Living donor liver transplantation in a pediatric patient with congenital absence of the portal vein
Jung-Man Namgoong
1, Shin Hwang
1, Gil-Chun Park
1, Hyunhee Kwon
1, Kyung Mo Kim
2, Seak Hee Oh
21
Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea,
2
Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Case Report
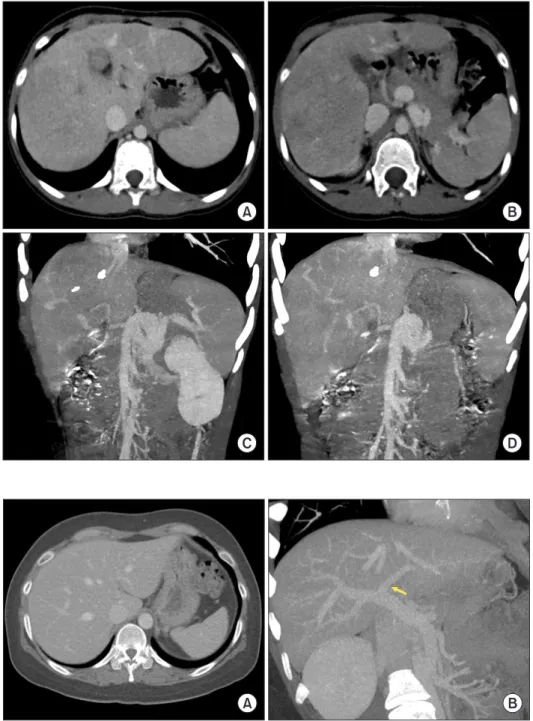
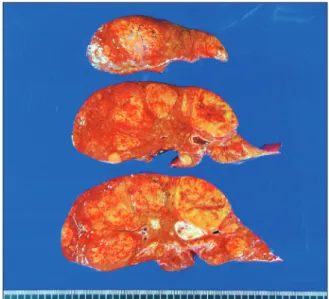
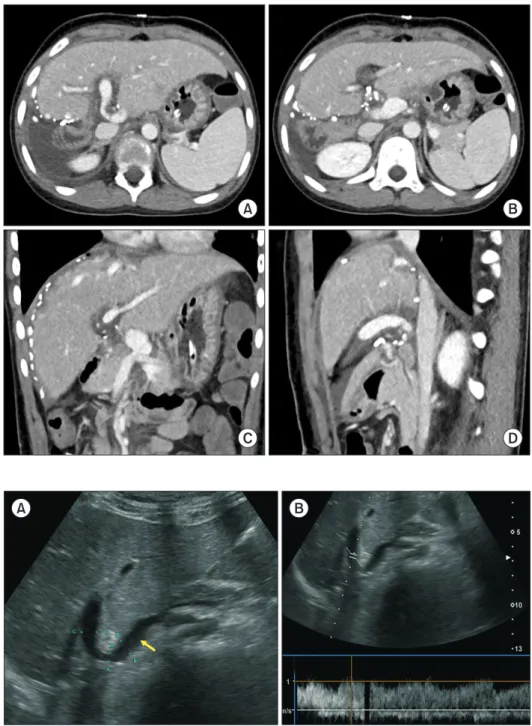
Congenital absence of the portal vein (CAPV) is a rare venous malformation in which mesenteric venous blood drains directly into the systemic circulation. We report a case of pediatric living donor liver transplantation (LDLT) for CAPV combined with focal nodular hyperplasia (FNH) and hepatocellular adenoma. A 9-year-old girl who had been diagnosed with multiple FNH had CAPV. Her blood ammonia level was raised to 137 µg/dL. However, she did not complain of any symptoms. To treat CAPV and FNH, we decided to per- form LDLT. The graft was a left liver graft from 39-year-old mother of the patient. Recipient hepatectomy was performed according to standard procedures of pediatric LDLT. Portal vein reconstruction was performed using interposition of an iliac vein homograft conduit to the superior mesenteric vein-splenic vein confluence. The CAPV-associated congenital splenorenal shunt was securely li- gated. The pathology report of the explant liver showed a 2 cm-sized hepatocellular adenoma and multiple FNH lesions measuring up to 7.1 cm. The patient recovered uneventfully from the LDLT operation. The reconstructed portal vein was maintained well without any hemodynamic abnormalities. In conclusion, as CAPV patients can have various vascular anomalies, combined vascular anomalies should be thoroughly assessed before and during liver transplantation operation. The most effective reconstruction techniques should be used to achieve satisfactory results following liver transplantation.
Key Words: Portal vein agenesis; Splenorenal shunt; Interposition graft; Iliac vein homograft; Liver tumor
INTRODUCTION
Congenital absence of the portal vein (CAPV) is a rare ve- nous malformation in which the mesenteric venous blood drains directly into the systemic circulation. The majority of patients with CAPV show no signs or symptoms of porto- systemic encephalopathy. They only show slightly abnormal results in liver function tests. Liver transplantation (LT) is indicated for patients with symptomatic CAPV refractory to medical treatments [1-4].
The congenital portocaval shunt (PCS) drains the entire
mesenteric venous blood either directly into the inferior vena cava (IVC) or the left renal vein. Thus, theoretically, there is no portal hypertension or collateral circulation [3-5]. As the liver with CAPV does not have sufficient portal inflow, the hepatic arterial flow is the main inflow of blood. If a patient cannot tolerate a medical treatment, LT should be taken into account for such a case. For patients with CAPV, their liver function profiles are not severely impaired with resultant low Pediatric End-stage Liver Disease scores. Because of the low chance for deceased donor liver transplantation in the current Korean setting, patients with CAPV need to consider living donor liver transplantation (LDLT). We herein present a case of pediatric LDLT using a left liver graft for CAPV and liver tumors.
CASE
A 9-year-old girl was referred to our hospital due to the diag- nosis of multiple liver mass. These liver lesions were diagnosed with focal nodular hyperplasia (FNH) on imaging studies and percutaneous liver biopsy. There was no abnormal finding on the diagnostic exome sequencing. The patient was prematurely
Received: February 2, 2021, Revised: February 11, 2021, Accepted: February 14, 2021
Corresponding author: Shin Hwang
Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Olympic-ro 43-gil 88, Songpa-gu, Seoul 05505, Korea
Tel: +82-2-3010-3930, Fax: +82-2-3010-6701, E-mail: shwang@amc.seoul.kr ORCID: https://orcid.org/0000-0002-9045-2531
Copyright Ⓒ The Korean Association of Hepato-Biliary-Pancreatic Surgery
This is an Open Access article distributed under the terms of the Creative Commons Attri- bution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.