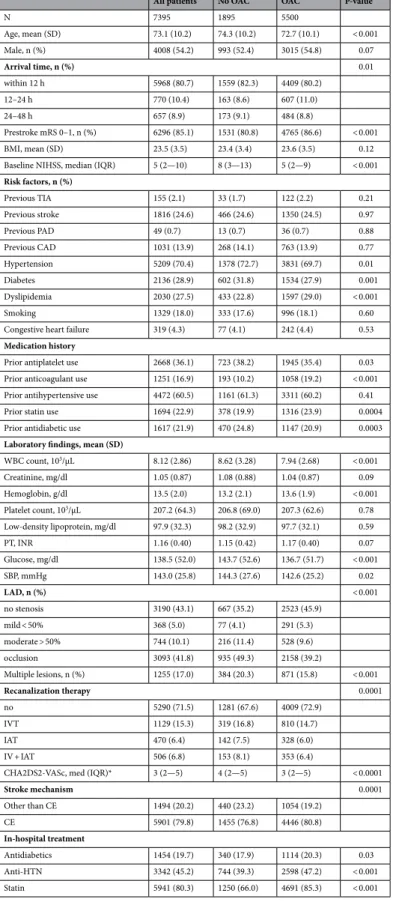
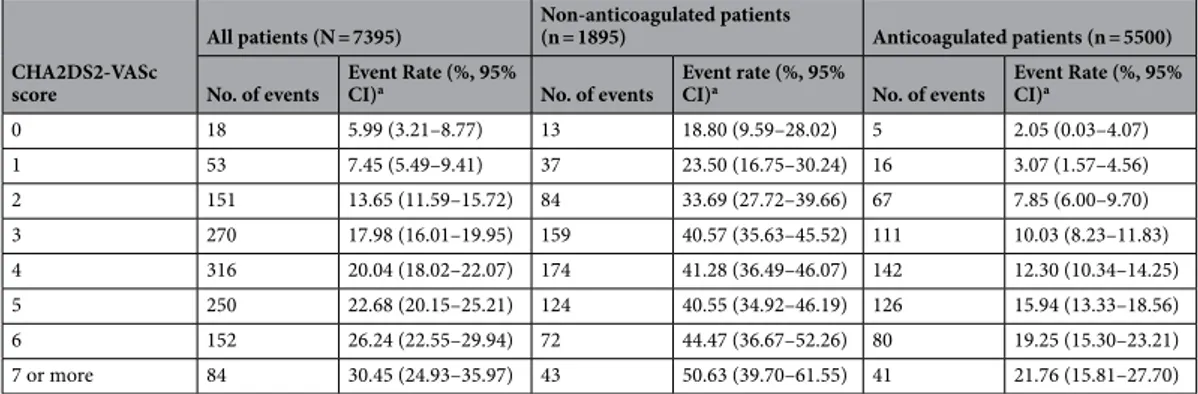
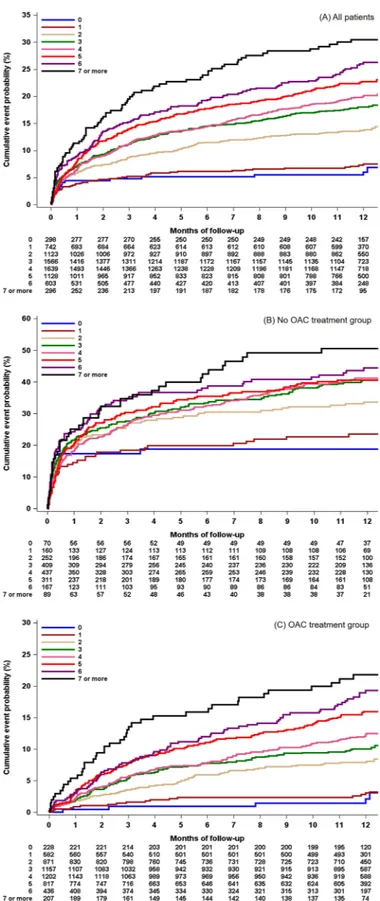
CHA2DS2-VASc score in acute ischemic stroke with atrial fibrillation: results from the Clinical Research Collaboration for Stroke in Korea
전체 글
수치
관련 문서
The aim of this study was to develop a training program for swallowing and to test its effect on swallowing capacity and nutritional status in stroke
Incident major cardiovascular events (coronary artery disease, ischemic stroke, hemorrhagic stroke and cardiovascular mortality) were set as primary end points.
Therefore, we investigated the association between migraine and major cardiovascular outcomes, including myocardial infarction (MI), ischemic stroke (IS), cardio-
"Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke
In addition, if the patients had a high MPV level (cut-off value of 7.95 fL) without low-dose aspirin therapy, they were at risk for ischemic stroke, especially in
The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third
Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council,
The clinical outcomes were assessed by measuring the Korea shoulder society (KSS) score, Mayo elbow performance (MEP) score and the postoperative complications.