in activity or expression of specific phosphatase, which can dephosphorylate p-Erk1/2 during the senescence process, we performed experiments as follows.
(1) We tested whether which phosphatase can dephosphorylate p-Erk1/2.
(2) We tested whether there is any change in activity of phosphatase during the senescence process.
(3) We tested whether increased level of reactive oxygen species (ROS) in senescent cell affects the phosphatase activity.
- Second, to investigate the effects of induction of p-Erk1/2 nuclear translocation on senescent cells, We tested the followings :
(1) whether TPA induces nuclear translocation of p-Erk1/2 in senescent cells. (2) whether TPA treatment induces cell cycle progression and reversal of senescence phenotype.
- Third, to investigate the role of PKCa in senescent process, We tested the followings : (1) whether PKCa down-regulation or inhibition reverses of senescence phenotype.
Ⅱ
. MATERIAL AND METHODS
A. Materials
Anti-actin antibody, H2O2, TPA, and GF109203X were purchased from Sigma Aldrich
(St. Louse, MO, U.S.A.). Anti-p-Erk1/2 antibody was from Cell Signaling (Beverly, MA); antibodies against PKCa, PKCbI, and p21WAF1 were from Santa Cruz (Santa Cruz, CA);
p53 and a-tubulin antibodies were from Oncogene (Boston, MA, U.S.A.). Donkey anti-mouse IgG (H+L) conjugated with Cy3 AffiniPure and Donkey anti-anti-mouse IgG (H+L) conjugated with FITC AffiniPure, used as a secondary antibody for immunocytochemistry, was from Jackson Laboratory (West Grove, PA, U.S.A.). DAPI (4',6-diamidine-2'-phenylindole dihydrochloride) and 2',7'-dichlorodihydrofluorescein diacetate (H2-DCFDA)
were from Molecular Probes (Eugene, OR, U.S.A.). All other reagents used were of molecular biology grade.
B. Human Diploid Fibroblast (HDF) Cell Culture
Foreskin from a 4-year-old boy was obtained during operation after parents' consent at Ajou University Hospital, and the foreskin was sliced to 1×5 mm with scissors and forceps. The minced tissue was rinsed with Dulbeccos' phosphate-buffered saline (D-PBS), treated with 5 ml 0.25% collagenase (C-5138, Sigma Chemical Co., St. Louis, MO, U.S.A.) for 1.5
h and then centrifuged at 1000×g (Brushless D.C. motor centrifuge, Vision Scientific Ltd., Seoul, South Korea) for 5 min. Pellet was treated with 10 ml D-PBS containing 0.0125% trypsin for 5 min at 37°C in 5% CO2 incubator. To terminate trypsin activity, 1 ml of FBS
(GIBCO-BRL 16000-044) was added and then vortexed for 10 min to separate individual cells. After centrifugation at 1000×g for 3 min, pellets were washed twice with 10 ml of D-PBS. Finally, the pellet was resuspended in 5 ml of Dulbeccos' modified Eagle's medium (DMEM, GIBCO-BRL 31600-034) containing 10% FBS, and the suspension was incubated for 7 days at 37°C in 5% CO2 incubator. In the presence of 2 mM CaCl2, keratinocytes
disappeared, while fibroblasts proliferated. Primary culture cells were regularly trypsinized and the number of population doubling (PD) was monitored for further experiments. Young, mid-old, and old cells used in the present experiments weredefined as the HDF with a doubling time of around 24 h, 7–10days, and over 2 weeks, respectively.
C. Assay of Senescence Associated β-galactosidase
Senescence-associated β-galactosidase (SA-β-gal) was assayed at pH 6.0 by slightly modified method of Dimri et al. (Dimri et al., 1995). Briefly, cells were washed twice with PBS, fixed to plates by 3% formaldehyde for 5 min, washed with PBS, and then incubated overnight in freshly prepared staining solution of 40 mM citric acid/sodium phosphate, pH 6.0, containing 1 mg/ml of X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2. Stain could be visible after 12 h of incubation at 37°C. By counting the numbers of
the blue and total cells per field of 0.5×0.5 cm under an inverted microscope, percentage of the cells stained blue was calculated to indicate how many cells were associated with senescence. The average counts of five fields were calculated.
D. Subcellular Fractionation
Cytoplasmic and nuclear extracts were prepared as describedpreviously (Nakamura et al., 2000) with minor modification. Briefly,the cells were washed twice with ice-cold PBS, and transferredto 1.5-mL eppendorf tubes. The cells were then centrifuged at300×g for 4 min at 4°C. After centrifugation, the pelletwas resuspended in 400 µL of cold buffer A [10 mM HEPES-KOH(pH 7.5), 10 mM KCl, 1 mM DTT, 1 mM PMSF, and 1 µg/mL leupeptin]. Afterincubation on ice for 15 min, 12.5 µL of 10% Nonidet P-40was added, the mixture was vortexed briefly, and incubated on ice for 10 min. The nuclei were pelleted by centrifugation at1500×g for 5 min at 4°C, whereas the supernatant (cytoplasmicextracts) was recovered by centrifugation at 13,000×g for 15min. Nuclei were washed with 1 mL of ice-cold buffer A twice,and then resuspended in 50 µL of ice-cold buffer B [20mM HEPES-KOH (pH 7.5), 0.4 M NaCl, 1 mM DTT, 1 mM PMSF, and 1 µg/mL leupeptin],followed by incubation on ice for 30 min. The mixture was thencentrifuged at 18,000×g for 5 min, and the supernatant was collectedas a nuclear extract. To determine subcellulardistribution of PKC isozymes, soluble and particulate fractionation wasperformed as described previously (Maloney et al., 1998) with minor modification. For soluble and particulate fractions, cells were washedtwice with ice-cold PBS and scraped into a homogenization buffercontaining
25 mM Tris/HCl (pH 7.4), 2 mM EDTA, 0.25M sucrose, 1 µg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF) and then lysed by sonication(Sonic Dismembrator 550, Fisher) twice at level 2 for 10 seconds. The lysateswere centrifuged at 500×g for 5 min, and the low-speed supernatantwas centrifuged again at 100,000×g for 1 hr. The high-speed supernatantconstituted the soluble fraction. The high-speed pellet waswashed three times and extracted in ice-cold homogenizationbuffer containing 1% Triton X-100 for 30 min. The Triton-soluble component (particulate fraction) was separated from the Triton-insoluble material by centrifugationat 100,000×g for 1 hr.
E. Immunoprecipitation
Immunoprecipitation with 1 mg of anti-PKCa antibody or anti-phospho-Erk1/2 antibody was performed with young and mid-old HDF celllysates (500 µg or 1 mg) in the immunoprecipitation buffer containing 50 mM Tris-HCl(pH 7.4), 150 mM NaCl, 0.5% deoxycholic acid, 1% NP-40, 1 mM PMSF, and 1 µg/ml leupeptinby the standard method. Whole cell lystates were pre-cleared with protein G-agarose beads (Invitrogen, Carlsbad, CA, U.S.A.) for 1h at 4°C before precipitation with either anti-PKCa or anti p-Erk1/2 antibodies overnight at 4°C. The immunoprecipitates were washed 3 times with immunoprecipitation buffer, and then subjected to kinase assay or immunoblot analysis with anti-PKC a or anti p-Erk1/2 antibodies.
F. Immunoblot Analysis
Young and old HDF cells were solubilized with RIPA buffer [50 mM Tris-HCl(pH 7.5), 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% deoxycholicacid, 50 mM sodium fluoride, 1 mM sodium vanadate, 1 mM PMSF,1 µg/ml leupeptin], and 40 µg of cell lysates were resolved on 8 to 12% SDS-PAGE in 25 mM Tris-glycine buffer. The gel-separated proteins were then transferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat skim milk in phosphate-buffered saline containing 0.05% Tween20 (PBST) for 1 hr and then incubated with antibodies against PKCa, PKCßI, p-Erk1/2, Lamin B, actin, a-tubulin, p21WAF1, and p53 at roomtemperature for 1 hr, or at 4°C overnight. Nitrocellulose
membranes were washed three times with PBST and then incubated with horseradish peroxidase–conjugated secondary antibodiesfor 1 hr. ECL (Amersham Bioscience, UK) kit was employed to visualize protein expressionlevels.
G. PKCa Activity Assay
The PKCa immunoprecipitates were washed twice with immunoprecipitation buffer and then twice with kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, 1 mM DTT, 2.5 mM EGTA, 1 mM NaF, 0.1 mM Na3VO4, 10 mM b-glycerophosphate), and resuspended in 20 ml of kinase buffer. The kinase assay was initiated by adding 40 ml of kinase buffer containing 5 mg of myelin basic protein (MBP) and 5 mCi of [g-32P]ATP. The reactions were performed at 30oC for 30 min. The reactions were terminated by adding SDS sample buffer
and boiled for 5 min. The reaction products were analyzed by SDS-PAGE and autoradiography.
H. Immunocytochemistry
HDF cells were cultured ona cover glass and fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) at 4℃ for 20 min, and then washed 3 times with PBST. Within 15 min, cells were incubated with 5% bovine serum albumin (BSA) in PBST for 1 h at room temperature, and a primary antibody (1:200 in PBS plus 3% BSA) was then applied overnight at 4℃. The secondary antibody (1:200 in PBST with 3% BSA) and DAPI were applied for 1h at room temperature, and the cells were observed under confocal microscope (Olympus Fluoview FV300, 40× UPIanApo objective and fluoview v. 5.0 software, Japan) or fluorescence microscope (Carl zeiss, Axioimager M1, x100 EC Plan-NEOFLUAR and AxioVision 4.5 software).
I. Measurement of ROS by FACS Analysis
H2O2 generation duringcellular senescence was measured by FACS analysis using 50
µMH2-DCFDA, (D-399,Molecular Probes, Eugene, OR, U.S.A.) in cell culture system.
Young and mid-old HDF cells (old cells were too large to analyze byFACS) were plated in a 60-mm culture dish with 25 and 70% confluence,respectively. The next day, media were changed, and the cellswere incubated in complete medium for another 24 h. The cellswere
then pretreated with 10 mM N-acetyl-L-cysteine (NAC) (Sigma)for 1 h, and H2-DCFDA
was added 10 min prior to cell harvest.ROS generation was determined with FACScan (BD Biosciences, San Jose, CA, U.S.A.)by measuring the fluorescence of DCF arising from the oxidationof H2-DCFDA.
J.
Protein Phosphatases 1 and 2A AssayTo determine the activityof PP1/2A, cells were washed twice with phosphate-free mediumand lysed with a buffer solution, containing 100 mM Tris-HCl(pH 7.4), 1 mM PMSF, and 1 µg/ml leupeptin, by sonication(Sonic Dismembrator 550, Fisher, Pittsburgh, PA, U.S.A.) twice at level 2 for 10 s.The whole cell lysates were centrifuged at 11,000 ×g for 10min, and the supernatant was used for the enzyme assay. To separatecytoplasmic and nuclear fractions, whole cell lysates were preparedwith STM (250 mM sucrose, 20 mM Tris-HCl, pH 7.5, 10 mM Mg2+)buffer using a Dounce homogenizer, and the lysate was
centrifugedat 800 xg for 10 min. The supernatant was denoted as the cytoplasmicfraction, and the pellet was resuspended in STM buffer afterrinsing the pellet twice. Nuclear fraction was prepared by sonicatingthe above pellet and centrifuging at 11,000 ×g for 10 min,and the supernatant was used as a nuclear fraction. Malachite Green Assay kit (Upstate Biotechnology, Lake Placid, NY, U.S.A.) was mixed with Thr(P) peptide (KRpTIRR) (purchased from Upstate Biotechnology, Inc.), as a substrate. An assay mixture(25 µl) containing 250 µM substrate and 2 µgof the above subcellular fractions was incubated at 30 °Cfor 10 min, and the reaction was stopped with 100 µl ofmalachite green phosphate
detection solution. The assay mixturewas left for another 15 min at room temperature for color development. In order to eliminate phosphate contamination arising from thecell extracts and the reagents, an assay mixture without thesubstrate or reagent was run for each measurement. Absorbanceat 630 nm was read using microplate reader (Benchmark, Bio-Rad, Hercules, CA, U.S.A.).Phosphate released by the enzyme reaction was determined fromthe standard curve prepared with 0.1 mM KH2PO4 solution.
K. Restoration of Protein Phosphatases 1 and 2A Activity by Thiol specificReagents
To investigate the role of ROS played in a significantly reduced PP1/2A activity during cellular senescence, young HDFcells were pretreated once with 1 mM H2O2, since the
PP1/2Aactivity in the mid-old cells had been reduced, most likelydue to accumulated H2O2,
and these cells were not pretreated withH2O2. The cells were washed with phosphate-free
medium within10 min, and the cell lysates were then prepared according tothe method described above. To examine whether the H2O2 oxidizedthe active site cysteine residue in
PP1/2A or not, DTT (1 mM),b-mercaptoethanol (0.1%), or NAC (10 mM) was individually addedto the young and mid-old cell lysates before the PP1/2A assay.All of the experiments with young and mid-old HDF cells wererepeated 3 times. All the thiol reagents were purchased fromSigma.
L.
[3H]-thymidine Incorporation AssayOld HDF cells were plated, and the monolayer was stabilizedfor 48 hr until 70% confluent, re-fed with a fresh medium, andincubated for another 24 hr. HDF young cells also were platedon a six-well plate and incubated for 24 hr until 70% confluent.TPA (50 ng/ml), epidermal growth factor (EGF; 10 ng/ml), PKCinhibitor (Go6976; 0.4 x 10-6 M, 0.8
x 10-6 M), or DMSO as avehicle was added individually to the culture medium, and thecells were harvested at 4, 8, 20, and 32 hr of incubation. Beforeharvest, the cells were treated with 2 µCi/ml [3H]thymidinefor 4 hr. The harvested cells were lysed with 200 µl of0.04 M
Tris-HCl buffer (pH 8.0) containing 0.12 M NaCl and 0.5%NP-40 by vortex mixing at 4°C for 20 min, and the lysatewas rinsed thoroughly with PBS. Radioactivity of the lysate(100 µl) was counted by a liquid scintillation counter(Microbeta Counter 1450; Perkin Elmer, Wellesley, MA, U.S.A.) using1 ml scintillation solution. Data presented are mean± SD of quadruplicate wells from more than three experiments.Multiple linear regression analyses were applied for the data analyses obtained from [3H]thymidine incorporation assay by
calculating B value in the equation composed of the affectingfactors and using P = 0.05 as cutoff for significance.
M. Statistics
All bars represent mean ± standard deviations of determinants. Experiments were repeated at least three times. Statistical analysis was performed by t-test.
Ⅲ
. RESULTS
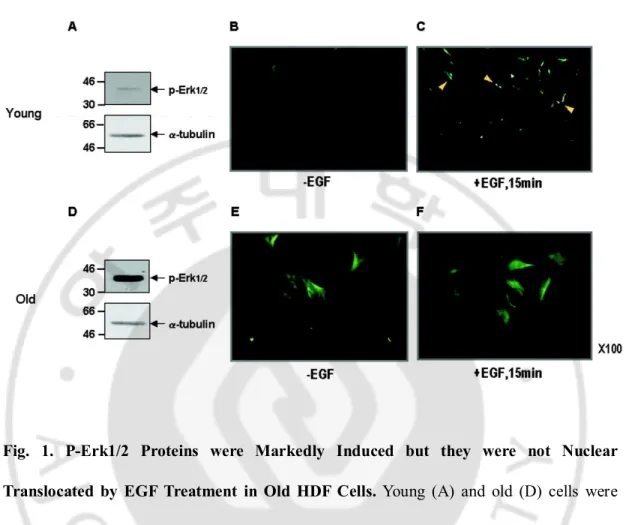
A. P-Erk1/2 Proteins were Markedly Induced in Senescent Cells.
We previously reported that the level of p-Erk1/2 was significantly increased in senescent HDF cells (Lim et al., 2000). By employing young HDF cells with a doublingtime of about 24 h and the old HDF cells with doubling timeof over 2 weeks, p-Erk1/2 levels were measured again by immunoblot analysis. Expression of p-Erk1/2 was significantly higher in the old cells (Fig. 1D) than the young cells (Fig. 1A). Moreover, immunocytochemical analysis revealed a significant induction ofp-Erk1/2 level in the young cells (Fig. 1, B and C), but not, in the old cells (Fig. 1, E and F). It should also benoted that the basal level of p-Erk1/2 was markedly differentbetween these cells (compare Fig. 1, B
versus E). Therefore, this phenomenon could be denoted as "senescence-associated
persistent induction of p-Erk1/2" or "SA-p-Erk1/2."
B. Various Phosphatase Can Dephosphorylate p-Erk1/2.
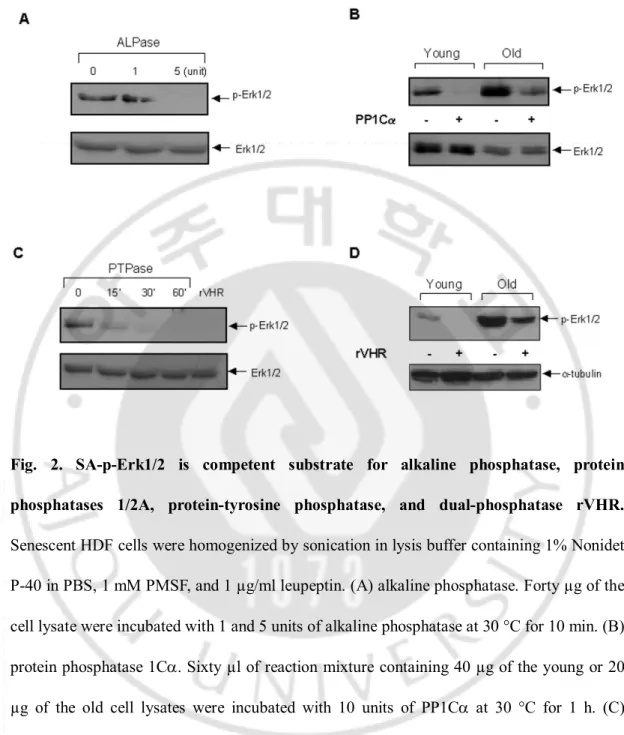
To discern phosphatases possiblyinvolved in the induction of SA-p-Erk1/2 in the old cells, wholehomogenates (40 µg) of the young and old HDF cells wereincubated with either a recombinant alkaline phosphatase (New England Biolabs, Beverly, MA, U.S.A.), protein phosphatase 1Ca (PP1Ca),phosphotyrosine phosphatase-b (PTP-b) (Upstate Biotechnology, Inc., Lake Placid,NY, U.S.A.), or recombinant vaccinia H1-related phosphatase (rVHR)
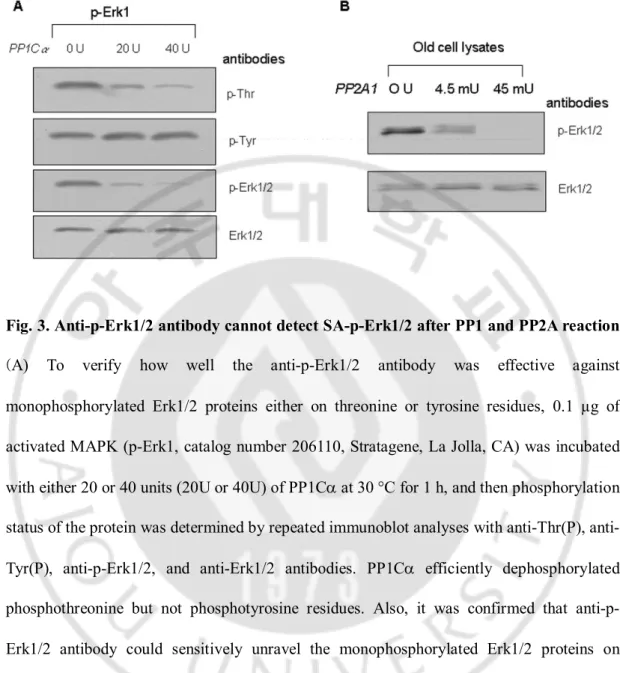
(generously donated by Dr. J. M. Denu at OregonHealth Sciences University), and the mixtures were analyzedby immunoblot with anti-p-Erk1/2 antibody. Five units of alkaline phosphatase (Fig. 2A), 10 units of PP1Ca (Fig. 2B), 20 unitsof PTP-b (Fig. 2C), and 0.2 ng of rVHR (Fig. 2D) completely removedphosphate from the p-Erk1/2 of the young cells after 30 minof incubation. On the other hand, both PP1Ca (10 units) andrVHR (0.2 ng) only partially removed phosphate in the oldcells under identical conditions as above (Fig. 2, B and D).These data indicate that SA-p-Erk1/2 might not only be regulatedby MAP kinase phosphatase (MKP) such as rVHR, but also by PP1 and PTP as well as thenonspecific alkaline phosphatase. In order to verify how well the anti-p-Erk1/2 antibody works against monophosphorylated Erk1/2 proteins either on threonine or tyrosine residue, 0.1 µg of activated MAPK (p-Erk1) was incubated with both20 and 40 units of PP1Ca at 30 °C for 1 h, and the phosphorylation status of the protein was then determined by repeated immunoblotanalyses with anti-Thr(P), Tyr(P), p-Erk1/2, and Erk1/2 antibodies.As shown in Fig. 3A, PP1Ca efficiently dephosphorylated Thr(P),but not Tyr(P) residue, confirming that anti-p-Erk1/2 antibodyreacted only with the -pTXpY-dual phosphorylated Erk1/2 proteins. Additionally, PP2A activity on p-Erk1/2 proteins was also examined by incubating the purified PP2A (Calbiochem, San Diego, CA, U.S.A.)with old cell lysates in 50 mM Tris-HCl (pH 7.4) containing2 mM EGTA, 0.1% b-mercaptoethanol, 1 mM PMSF, and 2 µg/ml leupeptin at 30 °C for 1 h. When the reaction mixture wasanalyzed by immunoblot with anti-p-Erk1/2 and Erk1/2 antibodies,PP2A was also found to be able to be dephosphorylated by p-Erk1/2(Fig. 3B). Therefore, these results suggest that not only thepurified PP1 but also the PP2A enzymes could efficiently regulatethe level of SA-p-Erk1/2, which was highly
Fig. 1. P-Erk1/2 Proteins were Markedly Induced but they were not Nuclear
Translocated by EGF Treatment in Old HDF Cells. Young (A) and old (D) cells were
homogenized in RIPA buffer, and 40 µg of the lysates were resolved on 10% SDS-PAGE and then immunoreacted with anti-p-Erk1/2 and a-tubulin antibodies, used as a loading control. Note the higher level of p-Erk1/2 in the old cells than the young cells. Young (B and C) and old (E and F) cells were treated with (C and F) or without (B and E) EGF (10 ng/ml) for 15 min, and immunocytochemistry with p-Erk1/2 antibody was performed to reveal the basal level of p-Erk1/2 between the young and old cells as well as its response to EGF treatment. Note the differences in the basal levels and the cell size between the young and old cells. Magnification, ×100.
Fig. 2. SA-p-Erk1/2 is competent substrate for alkaline phosphatase, protein phosphatases 1/2A, protein-tyrosine phosphatase, and dual-phosphatase rVHR.
Senescent HDF cells were homogenized by sonication in lysis buffer containing 1% Nonidet P-40 in PBS, 1 mM PMSF, and 1 µg/ml leupeptin. (A) alkaline phosphatase. Forty µg of the cell lysate were incubated with 1 and 5 units of alkaline phosphatase at 30 °C for 10 min. (B) protein phosphatase 1Ca. Sixty µl of reaction mixture containing 40 µg of the young or 20 µg of the old cell lysates were incubated with 10 units of PP1Ca at 30 °C for 1 h. (C) PTPase. The old cells were homogenized in the buffer containing 50 mM bis-Tris, 100 mM sodium acetate, 1 mM DTT, 0.01% bovine serum albumin, and 1% NP-40 (pH 7.0). The reaction mixture (60 µl) contained 20 units of PTP-b and 40 µg of the cell lysate, and was incubated at 30 °C up to 60 min. rVHR (0.2 µg) was added to the assay mixture as a positive
control. (D) rVHR. The young and old cells were homogenized in the lysis buffer, which was used as above for the PTPase. The assay mixture (60 µl), containing 40 µg of the cell lysate and 0.2 µg of rVHR, was incubated at 30 °C for 1 h. The reaction mixtures were resolved on 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane for immunoblot analysis with anti-p-Erk1/2 antibody. Expressions of Erk1/2 and a-tubulin were used for loading control.
Fig. 3. Anti-p-Erk1/2 antibody cannot detect SA-p-Erk1/2 after PP1 and PP2A reaction.
(A) To verify how well the anti-p-Erk1/2 antibody was effective against monophosphorylated Erk1/2 proteins either on threonine or tyrosine residues, 0.1 µg of activated MAPK (p-Erk1, catalog number 206110, Stratagene, La Jolla, CA) was incubated with either 20 or 40 units (20U or 40U) of PP1Ca at 30 °C for 1 h, and then phosphorylation status of the protein was determined by repeated immunoblot analyses with Thr(P), anti-Tyr(P), anti-p-Erk1/2, and anti-Erk1/2 antibodies. PP1Ca efficiently dephosphorylated phosphothreonine but not phosphotyrosine residues. Also, it was confirmed that anti-p-Erk1/2 antibody could sensitively unravel the monophosphorylated anti-p-Erk1/2 proteins on immunoblot analysis. (B) The activity of the purified PP2A was examined with 40 µg of old HDF cell lysates in the reaction mixture, containing 50 mM Tris-HCl (pH 7.4), 2 mM EGTA, 0.1% b-mercaptoethanol, 1 mM PMSF, and 2 µg/ml leupeptin, at 30 °C for 1 hr. Immunoblot analyses were done with anti p-Erk1/2 and Erk1/2 antibodies. PP2A also specifically dephosphorylated SA-p-Erk1/2 proteins that were increased in the old cells.
C. Activity of PP1/2A were Significantly Reduced duringCellular Senescence.
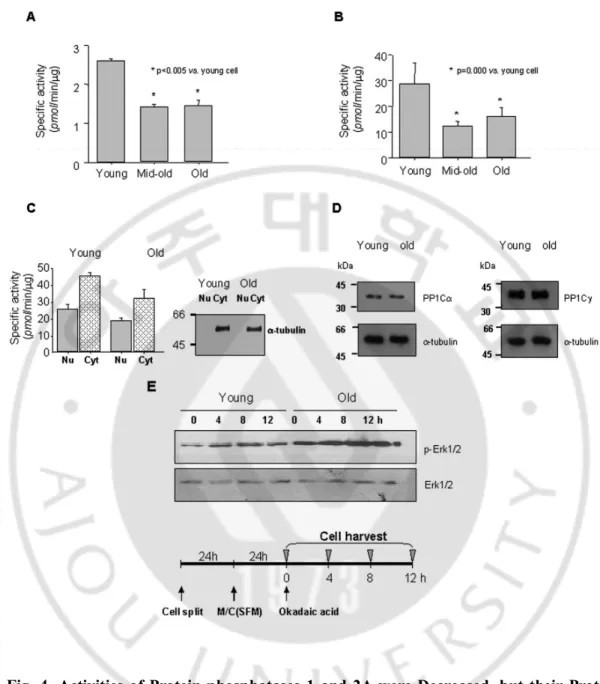
To investigate whether activities of phosphatases were altered during cellular senescence or not,alkaline phosphatase and PP1/2A were measured with the homogenatesof the young, mid-old, and old HDF cells. As illustrated inFig. 4, alkaline phosphatase activity was 2.61 ± 0.05,1.43 ± 0.06, and 1.45 ± 0.14 pmol of phosphate/min/µgof protein (Fig. 4A), and PP1/2A activities were 28.78 ± 8.05, 12.50 ± 1.59, and 16.03 ± 3.33 pmol of phosphate/min/µgof protein in the young, mid-old, and old cells (Fig. 4B), respectively. Both phosphatases activities were already significantly depressedin the mid-old cells as compared with the young cells.
Because we are herein primarily concerned with the trafficking of Erk1/2 between nucleus and cytoplasm and their phosphorylation status, we determined the changes of protein phosphatase activitiesin these two subcellular fractions during cellular senescence. Thus, nuclear and cytoplasmic fractions were prepared from theyoung and old cells, and possible contamination of nuclear fraction with cytoplasm was first determined by expression of a-tubulin.As shown in the right panel of Fig. 4C, the nuclear preparationwas free of a-tubulin component. With these preparations, boththe nuclear and cytoplasmic PP1/2A activities were found tobe decreased more in the old than in the young cells (left
panel).The possibility that the decrease of PP1/2A activity in theold cells was due to the less expression of catalytic PP1 subunitwas investigated by immunoblot analysis with anti-PP1Ca andanti-PP1Cg antibodies (Fig. 4D); however, no difference was found betweenthe young and old cells.
Next, in order to evaluate the specific effect of PP2A on theSA-p-Erk1/2 in vivo, the young and old HDF cells were treatedwith 40 nM okadaic acid, a specific PP2A inhibitor (Takai, 1988; Cohen, 1989; Wera and Hemmings, 1995). Thus, cells were harvested at 4, 8, and 12 h aftertreatment, and cell lysates were analyzed by immunoblotanalysis. As shown in Fig. 4E, inhibition of PP2A with okadaicacid significantly increased phosphorylation levels of Erk1/2proteins in both the young and old cells, strongly indicatingthat the change of PP1 and PP2A activities had significant effectson the SA-p-Erk1/2 in both young and old cells. In order tocompare the expression and their activities of PP1/2A in the young and old cells, total and specificactivity (pmol/min/µg) of PP1/2A, and amount of protein per cell were measured with16,500 cells. As shown in Table 1, the old cellshad 4 times more protein and 2.5 times higher total PP1/2A activityas well as much larger cell size than the young cells. Therefore,the specific activity in the old cells was only 60% of the youngcells, indicating that specific activity of PP1/2A was significantly decreased during cellular senescence.
Fig. 4. Activities of Protein phosphatases 1 and 2A were Decreased, but their Protein
Expression Levels were not changed During Cellular Senescence. (A) Significant
reduction of alkaline phosphatase activity in the mid-old HDF cells. Phosphatase activity was assayed according to the method described under "Material and Methods". Note statistically significant reduction of the activity in the mid-old and old cells. (B) Significant
decrease of PP1/2A activities in the mid-old and old cells as compared with the young cells. (C) Nuclear (Nu) and cytoplasmic (Cyt) distribution of PP1/2A activities in the young and old HDF cells. Nuclear and cytoplasmic fractions were prepared from the young and old cells, and PP1/2A activities were measured as described under "Experimental Procedures." To ascertain the purity of the nuclear fractions used for PP1/2A assay, a-tubulin immunoblot analysis was performed as a cytoplasmic marker. (D) Protein expression of PP1 isozyme in the young and old HDF cells. Immunoblot analyses with anti-PP1Ca and PP1Cg antibodies were performed with the whole cell lysates of the young and old HDF cells. The rest of the procedures were the same as described under "Experimental Procedures." (E) Inhibition of PP2A activity by okadaic acid increased p-Erk1/2 levels in both young and old cells. The young and old cells were plated, and serum-free medium was added 24 h later, and the cells were then cultured for another 24 h. Okadaic acid (40 nM) was added to the culture medium, and the cells were harvested at 0, 4, 8, and 12 h after the treatment. Cells lysates were immunologically analyzed as described under " Material and Methods".
Table 1. Comparison of protein phosphatase 1 and 2A expressed in young and old HDF cells
D. ROS was the Mechanism of Reduction of PP1/2A Activities during Cellular Senescence.
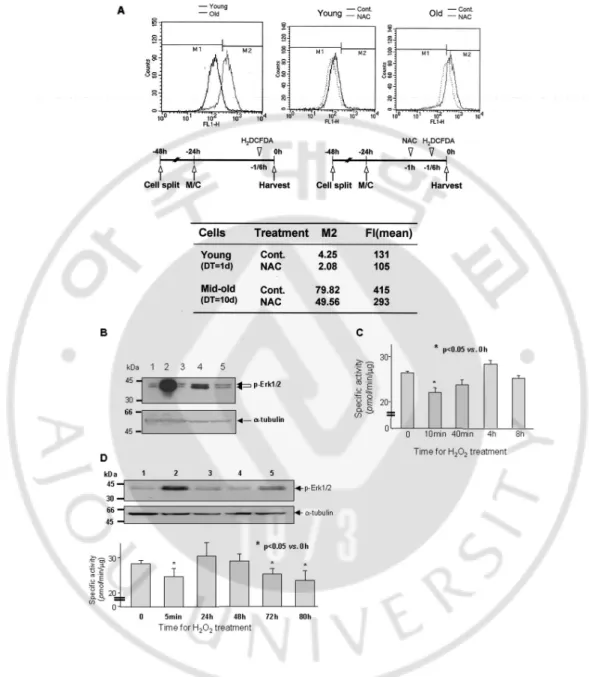
In order to furtherinvestigate the mechanism underlying the significant reduction of PP1/2A activities and elevated p-Erk1/2 level during cellularsenescence, ROS generation was measured during cellular senescenceby FACS analysis after incubating both the young and mid-oldHDF cells with H2-DCFDA. As shown in Fig. 5A, the mid-old cellscontained
about 20 times more ROS than the young cells, andthe ROS generation by both could efficiently be inhibited bypretreatment of the cells with 10 mM NAC. When young HDF cells were treatedonce with 1 mM H2O2, Erk1/2 phosphorylation was markedly increasedat
10 min, however, the increase was greatly attenuated in 40min. At the same time, when the cells were pretreated with 10 mM NAC for 1hr, phosphorylation of Erk1/2 protein was efficientlyinhibited (Fig. 5B). To discern whether H2O2 was responsiblefor the reduction of
PP1/2A activities or not, the enzyme activitieswere measured after treatment of the young cells once with 200µM H2O2. As seen in Fig. 5C, the enzyme activities weretransiently
inhibited in 10 min, but started to recover 40 minlater and finally returned to the control level in 8 h. To furtherinvestigate the regulation of p-Erk1/2 level and activity ofPP1/2A by H2O2 treatment, we measured the changes of the abovetwo parameters by immunoblot
analysis and the activity assay,respectively. As shown in Fig. 5D, when the young cells were repeatedly treated with 200 µM H2O2 at every 8 h, PP1/2Aactivities remained significantly
inhibited after 72 h; control HDF young cells had specific activity of 28.31 ± 0.97 pmol/min/µg protein, and it changed to 24.63 ±2.35, 30.50 ± 3.81, 29.06 ± 2.08, 25.38 ±
1.54, and 23.63 ± 2.68 pmol/min/µg protein at 5min, 24, 48, 72, and 80 h, respectively. Concomitant with thechanges of PP1/2A activities, phosphorylation of Erk1/2 proteinswas simultaneously affected by H2O2 treatment. These data indicatethat repeated damage of the
young cells by H2O2 exposure persistently inhibited PP1/2A activities, resulting in the
accompanying inductionof p-Erk1/2 levels.
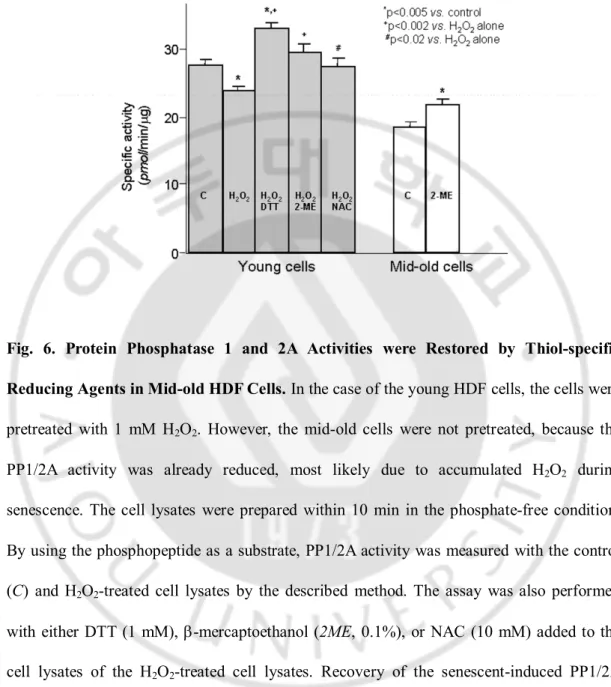
To elucidate whether decrease of PP1/2A activities in the mid-oldcells was due to the oxidation of cysteine residues in the enzyme molecules or not, thiol-specific reducing agents ,such as DTT,b-mercaptoethanol, and NAC, were added to the lysates of the young HDF cells, which had been pretreated with H2O2 for 10 min. Asshown in the left panel of
Fig. 6, the control PP1/2A activitywas decreased from 27.96 ± 0.76 to 24.04 ± 0.63 pmol/min/µg protein (p < 0.005) by the treatment withH2O2. However, the inhibition was
reversed to 33.38 ±0.66, 29.79 ± 1.26, and 27.63 ± 1.32 pmol/min/µgprotein by the addition of DTT, b-mercaptoethanol, and NAC, respectively. All three reagents significantly reactivated PP1/2A activityas compared with that of the H2O2 alone treatment (+, p <0.002,
and #, p < 0.02). However, only DTT, but not b-mercaptoethanoland NAC, significantly
increased PP1/2A activity of the youngcells more than the control (p < 0.005). Interestingly, whenthe assay was carried out with mid-old cell lysates, whose PP1/2Aactivity had already been reduced, most likely due to accumulatedH2O2 during senescence, b-mercaptoethanol
could significantlyincrease PP1/2A activity, as compared with the control (22.04± 0.80
versus 18.71 ± 0.76 pmol/min/µg protein,p < 0.005, right panel in Fig. 6), thus strongly
suggestingthat the high level of ROS found in senescent cells oxidizedthiol residues, which are important for phosphatase activity.
Fig. 5. Reactive oxygen species generated during cellular senescence-regulated PP1/2A activities and p-Erk1/2 levels. (A) Marked difference of ROS level between young and
mid-old HDF cells, measured by FACS analysis. The young and mid-old cells were seeded into culture dishes up to 25 and 70%, respectively. After 24 h, the monolayer was re-fed with
complete medium and then treated with H2DCF-DA 10 min prior to cell harvest. The cells
were pretreated with NAC 1 h prior to harvest and treated with H2DCF-DA for 10 min
before the harvest. Changes of fluorescence by generated ROS were measured by FACS analysis. Note 19-fold (4.25 versus 79.82) higher ROS in the mid-old cells than the young cells. (B) Treatment of HDF young cells with 1 mM H2O2 significantly increased Erk1/2
phosphorylation, but it was efficiently blocked by NAC pretreatment. Control HDF cells (lane 1) were treated with 1 mM H2O2 for 10 (lane 2) and for 40 min (lane 4), pretreated for
1 h with 10 mM NAC, and then treated with H2O2 for 10 (lane 3) and for 40 min (lane 5).
The cells were harvested, and p-Erk1/2 expression level was measured by immunoblot analysis. (C) To investigate whether ROS regulated PP1/2A activity or not, HDF young cells were treated once with 200 µM H2O2. The enzyme activity was transiently inhibited in 10
min; however, it was recovered in 40 min and then returned to control level in 8 h. D, simultaneous regulation of PP1/2A activities and Erk1/2 phosphorylation of young HDF cells by repetitive treatment with H2O2. Young HDF cells were treated with 200 µM H2O2
every 8 h and then harvested after 0 min (lane 1), 5 min (lane 2), 24 h (lane 3), 48 h (lane 4), 72 h (lane 5), and 80 h. The cell lysates were used for p-Erk1/2 immunoblot analysis (upper
panel) and PP1/2A activity assay (lower panel). Note the reciprocal changes of p-Erk1/2
(increase) and the PP1/2A activities (decrease) at 5 min. The reciprocal change was consistent by repeated treatments for 72 h.
Fig. 6. Protein Phosphatase 1 and 2A Activities were Restored by Thiol-specific Reducing Agents in Mid-old HDF Cells. In the case of the young HDF cells, the cells were
pretreated with 1 mM H2O2. However, the mid-old cells were not pretreated, because the
PP1/2A activity was already reduced, most likely due to accumulated H2O2 during
senescence. The cell lysates were prepared within 10 min in the phosphate-free condition. By using the phosphopeptide as a substrate, PP1/2A activity was measured with the control (C) and H2O2-treated cell lysates by the described method. The assay was also performed
with either DTT (1 mM), b-mercaptoethanol (2ME, 0.1%), or NAC (10 mM) added to the cell lysates of the H2O2-treated cell lysates. Recovery of the senescent-induced PP1/2A
activity was also evaluated with the mid-old cell lysates by addition of 0.1% b-mercaptoethanol to the control cell lysate. All the samples were triplicated, and the assays were performed more than three times.
E. TPA Treatment Reverses Senescence Phenotype
For cell proliferation, nuclear translocation of activated Erk1/2 is important. To investigate the consequence of nuclear translocation of p-Erk1/2, young and old HDF cells were treated with either TPA. As shown in Fig. 7, only TPA treatment induced nuclear translocation of p-Erk1/2 in young and old cells.
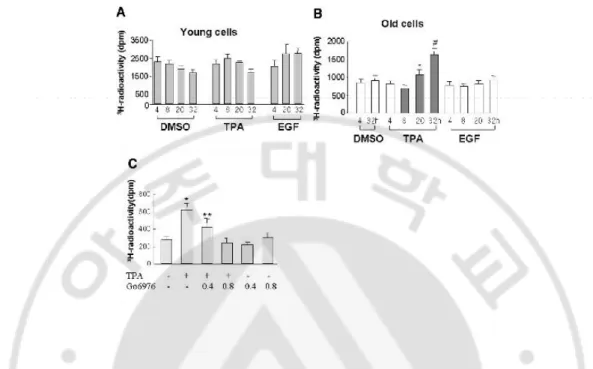
To elucidate the effect of TPA-induced cell cycle progression, [3H]-thymidine
incorporationassay was performed, and statistically significant inductionof DNA synthesis was found only in the old cells in 20 h (Fig.8B) as compared with no response in the young cells (Fig. 8A).Furthermore, when added to old HDF cells, the proteinkinase C (PKC) inhibitor, Go6976, inhibited significantly TPA-induced[3H]-thymidine incorporation (Fig.
8C), indicating an important role of PKC played in the nuclear translocation of p-Erk1/2
and induction ofDNA synthesis only in the senescent cells.
In addition to [3H]-thymidine incorporation, the expressions of severalother senescence
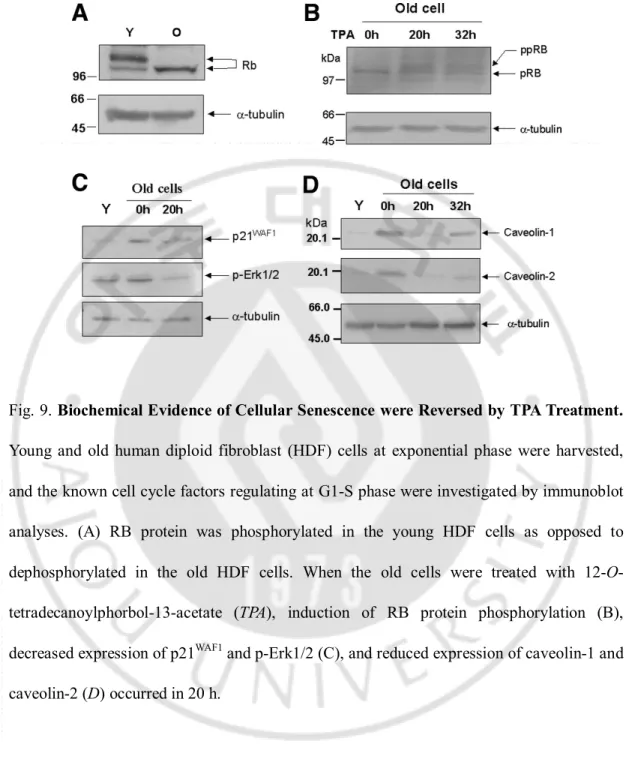
markers, including RB phosphorylation (Fig. 9B), induction of p21WAF1 and p-Erk1/2
expressions (Fig. 9C),and increased caveolin-1 and caveolin-2 expressions (Fig. 9D) were found to be reversed by TPA treatment for 20h. RB protein in the senescent HDF wasmostly dephosphorylated as compared with that in the young cells(Fig. 9A). These results are in accordance with previous findingsthat phosphorylated and under-phosphorylated forms of RB proteins are found in young cells, whereas only under-phosphorylatedRB protein is found in senescent cells (Futreal and Barrett, 1991) and in the long-termconfluent cultures (Munro et al., 2001). These findings strongly indicate thatTPA changednot only the old
morphologic phenotypes but also biochemicalmarkers.
F. TPA Activates PKC and Induces Translocation
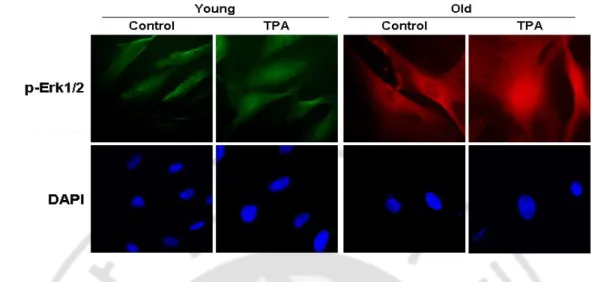
As TPA is well known as a PKC activator, PKC isozymes (a, -bI, -bII, -g, -d, -e, -h, -z) were investigated to find out which isozymes were activated by TPA treatment in HDF cells. As seen in Fig. 10A, PKCa and bI were activated and translocated from soluble fraction to particulate fraction in young and old HDF. Most senescent cells were arrested in G0/G1 cell cycle. The biological responses obtained by the PKCa activation are cell type specific. For example, overexpression of PKCa promotes proliferation in some types of cells (Ways et al., 1995; Mandil et al., 2001), but caused G1 cell cycle arrest (Sasaguri et al., 1993; Frey et al., 1997; Besson and Yong, 2000; Frey et al., 2000) and differentiation in other types. Therefore, we focused on the roles of PKCa in TPA-induced reversal of senescence phenotype and in senescence process. Also activated PKCa is known to translocate from cytoplasm to nucleus (Schmalz et al., 1998; Vijayan et al., 2004; Kenessey et al., 2006), therefore, we investigated intracellular localization change of PKCa with TPA treatment. As shown in Fig. 10B, TPA induced nuclear translocation of PKCa, and it was also confirmed by subcellular fractionation and immunoblot analysis (Fig. 10C). Therefore, TPA induced translocation of PKCa and p-Erk1/2.
Fig. 7. TPA treatment induces nuclear translocation of p-Erk1/2. Immunofluorescence
findings showing the nuclear translocation of p-Erk1/2 by 12-O-tetradecanoylphorbol-13-acetate (TPA). Young and old human diploid fibroblast cells were treated with TPA or vehicle (DMSO). The cells were fixed for 15 mins after treatment and immunocytochemistry was performed to show translocation of p-Erk1/2 to nuclei.
Fig. 8. 12-O-tetradecanoylphorbol-13-acetate (TPA) induced DNA synthesis only in the old, but not in the young, human diploid fibroblast cells. (A) Young cells were treated
with epidermal growth factor (EGF), TPA, or DMSO when the cell density reached 70%, and they were then incubated with [3H]-thymidine (2 µCi/ml) for 4 hr before harvest at the
indicated times. (B) Mid-old (doubling time, 10 days) cells with 70% cell density were stabilized for 48 h before being subjected to [3H]-thymidine incorporation assay. Note
significantly increased DNA synthesis after 20 h of TPA treatment (*P = 0.024 versus TPA 8 h; #P < 0.01 versus all other samples). (C) To evaluate the role of protein kinase C (PKC),
Go6976 (0.4 and 0.8 µM) was added to the mid-old cells, which were stabilized for 48 h, and the concentration-dependent inhibition of DNA synthesis was measured by the method described earlier. *P < 0.001 versus all other samples; **P < 0.005 versus all other samples.
Fig. 9. Biochemical Evidence of Cellular Senescence were Reversed by TPA Treatment. Young and old human diploid fibroblast (HDF) cells at exponential phase were harvested, and the known cell cycle factors regulating at G1-S phase were investigated by immunoblot analyses. (A) RB protein was phosphorylated in the young HDF cells as opposed to dephosphorylated in the old HDF cells. When the old cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), induction of RB protein phosphorylation (B), decreased expression of p21WAF1 and p-Erk1/2 (C), and reduced expression of caveolin-1 and
Fig. 10. TPA activated PKCa and induced nuclear translocation. (A) TPA activated PKC a and bI isozymes in young and old cells. Young and old cells were treated with 50 ng/ml TPA ,and soluble(S) and particulate(P) fractionation wasperformed after 15min. Thirty mg of fractionated protein were resolved on 8% SDS-PAGE and then hybridized with anti-PKCa and anti-PKCbI antibodies. (B) Immunocytochemistry for intracellular localization of PKCa was preformed. By TPA treatment, activated PKCa was translocated into nucleus in young
and old HDF. Young and mid-old cells were treated with 50 ng/ml TPA for 15 min. (C) Nuclear translocations of PKCa and p-Erk1/2 were simultaneously induced by TPA treatment. Young and mid-old cells were treated with TPA (50 ng/ml, 15 min), and nuclear(N) and cytosolic(C) fractionation was performed. TPA treatment induced nuclear translocation of PKC a and p-Erk1/2. Lamin was used as a nuclear fraction control.
G. Activated PKC a interacts with p-Erk1/2
As TPA induced both PKCa and Erk1/2 activation and nuclear translocation, we next investigated the possibility of interaction between PKCa and p-Erk1/2 (Fig. 11). Fifteen minutes after TPA treatment, interaction between PKCa and p-Erk1/2 was increased. However, 4 hr later, their interaction was normalized (Fig. 11A). There is a report that insulin-like growth factor-I induces interaction of PKCe with Erk 2 in MCF-7 cells (Bourbon et al., 2001). In the present study, we also demonstrated that activated PKCa increasingly bound to p-Erk1/2 after TPA treatment (Fig. 11A), and its vice versa (Fig. 11B).
H. Increased ROS level in old cells activates PKC
Since TPA is known not only as a PKC activator and a down-regulator of PKC (Lindner et al., 1991; Li et al., 1998; Lu et al., 1998) as well as reversal of senescence phenotype in 20 hr after TPA treatment, we measured PKC a expression level (Fig. 12). PKCa in the old cells was regulated from 8 hr after TPA treatment. p-Erk1/2 level was also down-regulated from 12 hr after TPA treatment. From these results, we suggested that the expression and activity of PKCa could be related with maintenance of senescence phenotype. To investigate whether there is any change in PKCa expression level or activity, young, mid-old, and old HDF cells were used for PKCa immunoblot analyses (Fig. 13A). Protein expression level was not significantly changed between young and old cells, however, activity of PKCa activity was significantly higher in the mid-old cells than young