의
의
의학
학
학 박
박
박사
사학
사
학
학위
위
위 논
논
논문
문
문
Reduction of tumorigenesis in androgen
receptor overexpressed Huh7 cells
through TGF-β
β
β
β1 induced cellular
senescence phenotypes
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
의
의
의 학
학
학 과
과
과
김
김
김 지
지
지 연
연
연
Reduction of tumorigenesis in androgen receptor
overexpressed Huh7 cells through TGF-β
β
β
β1 induced
cellular senescence phenotypes
by
Ji Yeon Kim
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the
Degree of
DOCTOR OF MEDICAL SCIENCE
Supervised by
In Kyoung Lim, M.D., Ph.D.
Department
Department
Department
Department of
of
of
of Medical
Medical
Medical Sciences
Medical
Sciences
Sciences
Sciences
The
The
The
The Graduate
Graduate
Graduate
Graduate School,
School,
School,
School, Ajou
Ajou
Ajou
Ajou University
University
University
University
김
김
김지
지
지연
연
연의
의 의
의
의
의학
학
학 박
박
박사
사
사학
학
학위
위
위 논
논
논문
문
문을
을
을 인
인
인준
준
준함
함
함.
.
.
심
심
심사
사위
사
위
위원
원
원장
장
장
이 재
이
이
재
재 호
호
호
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
송
송
송 계
계
계 용
용
용
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
윤
윤
윤 계
계
계 순
순
순
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
박
박
박 태
태
태 준
준
준
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
임
임
임 인
인
인 경
경
경
인
인
인
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
2
2
20
0
00
0
06
6
6년
년
년 1
1
12
2
2월
월
월 2
2
22
2
2일
일
일
-ABSTRACT-
Reduction of tumorigenesis in androgen receptor overexpressed
Huh7 cells through TGF-
β1 induced cellular senescence phenotypes
Purpose: Hepatocellular carcinoma (HCC) is a primary malignancy (cancer) of the liver.
HCC has been known as androgen-dependent tumor with incidence of five times higher and worse prognosis in male than female. Steroid hormones and their receptors play a major role in the development of many types of human and animal cancers. It has been known that androgen and its receptor (AR) are significantly related with hepatocarcinogenesis both in human and animals, however, the level of AR in hepatoma is variable and their exact effects are still poorly explained. Therefore, we investigated the role of androgen receptor (AR) in HCC development.
Method: To investigate the potential role of AR in hepatoma, Huh7-AR and Huh7-V cell
lines were established by transfection of Huh7 hepatocelluar carcinoma (HCC) cells with human androgen receptor (AR) in pCMV5 and its empty vector, respectively. And their in vivo and in vitro tumorigenesis were evaluated in nude mice as well as in cell culture systems.
Results: AR overexpressing Huh7 cells, Huh7-AR, and its control, Huh7-V cells, were
established. AR expression was measured by RT-PCR and immunoblot analyses. Huh7-AR cells, which were responsive to 5α-dihydrotestosterone (DHT), revealed a decreased tumorigenecity in nude mice in addition to reduced clonogenicity in vitro, as compared with
those of Huh7-V cells; Tumor volume and multiplicity of the HCC development in nude mice and invasiveness of the Huh7-AR cells, measured by Matrigel chamber analysis, were significantly reduced than those of Huh7-V cells. Interestingly, when cultured in the presence of DHT, Huh7-AR cells induced cellular senescence phenotypes, such as SA- β-galactosidase activity, nuclear actin translocation, cytoplasmic p-Erk1/2 sequestration and reduced telomerase activity. When underlying mechanisms were studied in more detail in the cell culture system with or without DHT, TGF-β1 mRNA expression was increased in the Huh7-AR cells and HCC tumors in the presence of DHT. At the same time, secretion of TGF-β1 was significantly higher in the Huh7-AR cells than that in Huh7-V cells. The effect of TGF-β1 on the induction of senescence of Huh7-AR cells was studied by infection of dominant negative TGFβ-RII adenovirus (TGFβRII DN-Ad); The reduced growth rate and other cellular senescence phenotypes were significantly recovered after TGFβRII DN-Ad infection, compared with the infection of its control viruses.
The above data strongly indicate that androgen receptor inhibits progression of HCC formation by Huh7-AR cells through the induction of senescence phenotypes via TGF-β1 expression in the presence of DHT.
Key words:
Huh7 hepatoma cells, Androgen receptor, Dihydrotestosterone, TGF-β1, Cellular senescenceTABLE OF CONTENTS
ABSTRACT ···i
TABLE OF CONTENTS ···iii
LIST OF FIGURES ···v
Ⅰ. INTRODUCTION ···1
A. Androgen receptor ···1
B. TGF-β signaling ···4
C. Cellular senescence ···6
D. Purpose of this study ···7
Ⅱ. MATERIALS AND METHODS ···9
A. Preparation of Huh7-AR cells and treatment ···9
B. Isolation of RNA and RT-PCR ···9
C. Assessment of androgen response of Huh7-AR cells by using MMTV promoter ···10
D. Immunocytochemistry ···11
E. Measurement of growth rate by cell counting ···11
F. Assay of tumorigenicity ···12
G. Tumor formation in nude mice and immunohistochemistry ···13
H. Immunoblot analysis ···13
I. Assay of senescence associated β-galactosidase ···14
K. Measurement of TGF-β1 secretion by MTT assay···15
L. Infection of dominant negative TGFβRII adenovirus ···16
M. Statistics ···16
Ⅲ. RESULTS ···17
A. Androgen receptor expression and androgen responsiveness of Huh7-AR cells ··17
B. Reduced tumorigenesis of Huh7-AR cells in vivo and in vitro ···18
C. Dihydrotestosterone induced senescence of Huh7-AR cells, negative regulation of tumorigenesis ···28
D. Increased TGF-β1 expression in HCCs by Huh7-AR cells in nude mice ··· ···28
E. Increase of TGF- β1 secretion in Huh7-AR cells ···29
F. Increased p27KIP1 expression in Huh7-AR cells, but decreased in cytoplasm of Huh7-AR cells after treatment with DHT ···36
G. Decreased c-myc, β-catenin and cyclin D1 expression in Huh7-AR cells after DHT treatment ···36
H. Recovery of senescence phenotypes in Huh7-AR cells by adenovirus infection of dominant negative TGFβRII construct ···41
Ⅳ. DISUSSION ···45
Ⅴ. CONCLUSION ···49
REFERENCE···51
LIST OF FIGURES
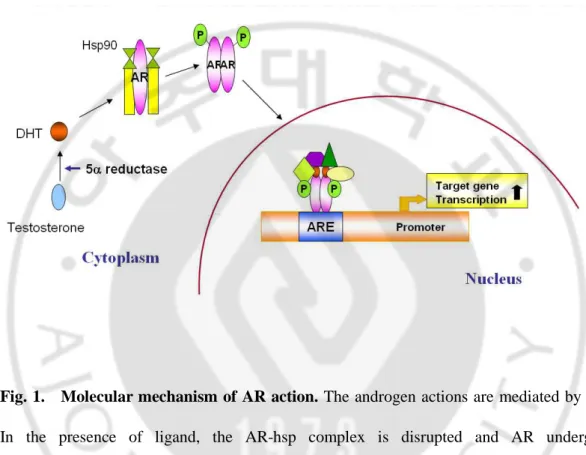
Fig. 1. Molecular mechanism of AR action ···3
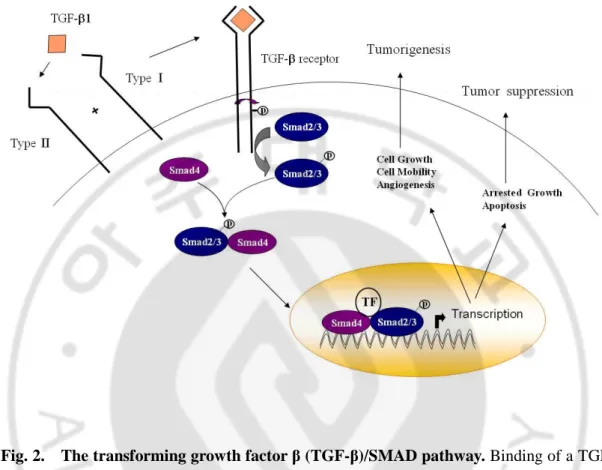
Fig. 2. The transforming growth factor β (TGF- β)/SMAD pathway ···8
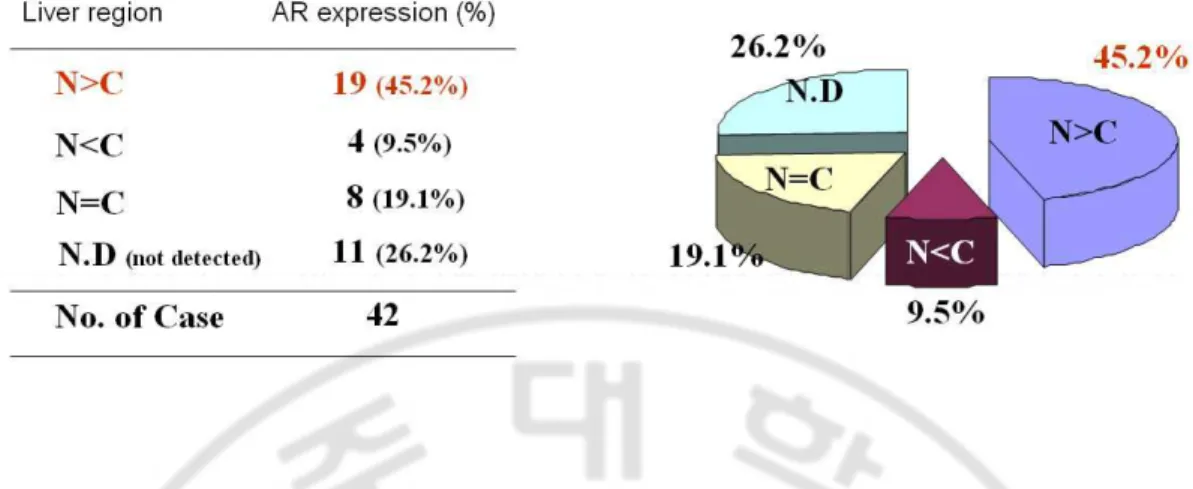
Fig. 3. AR expression in human livers was analyzed by RT-PCR···19
Fig. 4. Expression of androgen receptor in Huh7-AR cells ···20
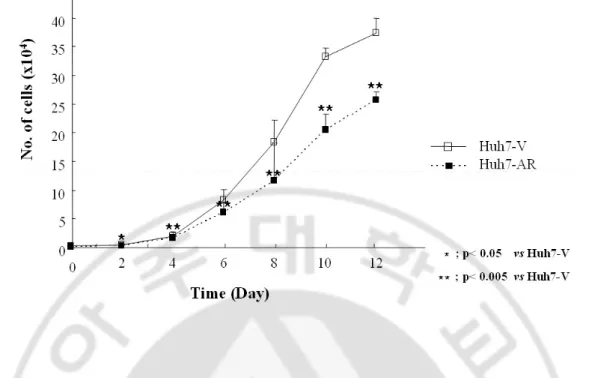
Fig. 5. Growth rate of Huh7- AR cells was decreased ···23
Fig. 6. Reduced tumorigenesis of Huh7-AR cells in vitro ···24
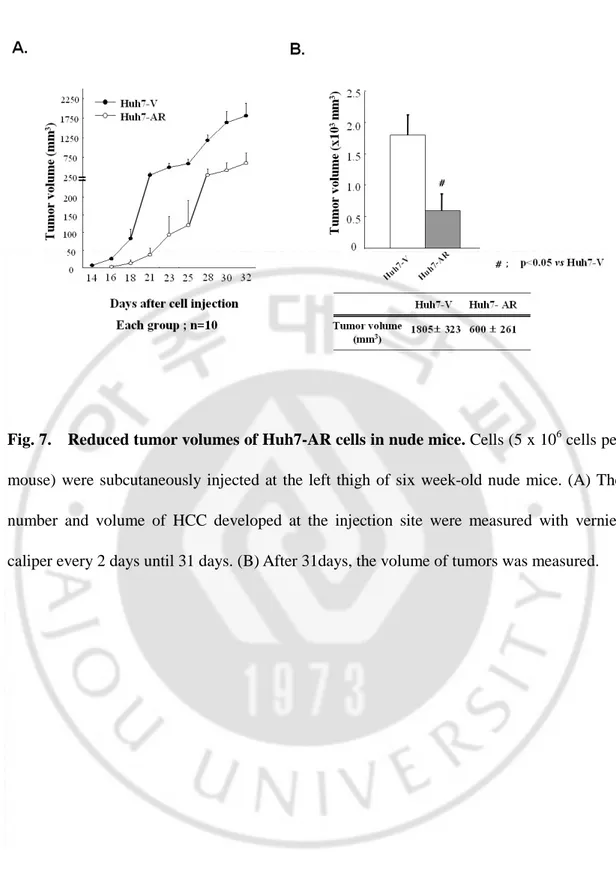
Fig. 7. Reduced tumor volumes of Huh7-AR cells in nude mice ···26
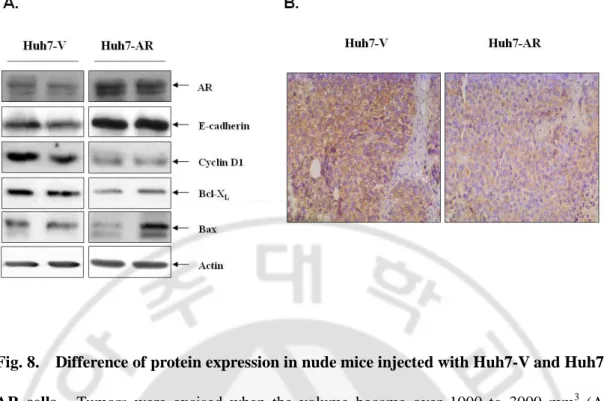
Fig. 8. Difference of protein expression in nude mice injected with Huh7-V and Huh7-AR cells ···27
Fig. 9. AR induced senescence phenotype ···30
Fig. 10. TGF- β1 expression was measured by RT-PCR···33
Fig. 11. Secretion of TGF- β1 in Huh7-AR cells ···35
Fig. 12. Increased p27KIP1 expression in Huh7-AR cells, but decreased its phosphorylation in cytoplasm of Huh7-AR cells after treatment with DHT ···38
Fig. 13. Decreased c-myc expression in Huh7- AR cells after DHT treatment ··· ···39
Fig. 14. Decreased cyclin D1 and β-catenin expression in Huh7-AR cells after DHT treasment···40
Fig. 15. Recovery of senescence phenotype in Huh7-AR cells after dominant negative TGFβRII adenovirus infection ···42
Fig. 16. Mechanism to decrease tumorigenesis of AR overexpression in Huh7 cells.
Ⅰ
Ⅰ
Ⅰ
Ⅰ. INTRODUCTION
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver. The cause of liver cancer usually includes cirrhosis or scarring of the liver. Cirrhosis may be caused by viral hepatitis, primarily hepatitis B and C, alcohol abuse, hemochromatosis, certain autoimmune diseases of the liver, and a whole host of other diseases that result in chronic inflammation of the liver, leading to scarring.
HCC has been known as androgen-dependent tumor with incidence of five times higher and worse prognosis in male than female (Ricca-Rosellini and Miglio, 1991; Tarao et al., 1993). Different gender frequency of HCC has been known to be due to the differences of sex hormones and/or the different status of hormone receptors in liver cells.
A. Androgen receptor
Steroid hormones and their receptors play a major role in the development of many types of human and animal cancers. Steroid receptors are coupled to heat – shock proteins and sequestered to the cytoplasm in the absence of ligand (Tsai and O'Malley, 1994). The classical steroid receptors are androgen receptor (AR), estrogen receptor (ER), progesterone receptor (PR), glucocorticoid receptor (GR), and mineralcorticoid receptor (MR). The AR is a member of the nuclear receptor superfamily of ligand dependent transcription factors. AR can be divided into four functional domains: the NH2-terminal transactivation domain (or
A/B domain), the DNA-binding domain (DBD), hinge region, and ligand-binding domain (LBD). An NH2-terminal activation function (AF-1) functions in a ligand-independent manner when artificially separated from the LBD, creating a constitutively active receptor. A ligand-dependent AF-2 function is located in the LBD, and mutation or deletion of the AF-2 domain dramatically reduces transcriptional activation in response to ligand.
Androgens mediate a wide range of developmental and physiological responses, and are especially important in male sexual differentiation, pubertal sexual maturation, the maintenance of spermatogenesis, and male gonadotropin regulation (Roy et al., 1999; Sheckter et al., 1989; McLachlan et al., 1996). The effects of androgens are mediated through the androgen receptor (AR), a 110-kDa ligand-inducible nuclear receptor that regulates the expression of target genes through binding to an androgen response element (Chang et al., 1988; Tilley et al., 1989). In the presence of the ligand testosterone and its more potent derivative dihydrotestosterone (DHT), the AR-hsp complex is disrupted and the AR undergoes conformational change that allows phosphorylation, dimerization, and translocation of the more stable ligand-receptor complex to the nucleus (Wong et al., 1993; Kemppainen et al., 1992). In nucleus, the dimerized AR-ligand complex interacts with coactivator molecules (e.g., ARA54, ARA70) (Kang et al., 1999) and initiates gene transcription of androgen response elements (AREs) (Fig. 1). AR binds the male sex steroid hormones, dihydrotestosterone (DHT) and testosterone, and regulates genes for male differentiation and development. The presence of an AR within the liver has increasingly attracted attention, especially with respect to its relationship with HCC.
Many studies suggest that HCC is an androgen-dependent tumor with an incidence five times higher in males, but few data are available on the AR mRNA levels in different physiological classes of human liver specimens (Tavian et al., 2002). The presence of androgen receptors within the tumor was significantly related to a smaller tumor size (Boix
et al., 1995).
Fig. 1. Molecular mechanism of AR action. The androgen actions are mediated by AR.
In the presence of ligand, the AR-hsp complex is disrupted and AR undergoes conformational change and translocation of the more stable ligand-receptor complex. In nucleus, the dimerized AR-ligand complex interacts with coactivator molecules (e.g., ARA54, ARA70) and initiates gene transcription of androgen response elements (AREs).
B. TGF-β signaling
The regulation of liver growth depends on the balance between stimulatory and suppressor factors. Among these are the hepatocyte growth factor (Zarnegar and Michalopoulos, 1989; Nakamura et al., 1989), epidermal growth factor (McGowan et al., 1981), and transforming growth factor-α (Bachem et al., 1989) (all of which stimulate cell proliferation), whereas transforming growth factor-β (TGF-β) is a potent inhibitor of epithelial cell growth.
The transforming growth factor-β (TGF-β) family, a potent modulator of the phenotype of epithelial cells (Massague, 1998), consists of three highly homologous genes in humans: TGF-β1, TGF-β2, and TGF-β3. These genes inhibit the growth of many cell types, including epithelial cells; however, they also stimulate proliferation of various mesenchymal cell types (Roberts and Sporn, 1992). Elucidation of the mechanism by which epithelial cells lose their sensitivities to the regulatory action of TGF-β during carcinogenesis remains to be explored (Sporn and Roberts, 1992). In addition to its overexpression in aged animal (Zhao et al., 2002), the involvement of TGF-β1 in the aging and senescent process has recently been demonstrated by its direct induction of cellular senescence in a few cell lines, such as human diploid fibroblasts, mammary epithelial cells, and lung carcinoma cells (Kordon et al., 1995; Katakura et al., 1999; Frippiat et al., 2001), Also, TGF-β1 has been found to induce cell death in hepatocytes and hepatoma cell lines through regulation of the cell cycle (Alexandrow and Moses, 1995; Fan et al., 1996; Fukuda et al., 1993; Oberhammer et al,
β1-induced growth arrest of Huh7 cells is accompanied with p27KIP1
expression. TGF- β1 endows hepatocytes with dual functions: to induce growth inhibition and also topromote malignant transformation.
TGF-β signals are carried out through an interaction with two transmembrane serine/threonine kinase receptors, TGFβRI and TGFβRII. The best-documented intracellular mediators of these receptors are a family of proteins known as Smads. Smads 2 and 3 are activators, Smad4 is a mediator, and Smad7 is an inhibitor of TGF-β responses. However, many of the mechanisms by which TGF-β controls apoptosis and the growth of epithelial cells remain poorly understood, partly because of the substantial complexity and diversity of TGF-β signalling mechanisms among various systems, tissues, and cell types. TGF-β signalling mediators, particularly Smads 2, 3, and 4, selectively associate to and cross-talk with a highly complex network of signaling molecules, many of which, including the androgen receptor (AR), are expressed in a tissue-specific manner.
We previously reported that androgen can directly regulate TGF-β1 expression through binding of AR to ARE in TGF-β1 promoter, and suggested that such activation might regulate the progression of HCC: AR mediated upregulation of TGF-β1 expression was confirmed by HCC developed in nude mice with AR-overexpressed Huh7-cells. In a recent study where dominant-negative TGFβRII (TGFβRII DN) in transgenic mice was targeted to the prostate with a C3 promoter, the loss of TGF-β1 signalling substantially lowered the levels of apoptosis and increased epithelial cell proliferation in the proximal duct of the prostate (Kundu et al., 2000). Importantly, the expression of TGFβRII DN in these cells inhibits TGF-β1 induced apoptosis in vitro and triggers their malignant transformation
(Yamamura et al., 2000). However, tumor-derived cell lines have frequently lost this antiproliferative response. Resistance to the TGF-β mediated growth inhibition seems to be a late event in tumor progression (Haddow et al., 1991), and it has been shown for a variety of human tumors or tumor cell lines that reduced antiproliferative response to TGF-β is due to mutations of the type II TGF-β receptor (Markowitz et al., 1995; Shin et al., 2000; Park et
al., 1994; Myeroff et al., 1995, Garrigue-Antar et al., 1995; Knaus et al., 1996; Kimchi et al.,
1988; Kalkhoven et al., 1995).
C. Cellular senescence
Cellular senescence has been suggested to be an important process in two distinct phenomena: in aging and carcinogenesis (Mathon and Lloyd, 2001; Shay and Roninson, 2004), which are not only due to its general effect of growth arrest, but also by sharing common mechanisms involved. Senescent cells continue to function metabolically, but will not respond to mitogens. Decreased growth rate, limited cell division, flat and large cell shapes, and tight binding of the cells to a culture dish are well-known characteristics of cells entered into senescence (Macieira-Coelho et al., 1975; Start et al., 1991). Cellular senescence marker includes senescence-associated β-galactosidase expression, the nuclear actin accumulation (Kwak et al., 2004), the cytoplasmic p-Erk1/2 accumulation (Lim et al., 2000) and reduction of telomerase activity. Replicative senescence also is thought to be a tumor-suppressive mechanism and an underlying cause of aging. There is much evidence to
senescence is thought to be tumor suppressive. Immortality increases greatly the susceptibility to malignant transformation in culture and in vivo (Ponten, 1976; Sager, 1991). Many tumors contain immortal cells or cells with an extended replicative life span (Kim et
al., 1994; Brenner et al., 1998). Cell senescence in culture is not only induced by an intrinsic
cell division counter-mechanism (replicative senescence), but can also be induced by extrinsic factors such as culture conditions (premature senescence). The only known cell division counter-mechanism is telomere shortening. Telomere shortening can either promote or protect against tumorigenesis in mice (Mathon and Lloyd, 2001). The elevated expression of telomerase in almost all tumours may provide a diagnostic and prognostic tool for cancer. Moreover, as tumour cells require telomerase expression for continued proliferation, inhibitors of telomerase activity may provide effective cancer therapies.
D. Purpose of this study
Recently, we found that the expressions of AR and TGF-β1 were increased in the surrounding liver, compared with that of HCC in human. Thus, there arose a question of whether there is any correlation between the male sex hormone and TGF-β1 for the development of HCC in animals and humans.
In this study, we examined the role of androgen receptor in HCC development. To investigate the basic role of male sex hormone and its receptor in hepatocarcinogenesis, AR overexpressing Huh7 cells, Huh7-AR, and its control, Huh7-V cells, were established, and their in vivo and in vitro tumorigenesis were evaluated in nude mice as well as in vitro cell
culture systems.
Fig. 2. The transforming growth factor β (TGF-β)/SMAD pathway. Binding of a TGF-
β family member to its type II receptor in concert with a type I receptor leads to formation of
a receptor complex and phosphorylation of a type I receptor. Thus activated, the type I receptor subsequently phosphorylates a receptor-regulated SMAD (R-Smad), allowing this protein to associate with Smad 4 and move into the nucleus. In nucleus, the SMAD complex associates with a DNA-binding partner, and this complex binds to specific enhancers in targets genes, thus activating transcription.
Ⅱ
Ⅱ
Ⅱ
Ⅱ. MATERIAL AND METHODS
A. Preparation of Huh7-AR cells and treatment
Huh7- AR and Huh7- V cells were prepared by stable transfection with pCMVhAR plasmid and its vector pcDNA3, respectively, in Huh7 cells by using LipofecAMINE in Dulbecco’s modified Eagle’s medium (GIBCO BRL, Rockvile, MD, USA). After transfection for 6 h, the culture medium was replaced with fresh medium and cultured overnight in a 37°C incubator supplemented with 5% CO2 in air. Subsequently, cells were trypsinized and plated in 100-mm culture plates in 10% FBS in DMEM for 24 h, and then selected with G418 (1 mg/ml) for 2 weeks. G418-resistant cells were pooled and named as Huh7-AR and Huh7-V cells. All the prepared cells were routinely maintained in G418 (0.5 mg/ml) containing media. To investigate the effect of male sex hormone, Huh7 or Huh7-AR cells were cultured in DMEM plus charcoal stripped serum (CSS) for 24 hr before adding 10 nM DHT.
B. Isolation of RNA and RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). One µg of RNAs was used for cDNA synthesis in 10µl of reaction mixture using Moloney murine leukemia virus reverse transcriptase (Bioneer, Daejeon, Korea) and oligo dT as primers. AR
(446bp), TGF-β1 (330-bp) and GAPDH (195-bp) cDNA fragments were amplified using the
following primers: sense, 5’- CCTTTGCTGCCTTGTTATCT-3’, antisense,
5’-GCATGCAATGATGCGATCAA-3’ for hAR, sense, 5’-GCCCTGGACACCAACTATT-3’,
antisense, 5’-TCAGCTGCACTTGCAGGAG-3’ for TGF-β1 and sense, 5’-CCATG GAGAA
GGCTGGGG-3’, antisense, 5’-CAAAGTTGTCATGGATGACC-3’ for GAPDH
amplification. AR was amplified by 35cycles at 50°C, TGF-β1 was amplified by 25 cycles at 50°C, and 24 cycles at 61°C for GAPDH. PCR products were then examined by 1.2 % or 1.5 % agarose gel electrophoresis.
C. Assessment of androgen response of Huh7-AR cells by using MMTV promoter
Huh7-AR cells were seeded into six-well plates at 50% confluency, and were transfected by using LipofecAMINE with 2㎍ of MMTV-Luc plasmid and 0.5㎍ of pRL-TK (Promega Corp., Madison, WI) as an internal control. After washing with phosphate-buffered saline (PBS), the cells were then incubated for 28 hr in media containing 10% CSS in the absence or presence of 5α-dihydrotestosterone (10nM; DHT). The cells were lysed, and luciferase activity of cell extracts was measured by luminometer (TD-20/20, Turner BioSystems, Sunnyvale, CA, USA). The activity is represented as relative light units according to the Dual-Luciferase Reporter Assay System (Promega Corp., Madison, WI, USA). All transfection experiments and luciferase assays were carried out in triplicate and also repeated at least twice.
D. Immunocytochemistry
Huh7 and Huh7-AR cells were cultured on cover slides and fixed with 4% formaldehyde for 30 min at 4°C. Cells were then rinsed three times with TBS (50 mM Tris-HCl, pH 7.4, 150 mM NaCl) in 0.1% Triton X-100, and incubated in blocking buffer (TBST+5% bovine serum albumin) for 60 min at room temperature. Cells were then incubated overnight at 4°C with anti- AR (1:100, Santa Cruz, CA, USA), and anti-actin (1: 300, Sigma, ST. Louis, Missouri, USA), rinsed three times with TBS, and then incubated with anti-mouse secondary antibodies conjugated to Texas - red or Cy3 (1:200) in 3% bovine serum albumin in TBS for 90 min at room temperature. The cells were rinsed and mounted with glycerol. Images were viewed using a confocal microscope (Olympus Corp., Tokyo, Japan).
E. Measurement of growth rate by cell counting
To measure cellular growth rates of the Huh7 cells which constitutively expressed AR gene, 2.5 x 103 cells were seeded into 24-well plates, and then further cultured for 14 days. At indicated times, cells were harvested and counted with hemocytometer, and culture medium was changed ever 3 days during experiment. The average counts from duplicate experiments are presented.
F. Assays for tumorigenicity
For colonogenic assay, Huh7-V and Huh7-AR cells (4 x 102/35-mm plate) were maintained at 37°C for incubation times with DMEM serum free medium which was changed every 3 days during experiment. After 9 days, grown colonies were stained with crystal violet.
Soft agar colony forming assay was done as described previously (Conzen et al., 2000). Briefly, Huh7-V and Huh7-AR cells were plated subconfluently in six-well plates in 0.6% agarose on a 1.0% agarose bed. The culture medium was added every 3 days. After 2 weeks, grown colonies were counted under optical microscope (Olympus Corp., Tokyo, Japan).
Invasion assay with Matrigel -Cells were tested for invasive ability using the basement membrane Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) in vitro in Transwell chambers (Coring Costar, Cambridge, MA), as described previously (Albini et al., 1987; Kim et al., 2003), using 24-well Transwell chamber inserts (Corning Costar, Cambridge, MA) with 8-㎛ porosity polycarbonate filters. In brief, DMEM medium supplemented with 5% FBS was placed in the lower well. Cells suspended in DMEM medium containing 1% FBS were added to the upper chambers (2.5 x 104 cells/well) and incubated for 24 hr at 37°C in 5% CO2 incubator. Cells were fixed and stained with 1% toluidine blue in 1% borax . Non-invading cells on the upper surface of the filter were removed by wiping out with a cotton swab, and the filter was excised and mounted on a microscope slide. Invasiveness was quantified by counting cells on the lower surface of the filter.
G. Tumor formation in nude mice and immunohistochemistry
To investigate the effect of testosterone on in vivo tumorigenesis of V and Huh7-AR cells, 6 week-old male nude mice (CBy. Cg-Foxn1nu, Jackson Laboratory, Bar Harbor, ME) were used and maintained for 2 weeks. Cells (5 x 106 cells per mouse) were then subcutaneously injected at the left thigh of mice. The number and volume of HCC developed in the injection site were measured every 2 days with vernier caliper until 31days. Tumors were excised when the volume became over 1000 to 3000 mm3, and paraffin block was prepared after formalin fixation. Anti- β-catenin (1:100, Zymed, San Francisco, CA, USA) was incubated for 2hr at room temperature, and Envision+ detection kit (DAKO, Glostrup, Denmark) was then applied for 40 min according to the instruction, and hematoxyin counter stain was employed. Tumors were used for protein and RNA preparation to evaluate AR and TGF-β1 expression.
H. Immunoblot analysis
Tissues were homogenized in RIPA buffer A (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, and 1 µg/ml leupeptin). The homogenate was centrifuged at 800 x g for 10 min at 4°C. Supernatant was used as tissue lysates. For cell lysates, culture plates were washed with cold PBS, and total cell lysates were prepared in RIPA buffer containing protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 µg/mL leupeptin, 1 mM
Na3VO4 and 1mM NaF). Protein concentrations were determined by the Bradford method (Bio-Rad Protein assay, Richmond, CA, USA). Tissue lysates and cell lysates were separated by 8% SDS-PAGE, and the proteins were then transferred onto Immuno-blot PVDF membrane. Each blot was individually incubated with each of the following antibodies: anti- AR (1:1000, Santa Cruz, CA, USA), anti-E-Cadherin (1:1000, Santa Cruz), anti-p27KIP (1:1000, BD bioscience pharmingen, San Diego, CA, U.S.A.), anti-p- p27KIP (1:1000, Santa Cruz), anti-Bcl-XL (1:1000, Santa Cruz), anti-Bax (1:1000, Santa Cruz), anti-c-myc (1:1000, Santa Cruz) and anti-cyclin D1 (1:1000, Santa Cruz). Expression of β-actin and laminB1 was used as a loading control. Membranes were washed with 0.1% Tween 20 in PBS and incubated for 60 min with anti-rabbit and anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies (Amersham Bioscience, Piscataway, NJ, USA). After washing with Tween 20/PBS, membranes were subjected to enhanced chemiluminescence (ECL) detection analysis (Amersham Bioscience). To reprobe with other primary antibodies, the membranes were stripped using the erasing buffer (50 mM Tris-HCl pH 6.8, 2% SDS, 0.1M β-mercaptoethanol) for 15 min at 65°C.
I. Assay of senescence associated β-galactosidase
Senescence-associated β-galactosidase (SA-β-gal) was assayed at pH 6.0 by slightly modified method of Dimri et al. (Dimri et al., 1995). Briefly, cells were washed twice with PBS, fixed to plates by 3% formaldehyde for 5 min, washed with PBS, and then incubated
containing 1 mg/ml of X-gal (5-bromo-4-chloro-3-indolyl- β-D-galactopyranoside), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2. Stain could be visible after 12 hr of incubation at 37°C. By counting the numbers of the blue and total cells per field of 0.5 x 0.5 cm under an inverted microscope, percentage of the cells stained blue was calculated to indicate how many cells were associated with senescence.
J. Other senescence markers
It has been known that nuclear actin accumulation is a more sensitive and earlier event than the appearance of senescence-associated β-galactosidase (SA-β-gal) activity (Kwak et
al., 2004). Therefore, induction of senescence was confirmed by measuring the degree of
nuclear actin accumulation. Also, SA-p-Erk1/2 proteins accumulated in the cytoplasm of AR expressing cells were subjected to immunocytochemical and immunoblot analysis. Telomerase activity was examined by TRAP (Telomeric repeat amplification protocol) containing telomerase reaction and amplification step. PCR products were then examined by 10 % polyacrylamide gel electrophoresis and visualized with SyBr green staining.
K. Measurement of TGF-β secretion by MTT assay
Huh7-V cells and Huh7-AR cells were plated at 1.0 x 105 cells per 60-mm culture dish with complete medium. After 18hr of incubation, culture medium was changed with serum free medium and incubated for 2 days. Conditioned culture medium was prepared through
filteration by 0.45㎛ syringe filter. Mv1Lu cells (5.0 x 103 cells per 96-well plate) were cultured in DMEM plus 10% FBS for 18hr, and the medium was replaced with serum free medium containing 0 ~ 1000 pg/ml recombinant hTGF-β1 or conditioned medium of Huh7-V and Huh7-ARcells. After 4 days of incubation, TGF-β1 secretion was measured by MTT assay.
L. Infection of dominant negative TGF-βRII adenovirus
Lac Z adenovirus and TGFβRII dominant negative (TGFβRII DN) adenovirus were kindly provided by Dr. Tae Jun Park (Ajou University, School of Medicine). Huh7-V cells and Huh7-AR cells at 2.5 x 103 cells were plated into 24-well plates. After 24hr, the Huh7-V cells and Huh7-AR cells were found to be infected with 100MOI Lac Z adenovirus or TGFβRII DN adenovirus, and they were then further cultured for 7 days. At indicated times, cells were harvested and counted with hemocytometer; culture medium was changed ever 3 days during experiment. The average counts from triplicate experiments are presented.
M. Statistics
All bars represent mean ± standard deviations of determinants. Experiments were repeated at least three times. Statistical analysis was performed by t-test.
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS
Androgen receptor expression and androgen responsiveness of Huh7-AR cells
To investigate the phisiological role of male sex hormone and its receptor in hepatocarcinogenesis, AR expression was analyzed in human livers by RT-PCR. Interestingly, more AR expression was observed in non-cancer region than cancer (Fig. 3).
Next, AR overexpressing Huh7 cells, Huh7-AR, and its control, Huh7-V cells, were established, and AR expression was measured by RT-PCR (Fig. 4A) and immunoblot (Fig. 4B) analyses. As seen in the figures, the vector transfected Huh7 cells (Huh7-V) failed to express AR at both the mRNA and protein levels, however, the expression was variable, depending on the levels of AR expression, e.g. single clones (Huh7-AR3) and Huh7-AR mixture (Huh7-AR). To test androgen response in the estabilished cells, Huh7-AR cells were transfected with MMTV-Luc plasmid, using the murine mammary tumor virus (MMTV) promoter sequence as a positive control of androgen responsiveness. In the presence of 10nM DHT, AR expressing cells increased luciferase activity more than that of the Huh7-V (Fig. 4C). To investigate the response of AR to DHT treatment, immunocytochemistry was performed; When treated with 10nM DHT for 2~8 hr, AR was found in the nuclei of Huh7-AR cells, however, it was diffused over the cytoplasm in 8hr (Fig. 4D).
Reduced tumorigenesis of Huh7-AR cells in vivo and in vitro
To evaluate the effect of AR on the growth of HCC cells, the growth rates of Huh7-V and Huh7-AR cells were measured by cell counting. Reduced growth rate of Huh7-AR cells was observed, as compared with that of Huh7-V cells (Fig. 5). To investigate the degree of tumorigenecity of the Huh7-AR cells, clonogenic assay, soft agar colony formation and Matrigel invasion assaies were performed, and the results showed reduced tumorigenicity of the Huh7-AR cells, as compared with that of the Huh7-V cells (Fig. 6A, 6B, 6C).
As shown in Fig. 7, tumor volume of Huh7-V was significantly larger than that of Huh7-AR cells 31days after inoculation. HCC growth of Huh7-AR cells in nude mice was inhibited, in contrast to the HCC of Huh7-V cells. This finding is in good agreement with recent reports that AR induces apoptosis in prostate cancer cells and likely reduces in vivo prostate cancer growth.
Immunoblot analyses revealed that Bcl-XL and cyclin D1 expressions in the Huh7-AR HCC were inhibited, whereas the expression of E-cadherin was increased (Fig. 8).
The above results are well accordant with the suggestion that overexpression of AR may result in the reduced tumorigenesis.
Fig. 3. AR expression in human livers was analyzed by RT-PCR. To investigate the role
of male sex hormone and its receptor in hepatocarcinogenesis, AR expression was measured by RT-PCR in human livers. (N; normal liver, C; cancer)
Fig. 4. Expression of androgen receptor in Huh7-AR cells. To investigate the effect of
male sex hormone, Huh7-V or Huh7-AR cells after transfection were cultured in DMEM plus charcoal stripped serum (CSS) for 24 hr before adding 10nM DHT. AR expression in Huh-V and Huh7-AR cells was measured by RT-PCR (A) and Western blot analysis (B). (C) Androgen response of Huh7-AR cells by MMTV- promoter assay. Huh7-V and Huh7-AR cells were transfected by using LipofecAMINE with 2㎍ of MMTV-Luc plasmid and 0.5㎍ of pRL-TK (Promega) as an internal control. After washing with PBS, the cells were then incubated for 28 hr in medium containing 10% CSS in the absence or presence of 5 α-dihydrotestosterone (10nM; DHT). The cells were lysed, and luciferase activity was measured according to the Dual-Luciferase Reporter Assay System, and represented as relative light units. All transfection experiments and luciferase assays were carried out in triplicate and also repeated at least twice. (D) The localization of AR was examined by immunocytochemistry after treatment with 10nM DHT. Huh7-AR cells were cultured in DMEM plus 10% charcoal stripped serum (CSS) for 24 hr before adding 10nM DHT.
Fig. 5. Growth rate of Huh7-AR cells was decreased. Huh7-V and Huh7-AR cells (2.5 x
103 cells) were seeded into 24-well plates, and cultured further for 12 days. At the indicated times, cells were harvested and counted with hemocytometer. Culture medium was changed every 3 days during experiment. Mean ± S.D. from two independent experiments. *, **, p value by t-test.
Fig. 6. Reduced tumorigenesis of Huh7-AR cells in vitro. (A) Tumorigenicity of the
Huh7-AR cells was determined by clonogenic assay. Huh7-V and Huh7-AR cells (4 x 102/35-mm plate) were maintained at 37°C for 9 days with medium which was changed every other day. Grown colones were stained with crystal violet. CM; DMEM+10% FBS, CS; DMEM+10% CSS, CD; DMEM+10% CSS+ 10nM DHT. Treated with 10nM DHT every day. (B) For soft agar assay, Huh7-V and Huh7-AR cells were plated subconfluently in six-well plates in 0.6% agarose on a 1.0% agarose bed. Every 3 days, the culture medium was added. After 2 weeks, grown colonies were counted by an optical microscope. (C) Invasion assay into Matrigel - Invasive ability of cells was tested through the basement membrane Matrigel in Transwell chambers with 8-㎛ porosity polycarbonate filters. DMEM medium supplemented with 5% FBS was placed in the lower well. Cells suspended in DMEM medium containing 1% FBS were added to the upper chambers (2.5 x 104 cells/well) and incubated for 24 hr at 37°C in 5% CO2 .
Fig. 7. Reduced tumor volumes of Huh7-AR cells in nude mice. Cells (5 x 106 cells per mouse) were subcutaneously injected at the left thigh of six week-old nude mice. (A) The number and volume of HCC developed at the injection site were measured with vernier caliper every 2 days until 31 days. (B) After 31days, the volume of tumors was measured.
Fig. 8. Difference of protein expression in nude mice injected with V and Huh7-AR cells. Tumors were excised when the volume became over 1000 to 3000 mm3 (A) Proteins were extracted from each animal in order to evaluate the expression of AR, E-cadherin, cyclinD1, Bcl-XL, Bax and actin by Western blot analysis, (B) β-Catenin expression by immunohistochemistry.
Dihydrotestosterone induced senescence of Huh7-AR cells, negative regulation of tumorigenesis
When cultured longer in the complete medium (CM), Huh7-AR cells became larger, and stress fibers were significantly expressed. When the expression of SA-β-gal was examined to confirm the senescence-like changes in the Huh7-AR cells, the number of positive cells was significantly higher in the AR maintained in CM than that of Huh7-V in CM (Fig. 9A). When other senescence markers, such as nuclear actin accumulation and cytoplasmic pErk1/2 accumulation, were examined by immunocytochemistry, the Huh7-AR cells cultured in CM revealed actin accumulation in nucleus (Fig. 9B) and pErk1/2 accumulation in cytoplasm (Fig. 9C). As shown in Fig. 9D, telomerase activity was found to be reduced in the Huh7-AR cells, as compared with Huh7-V cells which had been maintained in CM, but not DMEM+10% charcoal stripped serum (CS). Therefore, overexpression of AR seems to be able to induce senescence phenotypes.
Increased TGF-ββββ expression in HCCs by Huh7-AR cells in nude mice
Previously, we reported that androgen regulates transcription of TGF-β1 through direct binding of DHT-AR complex to the potential ARE within TGF-β1 promoter region. Therefore, we selected TGF-β1 as a regulating factor of tumor formation in the HCC of Huh7-AR cells (Yoon et al., 2006). When TGF-β1 expression was measured by RT-PCR, it
also similar in HCCs developed in nude mice; all HCC tissues developed from the Huh7-AR cells (lanes 5–8, Fig. 10B) expressed higher level of TGF-β1 than the HCC of Huh7-V cells (lanes 1–4, Fig. 10B). The expression of TGF-β1 was also increased in human livers with the increased AR expression (Fig. 10C).
Increase of TGF- β1 secretion in Huh7-AR cells
To further confirm increased TGF-β1 expression in AR- expressing cells, we measured the secretion of TGF-β1 by MTT assay. TGF-β1 secretion was much higher in the Huh7-AR cells than that in the Huh7-V cells (Fig. 11).
These results suggest that, when AR was overexpressed in Huh7 cells, androgen receptor can inhibit tumorigenesis by induction of senescence phenotypes via the increased TGF-β1 expression.
Fig. 9. AR induced senescence phenotype. (A) Huh7-AR cells were stained for detection
of SA-β gal activity. Nuclear actin accumulation (B) and cytoplasmic p-Erk1/2 accumulation (C) were examined by immunocytochemistry. (D) Telomerase activity was reduced in the Huh7-AR cells, compared with Huh7-V cells. Huh7-V and Huh7-AR cells were maintained with CM (; DMEM+10% FBS) or CS (DMEM+10% CSS) medium.
Fig. 10. TGF-β1 expression was measured by RT-PCR. (A) TGF-β1 mRNA expression
was increased in Huh7-AR cells. (B) TGF-β1expression was increased in HCCs developed by Huh7-AR cells in nude mice. Each lane indicates the data for different animals. (C)
Fig. 11. Secretion of TGF-ββββ1 in Huh7–AR cells. Mv1Lu cells (5.0 x 103 cells per 96-well plate) were cultured in DMEM plus 10% FBS. After 18 hr, the medium was changed with serum free medium containing 0 ~ 1000 pg/ml recombinant hTGF-β1 or the same volume of the conditioned medium harvested from Huh7-V or Huh7-ARcells. After 4 days of incubation, TGF- β1 secretion was measured by MTT assay based on the cell toxicity induced by treatment of the cells with the known amount of recombinant hTGF-β1.
Increased p27KIP1 expression in Huh7-AR cells, but decreased its phosphorylation in cytopalsm of Huh7-AR cells after treatment with DHT
To confirm whether TGF- β1 could reduce the tumorigenicity of AR expressing cells, p27KIP1, downstream target of TGF-β1, expression was examined.
TGF- β1-induced growth arrest of Huh7 cells was accompanied with p27KIP1 expression. When the related CDKI was examined, p27KIP1 expression in Huh7-AR cells was increased (Fig. 12A), suggesting the TGF- β1 associated growth arrest. When Huh7-V cells were treated with 10nM DHT, p-p27 KIP1 translocation to the cytoplasm was slightly increased for 4 hr, whereas the translocation in Huh7-AR cells was the same regardless of DHT treatment or not (Fig. 12B). These data strongly suggest that AR can induce senescence of Huh7-AR cells when testosterone is present.
Decreased c-myc, β-Catenin and cyclin D1 expression in Huh7-AR cells after DHT treatment
To investigate the effect of TGF- β1 downstream, cells were treated with 10nM DHT, and expression of myc was determined by immunoblot analysis. DHT treatment reduced c-myc expression of Huh7-AR cells in 4 hr, as compared with untreated control, but not in Huh7-V cells (Fig. 13A). To further elucidate the effect, these cells were treated with DHT with or without cyproterone acetate, the anti - androgen. As shown in Fig. 13B, CPA could
cyclin D1 might be involved in tumorigenicity of AR-expressing cells, the levels of β-catenin and c-myc proteins were analysed by Western blotting. Expressions of β-Catenin and cyclin D1, related to HCC development in AR cells, were also reduced with DHT treatment. These results also strongly support that AR expression can inhibit HCC formation through increasing TGF-β and p27KIP1 expressions and reducing the c-myc, β-Catenin and cyclin D1 expressions.
Fig. 12. Increased p27KIP1 expression in Huh7-AR cells, but decreased its phosphorylation in cytoplasm of Huh7-AR cells after treatment with DHT. (A) Empty
vector or AR – transient transfected cells were treated with 10nM DHT for 1 hr and AR and p27KIP1 expressions were analyzed by Western blot analysis. (B) Huh7-V and Huh7-AR cells were treated with 10nM DHT or EtOH. After 4 hr, cells were harvested and fractionated to nuclear and cytoplasmic fractions for analysis of phosphorylated p27KIP1 and
Fig. 13. Decreased c-myc expression in Huh7-AR cells after DHT treatment. (A)
Huh7-V and Huh7-AR cells were treated with 10nM DHT for indicated times, and c-myc expression was determined by immunoblotting. Jurkat cell lysates were used for positive control. (B) The cells were treated with DHT (as androgen) and cyproterone acetate [(CPA), as anti-androgen] or not. After 4 hr, AR and c-myc expressions were determined. (-); Not treated, (+); treated
Fig. 14. Decreased cyclin D1 and β-catenin expression in Huh7-AR cells after DHT treatment. Huh7-V and Huh7-AR cells were treated with 10nM DHT for indicated times,
Recovery of senescence phenotypes of Huh7-AR cells by adenovirus infection of dominant negative TGFβRII construct
To further elucidate the reduced tumorigenesis of Huh7-AR cells by TGF-β1 expression, we used a TGFβRII dominant negative adenovirus to block the TGF-β1 signal. Thus, Huh7-V and Huh7-AR cells were infected with β-galactosidase expressing adenovirus (LacZ -Ad) and dominant negative TGFβRII expressing adenovirus (TGFβRIIDN-Ad) (Fig. 15A). Infection efficiency of adenovirus was determined by X-gal staining or TGFβRII immunocytochemistry. It was almost 100 percent infection efficiency of Lac Z-Ad or TGFβRIIDN-Ad in Huh7-V cells and Huh7-AR cells (Fig. 15B). When Lac Z-Ad was infected, the growth of AR cells was reduced, as compared with that of Huh7-V cells. Blocking of TGF-β1 signal with TGFβRIIDN-Ad recovered the reduced growth of Huh7-AR cells (Fig. 15C), and nuclear actin accumulation (Fig. 15D) as well as cytoplasmic pErk1/2 accumulations in Huh7-AR cells (Fig. 15E). These results led us to suggest that AR overexpression could reduce the tumorigenicity of AR overexpressing cells through TGF-β1 induced cellular senescence phenotypes.
Fig. 15. Recovery of senescence phenotype in Huh7-AR cells after dominant negative TGFβRII adenovirus infection (A) Dominant negative TGFβRII adenovirus Scheme (B)
Infection efficiency was determined by X-gal staining or immunocytochemistry. (C) Huh7-V and Huh7-AR cells were infected with LacZ-Ad or TGFβRIIDN-Ad and then further cultured for 7 days. At indicated times, growth rates were determined by cells counting, and culture medium was changed every 3 days during experiment. Mean ± S.D. from two independent experiments. *, **, p value by t-test. (D, E) Under the same condition as in (C), actin localization and p-Erk1/2 expression were determined by immunostaining and immunoblotting.
Ⅳ
Ⅳ
Ⅳ
Ⅳ. DISCUSSION
In the present study, the potential role of androgen and AR in hepatoma development was investigated after establishment of Huh7-AR and Huh7-V cell lines by transfection of human androgen receptor (AR) in pCMV5 and its empty vector to Huh7 hepatocelluar carcinoma (HCC) cells. Previously, Boix et al. reported that the presence of androgen receptors within the tumor was significantly related to a smaller tumor size: 22 of the 29 nodules < or = 3 cm contained androgen receptors, while only six of the 14 tumors larger than 3 cm (p < 0.05) contained the receptor (Boix et al., 1995).
Reduced growth rate and tumorigenicity of Huh7-AR cells were observed, compared with the Huh7-V cells (Fig. 5, 6A, 6B, 6C). When we injected Huh7-AR or Huh7-V (5 x 106 cells/mouse) cells to control and orchiectomized (OX) nude mice, the volume of Huh7-V tumor was decreased in OX mice (Data not shown). It is well known that development of HCC, including latency, frequency and multiplicity of tumors, in OX animal is significantly decreased compared with those in intact animals (Tsubura et al., 1990). Orchiectomy resulted in a significant reduction of hepatocellular carcinomas (15% in castrated rats versus 81% in control rats) (Rao and Kashireddy, 2002). Tumor volume of Huh7-V was larger than that in Huh7-AR cells 31 days after inoculation. HCC growth of Huh7-AR cells in control nude mice was inhibited, as opposed in the HCC of Huh7-V cells (Fig.7). And the voulum of Huh7-AR induced tumor was significantly smaller than that of Huh7-V tumor in control mice, whereas Huh7-AR tumor was large in the orchiectomized mice (Data not shown). In
vivo study, we suggested that the Huh7-AR tumor was androgen-independent, in contrast to
androgen-dependent in the Huh7-V tumors. Immunohistochemical analyses revealed that E-cadherin expression was inhibited in the Huh7-AR tumors of the control nude mice (Fig. 8A), however, in vivo expression of β-catenin, Bcl-XL and cyclin D1 in the Huh7-AR tumor was decreased (Fig. 8B).
When cultured longer, the Huh7-AR cells became larger and formed significant stress fibers in complete medium (CM). When the expression of SA-β-gal, nuclear actin accumulation, and cytoplasmic p-Erk1/2 accumulation were examined to confirm senescence change in the Huh7-AR cells, the number of positive cells was significantly higher in the Huh7-AR in CM than that of Huh7-V in CM (Fig. 9A). As shown in Fig. 9D, telomerase activity was found to be reduced in the Huh7-AR cells, compared with Huh7-V cells which were maintained with CM, but not DMEM+10% charcoal stripped serum (CS). Therefore, AR overexpression seems to be able to induce senescence phenotype and androgen induced senescence of Huh7-AR cells, which is a negative regulation of tumor progression.
We earlier reported that androgen regulates transcription of TGF-β1 through direct binding of DHT-AR complex to the potential ARE within TGF-β1 promoter region. Therefore, we selected TGF-β1 as a factor of tumor formation by AR overexpression. When TGF-β1 expression was measured by RT-PCR, the mRNA expression of TGF- β1 was higher in the Huh7-AR cells and tumors than those in Huh7-V cells, as seen in Fig 10A and 10B.
dual functions: to induce growth inhibition and also topromote malignant transformation. In summary, in spite of higher expression of TGF-β1, Huh7-AR cells failed to undergo epithelial mesenchymal transition, but rather became senescent with concomitant expression of p27KIP1 in the presence of androgen.
To further elucidate reduced tumorigenesis and induced senescence phenotype by
TGF-β1, we used a TGFβRII dominant negative adenovirus to block the TGF-β1 signal. When
infected with Lac Z- Ad was, the growth of AR cells was reduced, compared to Huh7-V cells. Blocking of TGF-β1 signal with TGFβRIIDN-Ad could recover the reduced growth of Huh7-AR cells (Fig. 15C), and nuclear actin accumulation (Fig. 15D) and p-Erk1/2 accumulation were not observed in Huh7-AR cells (Fig. 15E).
Therefore, all of the above results strongly indicate that induction of senescence in AR expressing HCC may act as a negative regulator of tumor progression in the presence of testosterone.
Fig. 16. Mechanism to decrease tumorigenesis of AR overexpression in Huh7 cells.
Huh7-AR cells were established by transfection of human AR in Huh7 HCC cells. When Huh7-AR cells were cultured with DHT, TGF-β1 expression was increased. Because of increased TGF-β1 expression, tumorigenicity was decreased and growth rates were reduced. Also, when cultured in the presence of DHT, cellular senescence phenotypes was induced in Huh7-AR cells, such as SA-β-galactosidase activity, nuclear actin translocation, cytoplasmic p-Erk1/2 sequestration and reduced telomerase activity,
Ⅴ
Ⅴ
Ⅴ
Ⅴ. CONCLUSION
Steroid hormones and their receptors play a major role in the development of many types of human and animal cancers. It has been known that androgen and its receptor (AR) are significantly related with hepatocarcinogenesis both in human and animals, however, the level of AR in hepatoma is variable, and their exact effects are still poorly explained.
To investigate the potential role of AR in hepatoma, Huh7-AR and Huh7-V cell lines were established by transfection of human AR in pCMV5 and its empty vector to Huh7 hepatocelluar carcinoma cells, respectively. Huh7-AR cells, responsive to 5α -dihydrotestosterone (DHT), revealed a decreased tumorigenicity in nude mice and reduced clonogenicity in vitro compared with those of Huh7-V cells; The tumor volume and multiplicity of the HCC, and invasiveness of the Huh7-AR cell, which was measured by Matrigel chamber analysis, were significantly reduced, compared with those of Huh7-V cells.
When underlying mechanisms were evaluated in the cell culture system with or without DHT, TGF-β1 expression was increased in the Huh7-AR cells and HCC tumors in the presence of DHT. Interestingly, Huh7-AR cells induced cellular senescence phenotypes, such as SA-β-galactosidase activity, nuclear actin translocation, cytoplasmic p-Erk1/2 sequestration and reduced telomerase activity, when cultured in the presence of DHT.
Huh7-AR cells secreted more TGF-β1than Huh7-V cells. After infection of dominant negative TGFβRII to Huh7-V and Huh7-AR cells, the reduced growth rate and cellular senescence phenotypes observed in the Huh7-AR cells could be recovered by inhibition of
TGF-β1 using the adenovirus infection.
The above data strongly indicate that overexpression of androgen receptor inhibits tumorigenesis of Huh7-AR cells in the presence of DHT via induction of senescence phenotypes by TGF-β1 expression.
REFERENCES
1. Alexandrow MG, Moses HL: Transforming growth factor β and cell-cycle regulation.
Cancer Res 55: 1452–1457, 1995
2. Bachem MG, Riess U, Gressner AM: Liver fat storing cell proliferation is stimulated by epidermal growth factor/ transforming growth factor a and inhibited by transforming growth factor β. Biochem Biophys Res Commun 162(2): 708-714, 1989
3. Boix L, Castells A, Bruix J, Sole M, Bru C, Fuster J, Rivera F, Rodes J: Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol 22(6):616-622, 1995
4. Brenner AJ, Stampfer MR, Aldaz CM: Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17: 199–205, 1998
5. Chang C, Kokontis J, Liao S: Molecular cloning of the human and rat complementary DNA encoding androgen receptors. Science 240: 324–326, 1988
6. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
Peacocke M, Campisi J:
A biomarker that identifiessenescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363-9367, 1995
7. Fan G, Ma X, Kren, BT, STEER CJ: The retinoblastoma gene product inhibits TGF-beta-1-induced apoptosis in primary rat hepatocytesand human Huh-7 hepatoma cells.
Oncogene 12: 1909–1919, 1996
8. Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O: Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem 276: 2531– 2537, 2001
9. Fukuda K, Kojiro M, Chiu J-F: Induction of apoptosis by transforming-growth-factor-beta-1-induced apoptosis in rat hepatoma cell line McA-RH7777: a possible association with tissue transglutaminase expression. Hepatology 18: 945–953, 1993
10. Garrigue-Antar L, Munoz-Antonia T, Antonia SJ, Gesmonde J, Vellucci VF, Reiss M : Missense mutations of the transforming growth factor beta type II receptor in human head and neck squamous carcinoma cells. Cancer Res 55: 3982-3987, 1995
gene activation. Oncogene 6: 1465-1470, 1991
12. Kalkhoven E, Roelen BA, de Winter JP, Mummery CL, van den Eijnden-van Raaij AJ, van der Saag PT, van der Burg B: Resistance to transforming growth factor beta and activin due to reduced receptor expression in human breast tumor cell lines. Cell Growth
Differ 6: 1151-1161, 1995
13. Kang HY, Yeh S, Fujimoto N, Chang C: Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J Biol
Chem 274(13): 8570-8576, 1999
14. Katakura Y, Nakata E, Miura T, Shirahata S: Transforming growth factor beta triggers two independent-senescence programs in cancer cells. Biochem Biophys Res Commun 255: 110–115, 1999
15. Kemppainen JA, Lane MV, Sar M, Wilson EM: Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem 267(2): 968-974, 1992
16. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW: Specific association of human telomerase activity with immortal cells and cancer. Science 266: 2011–2015, 1994
17. Kimchi A, Wang XF, Weinberg RA, Cheifetz S, Massague J: Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science 240: 196-199, 1988
18. Knaus PI, Lindemann D, DeCoteau JF, Perlman R, Yankelev H, Hille M, Kadin ME, Lodish HF: A dominant inhibitory mutant of the type II transforming growth factor beta receptor in the malignant progression of a cutaneous T-cell lymphoma. Mol Cell Biol 16: 3480-3489, 1996
19. Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH: Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol 168: 47–61, 1995
20. Kundu SD, Kim IY, Yang T, Doglio L, Lang S, Zhang X, Buttyan R, Kim SJ, Chang J, Cai X, Wang Z, Lee C: Absence of proximal duct apoptosis in the ventral prostate of transgenic mice carrying the C3 (1)-TGF-beta type II dominant negative receptor.
Prostate 43(2): 118–124, 2000
21. Kwak IH, Kim HS, Choi OR, Ryu MS, Lim IK: Nuclear Accumulation of Globular Actin as a Cellular Senescence Marker. Cancer Res 64: 572–580, 2004
nuclear accumulation of actin proteins during cellular senescence in human diploid fibroblasts. Mech Ageing Dev 119: 113–129, 2000
23. Macieira-Coelho A, Loria E, Berumen L: Relationship between cell kinetic changes and metabolic events during cell senescence in vitro. Adv Exp Med Biol 53: 51–65, 1975
24. Markowitz SD, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268, 1336-1338, 1995
25. Massague J: TGF-β signal transduction. Ann Rev Biochem 67: 753–791, 1998
26. Mathon NF, Lloyd AC: Cell senescence and cancer. Nat Rev Cancer 1: 203–213, 2001
27. McGowan JA, Strain AJ, Bucher NLR: DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol 180: 353-363, 1981
28. McLachlan RI, Wreford NG, O’Donnell L, de Kretser DM, Robertson DM: The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J
29. Myeroff LL, Parsons R, Kim SJ, Hedrick L, Cho KR, Orth K, Mathis M, Kinzler KW, Lutterbaugh J, Park K, Barg YL, Lee H, Park JG, Lynch A, Roberts B, Vogelstein SA, Markowitz A: A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer
Res 55: 5545-5547, 1995
30. Nakamura T, Nishizawa T, Hagiya M, Seki T, Sugimura M, Tashiro K, Shimizu S: Molecular cloning and expression of human hepatocyte growth factor. Nature 342: 440-443, 1989
31. Oberhammer FA, Bursch W, Parzefall W, Beir P, Erber E, Stadler M, Schulte-Hermann R: Effect of transforming growth factor β on cell death of cultured rat hepatocytes.
Cancer Res 51: 2478–2485, 1991
32. Oberhammer FA, Pavellea M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, Schulte-Hermann R: Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA 89: 5408–5412, 1992
33. Park K, Kim SJ, Bang YJ, Park JG, Kim NK, Roberts AB, Sporn MB: Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric
Sci USA 91: 8772-8776, 1994
34. Ponten J: The relationship between in vitro transformation and tumor formation in vivo.
Biochim Biophys Acta 458: 397–422, 1976
35. Rao MS, Kashireddy P: Effect of castration on dehydroepiandrosterone-induced hepatocarcinogenesis in male rats. Anticancer Res 22(3): 1409-1411, 2002
36. Ricca-Rosellini, Miglio: Epidemiology of hepatocellular carcinoma. Minerva
Gastroenterol Dietol 37(2): 73-84, 1991
37. Roberts AB, Sporn MB: Mechanistic interrelationships between two superfamilies: The steroid/retinoid receptors and transforming growth factor- β. Cancer Surv 14: 205-220, 1992
38. Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B: Regulation of androgen action. Vitam Horm 55: 309–352, 1999
39. Sager R: Senescence as a mode of tumor suppression. Environ. Health Perspect 93: 59– 62, 1991
Oncogene 23: 2919–2933, 2004
41. Sheckter CB, Matsumoto AM, Bremmer WJ: Testosterone administration inhibits gonadotropin secretion by an effect directly on the human pituitary. J Clin Endocrinol
Metab 68: 397–401, 1989
42. Shin KH, Park YJ, Park JG: Mutational analysis of the transforming growth factor beta receptor type II gene in hereditary nonpolyposis colorectal cancer and early-onset colorectal cancer patients. Clin. Cancer Res 6: 536-540, 2000
43. Sporn MB, Roberts AB: Transforming growth factors- β: Recent progress and new challenges. J Cell Biol 119: 1017-1021, 1992
44. Start RD, Loomes RS, Shortland JR: The relationship between donor age and the growth characteristics of human smooth muscle cultures of aorta and stomach. Int J Exp Pathol 76: 647–654, 1991
45. Tavian D, De Petro G, Pitozzi A, Portolani N, Giulini SM, Barlati S: Androgen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinoma. Histol
46. Tarao K, Ohkawa S, Shimizu A, Harada M, Nakamura Y, Ito Y, Tamai S, Hoshino H, Okamoto N, Iimori K: The male preponderance in incidence of hepatocellular carcinoma in cirrhotic patients may depend on the higher DNA synthetic activity of cirrhotic tissue in men. Cancer 72(2): 369-374, 1993
47. Tilley WD, Marcelli M, Wilson JD, McPhaul MJ: Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA 86: 327–331, 1989
48. Tsai MJ, O'Malley BW: Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63: 451-486, 1994
49. Tsubura A, Izuno Y, Shoji T, Kusunose N, Morii S: Influence of strain and sex on the local development of mammary tumors induced by direct application of DMBA powder to rat mammary glands. Acta Pathol Jpn 40(1):9-13, 1990
50. Wong CI, Zhou ZX, Sar M, Wilson EM: Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J Biol Chem 268(25): 19004-19012, 1993
51. Yamamura Y, Hua X, Bergelson S, Lodish HF: Critical role of smads and AP-1 complex in TGF-{beta}-dependentapoptosis. J Biol Chem 275(46): 36295-36302, 2000
52. Yu L, Nagasue N, Yamaguchi M, Chang YC: Effects of castration and androgen replacement on tumour growth of human hepatocellular carcinoma in nude mice. J
Hepatol 25(3):362-369, 1996
53. Yoon G, Kim JY, Choi YK, Won YS, Lim IK: Direct activation of TGF-β1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma Cells and its tumor in nude mice. J Cell Biochem 97:393–411, 2006
54. Zarnegar R, Michalopoulos M: Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res 49: 3314-3320, 1989
55. Zhao H, Patra A, Tanaka Y, Li LC, Dahiya R: Transforming growth factor-beta(s) and their receptors in aging rat prostate. Biochem Biophys Res Commun 294: 464–469, 2002