online©ML Comm ORIGINAL ARTICLE
Vascular Neurology 2009;1:46-52 ISSN 2092-6855
Effect of Hypothermia on the Blood Brain Barrier Integrity after Focal Cerebral Ischemia
Soon Jung Suh,1 Yangha Hwang,2 Hyung Soo Han1
1Departments of Physiology, 2Neurology, Kyungpook National University School of Medicine, Daegu, Korea
Received April 22, 2009 Accepted April 30, 2009 Correspondence Hyung Soo Han, MD, PhD Department of Physiology, Kyungpook National University School of Medicine,
101 Dongin 2-ga, Jung-gu, Daegu 700-422, Korea Tel +82-53-420-4814 Fax +82-53-424-3349 E-mail hshan@knu.ac.kr
Background Blood Brain Barrier (BBB) is a robust barrier between central nervous system and peripheral circulation that regulate and maintain the microenvironment of the brain. The BBB is characterized by the presence of continuous endothelial cell tight junctions (TJs) that result in specifically regulated restrictive permeability. A number of studies indicated that phos- phorylation of TJ proteins plays a critical role in the BBB tightness. Mild hypothermia is known to protect BBB in cerebral ischemia. In this study, we examined how hypothermia modulates TJ proteins in stroke.
Methods We used 290-320 g of male Sprague -Dawley rats. Middle cerebral artery occlu- sion (MCAO) was used to induce focal cerebral ischemia. Ischemia was kept for 2 hours after MCAO onset. Normothermic rats were maintained at 37℃ of rectal temperature during ischemia while the temperature of hypothermic rats was maintained at 33℃. The animals were killed by carbon dioxide at 2, 6 or 24 hours after MCAO onset and prepared for other analysis.
Results FITC-dextran (FITC-D) extravasation and decrease of endothelial barrier antigen (EBA) expression was observed in MCAO brains. Even though there was no quantitative change of occluding, claudin, and ZO-1, phosphorylation of occludin and ZO-1 was increase in MCAO.
Interaction between occluding and ZO-1 was decreased by stroke. Hypothermia attenuated stroke induced changes of FITC-D and EBA. But there was no significant hypothermic effect on TJ proteins.
Conclusions Hypothermia protected BBB disruption by stroke but TJ proteins were not
affected by hypothermia. Vascular Neurology 2009;1:46-52
Key Words Stroke, Hypothermia, BBB, Tight junction protein.
Introduction
Blood Brain Barrier (BBB) is a physical and metabolic barrier between the central nervous system (CNS) and the peripheral blood circulation that serves to regulate and protect the microenvironment of the brain. BBB is mainly character- ized by the presence of continuous endothelial cell tight junc- tions (TJs) that result in specifically regulated restrictive permeability. The proteins of the junctional complexes have a special importance in maintaining the tightness of BBB.
The first protein discovered to be localized to TJs was occlu- din1 which later was followed by the members of the claudin family2 and the junctional adhesion molecules.3 The trans- membrane protein occludin binds to the members of the zonula occludens protein family (ZO-1, -2, and -3), cingulin
and other proteins which connect the junctional complex to the cytoskeleton. Claudins are a large family of transmem- brane proteins that contribute to the formation of TJs. The first members of the claudin family, claudin-1 and -2, were identified from occludin-containing chicken liver junctional fractions, and there are now 24 members of this family.2,4 ZO-1 was discovered first and ZO-2 and -3 were later iso- lated as proteins that co-immunoprecipitated with ZO-1.5-7 ZO-1, -2, and -3 are peripheral membrane associated com- ponents of the cytoplasmic plaque of TJs and are found ubi- quitously within TJs of epithelial and endothelial cells.
BBB dysfunction is a critical event in the development and progression of several CNS diseases. In case of ischemic stroke8 and traumatic brain injury,9 increased BBB permea- bility is the consequence of the pathogenesis. Cerebral is-
chemia is a complex insult that involves loss of blood flow as well as depletion of oxygen and essential nutrients10 and is associated with increased microvascular permeability,11,12 A number of studies using in vitro models of the BBB have in- dicated that hypoxia and hypoxia/reoxygenation lead to in- creased permeability or disruption of paracellular tightness of
BBB.13-15 Hypoxic stress may increase permeability via the
transcellular route as well.16,17 In vivo experimental models indicate that TJ disruption is likely involved in the progres- sion of ischemic brain injury.
A number of studies indicated that phosphorylation of TJ proteins plays a critical role in endothelial permeability regu- lation. It is demonstrated that transcriptional level and post- translational modifications such as phosphorylation of occlu- din are precisely regulated in the endothelium of the BBB and changes of occludin are related with the permeability changes as well.18,19
Mild hypothermia is well known to protect the brain in cere- bral ischemia both clinically20 and experimentally.21,22 Its neu- roprotective effects have often been attributed to decrease in cerebral blood flow and metabolic requirement for oxygen,23 alteration in neurotransmitter release,24 and many other mech- anisms including maintenance of the BBB permeability.25
The purpose of this study was to evaluate the hypothermic effect on BBB dysfunction and to investigate the phosphoryla- tion status of TJ proteins and interactions between TJ pro- teins following transient middle cerebral artery occlusion (MCAO).
Materials and Methods
Focal cerebral ischemia model
Experiments were carried out according to the guidelines for the care and use of laboratory animals approved by our university Administrative Panel on Laboratory Animal Care.
Animals were housed under diurnal lighting conditions in a temperature-controlled environment and with free access to food and water until the day of the experiment. Male Sprague- Dawley rats weighing between 290 and 320 g were anes- thetized with enflurane during surgical procedures. Ischemia was induced using an occluding intraluminal suture. An un- coated 30 mm segment of 3-0 nylon monofilament suture with a flame-rounded tip was inserted into the stump of the common carotid artery and advanced into the internal carotid artery approximately 19 or 20 mm from the bifurcation, to occlude the ostium of MCA. At the end of the ischemic pe- riod, the suture was removed and the animal was sacrificed.
Sham-operated animals were treated in the same manner as the ischemic animals, but no ischemia was applied. During surgery, rectal temperature of normothermic rats was main-
tained at 37℃, corresponding to a brain temperature of 38℃.
Mild hypothermia (33℃ rectal temperature corresponding to brain temperature of 33℃) was induced as previously described, using a method that consistently produces neuro- protection.22,26 In brief, total body cooling was achieved by spraying alcohol onto the animal and cooling it to the desired temperature with a fan. Cooling required approximately 10 minutes to achieve the target temperature in the hypothermic groups. Cooling began immediately after the suture was re- moved and kept for 2 hours. The animals were killed by car- bon dioxide at 2, 6, or 24 hours after ischemic onset and pre- pared for further analysis. Infarct size was measured using 2, 3, 5 - triphenyl tetrazolium chloride (TTC) stained brain sec- tions at 24 hours after MCAO.
BBB permeability
The permeability of the BBB was evaluated by dextran extravasation. Fluorescein isothiocyanate (FITC) labeled 70 kDa dextran (FITC-D, 2.5 mg/mL, 0.5 mL/rat, Sigma) was administered intravenously through the tail vein. After 1 hour, the animal was sacrificed and the brain was rapidly removed and placed in 20% sucrose at 4℃ for 24 hours.
Frozen sections (25 μm) were cut and slides were prepared for image analysis of FITC.
Immunohistochemistry
6 μm thick sections were cut from paraffin-embedded brains. After deparaffinization, sections were treated for en- dogenous peroxidases with 0.03% H2O2 and incubated in blocking solution containing 1% bovine serum albumin (BSA), 5% normal horse serum and 0.3% Triton X-100 in PBS. Sections were then incubated with primary antibody against endothelial barrier antigen (EBA, 1:2,000, Sternberger Monoclonals) flowed by the anti-mouse immunoglobulin G (IgG) secondary antibody (1:200, Vector Labs). Antibodies were detected using the Elite Vectastain ABC kit (Vector Labs) and colorized with 0.05% diaminobenzidine (DAB;
Vector Labs).
Western blot analysis
The ischemic hemisphere of brain was divided into cortex and subcortex. Each of the tissue was homogenized in lysis buffer (20 mM Tris-HCl pH 8.0, 137 mM NaCl, 10% glyc- erol, 2 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, 5 mM NaF). The lysate was centrifuged at 12,000 rpm for 20 minutes at 4℃. The proteins in supernatant were quantified with Bradford method using a Bio-Rad protein assay kit (Bio- Rad Laboratories). A 30 μg protein sample was boiled for 5 minutes at 100℃ in gel loading buffer (0.5 M Tris-HCl, glycerol, 10% SDS, 0.5% bromophenol blue) and run on
10% polyacrylamide gels. Proteins analyzed on gel electro- phoresis were transferred to polyvinylidene difluoride (PV- DF) membranes at 90V for 90 minutes. The membranes were then blocked with 5% skim milk in Tris-buffered saline (50 mM Tris, 10 mM NaCl pH 7.4) for 1 hour at room tem- perature and incubated overnight at 4℃ with the primary an- tibodies against occludin, claudin-1 and ZO-1 (1:100, Zym- ed). After washing with Tris buffered saline-Tween 20 solu- tion (50 mM Tris, 10 mM NaCl pH 7.4, and 1% Tween 20), the membranes were incubated for 1 hour at room temper- ature with peroxidase conjugated secondary antibody (1:5,000, Vector Labs) and washed again. Proteins were visualized by the ECL system. Band density was semiquantitatively an- alyzed with NIH Image program.
Immunoprecipitation
Protein samples were immunoprecipitated with some mod- ifications.27 This method allowed determination of the state of phosphorylation of the tyrosine residues of TJ proteins and interaction between TJ proteins. Briefly, protein extracts in lysis buffer were incubated with phosphorlated-tyrosine (p- Tyr) antibody (1:1,000, Sigma) and occludin antibody (1:
1,000, Zymed) at 4℃ overnight. Protein A/G Plus-Agarose (0.5 mL agarose/2.0 mL, Santa Cruz) was added to the sam- ple to a concentration of 10% (w/v) and incubated at 4℃
for 4 hours. The immunoprecipitate was centrifuged at 1,000 g and 4℃ for 5 minutes. The pellet was resuspended in wa- shing lysis buffer and these steps were repeated three times.
After the last centrifugation, the pellet was dried, resuspended in sample buffer (50 mM Tris, 2% SDS, 10% mercaptoe- thanol, 2% bromophenol blue) and boiled for 5 min before electrophoresis.
Statistical analysis
Values were expressed as means±S.D. Statistical analysis for multiple comparisons was performed with using one-way ANOVA, followed by the LSD post-hoc analysis using SPSS software (SPSS 12.0 KO for Windows). p<0.05 was used as criterion for the statistical significance.
Results
Hypothermic effect on the infarct size and BBB permeability after focal cerebral ischemic injury
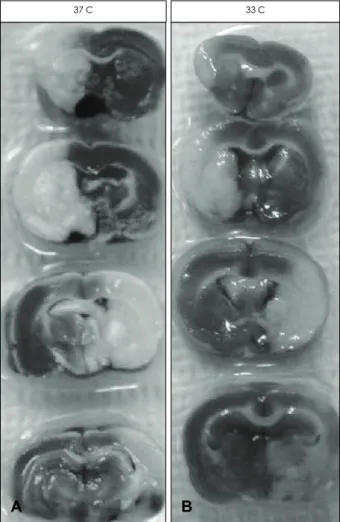
Infarct size following MCAO was decreased by hypother- mia as it is known. We repeated this known fact just to show our data is reproducible (Fig. 1). 1 hour after FITC-D admin- istration, brains were isolated and fluorescent image of the brain slices was observed under microscope. In normal brain, FITC-D was washed out from the blood vessels during 1 hour
of circulation after injection and no remaining FITC signal was observed (Fig. 2A). Since dextran (70 KDa) is not per- meable to normal BBB, it always stays inside of the cerebral vessels and easily moves away during circulation. MCAO brain showed significant amount of FITC signal in the brain parenchyma which indicates that FITC-D penetrated through BBB and was not cleared off completely (Fig. 2B). After is- chemic insult damages BBB, the integrity of BBB is disturbed and dextran can easily passes into the brain parenchyma. Since dextran is big and moving back into the blood circulation is not easy, it stays outside of the vessels for a long time as it is shown in MCAO brain. Signal intensity from hypothermic MCAO brain (Fig. 2C) was less than normothermic MCAO brain suggesting BBB integrity is not severely affected.
EBA expression in the endothelium
EBA is one of the typical marker proteins of BBB intactness and its expression in the brain vessels was observed from the
Figure 1. Hypothermia protects the brain against experimental ischemic stroke. Infarct size was decreased in hypothermic brain when the sections were stained with 2, 3, 5 - triphenyl tetrazolium chloride (TTC). The sections used here are from the brain ob- tained at 24 hours after ischemic insult. 37C: normothermic is- chemia and 33C: mild hypothermia.
B A
37 C 33 C
six selected regions in the ipsilateral hemisphere (Fig. 3A).
Area I, V and VI are located in the cortical area and area II to IV are located in the subcortical area. EBA immunostaining was observed in the cortex and subcortex in the ipsilateral hemisphere of each group (Fig. 3B con). The number of EBA immunoreactivity in the ipsilateral cortex (CI, dark bar) and subcortex (SI, light bar) was severely decreased at 24 hour (Fig. 3C). In addition, EBA stained endothelial cells demon- strated morphological abnormality compared with those in the normal brain area (Fig. 3B, 3C 37). These changes ob- served in normothermic MCAO were attenuated by mild hypothermia (Fig. 3B, 3C, 3C 33).
Western blot analysis of tight junction proteins The amount of typical TJ proteins such occludin, ZO-1 and
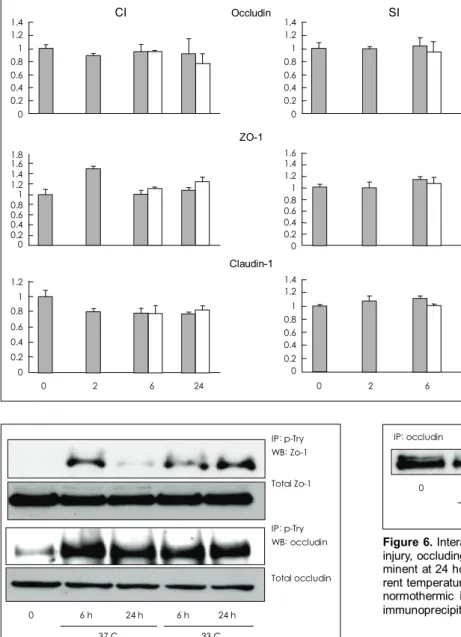
claudin-1 was measured in the normal (dark bars at 0 hour), MCAO (dark bars at 2, 6 & 24 hour) and hypothermic MC- AO (light bars at 6 & 24 hour) brain tissues. Total amount of these TJ proteins in the ipsilateral cortex (CI) and sub- cortex (SI) was not significantly altered by ischemia or hy- pothermia (Fig. 4).
Phosphorylation of tight junction proteins Phosphorylation of 2 TJ proteins such as ZO-1 and oc- cluding was investigated in the brain tissues. Since ZO-1 and occluding are phosphorylated at the tyrosine residue, the total protein was immunoprecipitated with antibody against p-Tyr residue first and then precipitated proteins were demonstrat- ed by specific antibody against ZO-1 and occludin by Western blot analysis. Phosphorylated ZO-1 was increased by MCAO
Figure 2. BBB permeability was evaluated using FITC labeled dextran. In the normal brain signal from FITC is not observed (A). In the ischemic brain extracted at 24 hours after ischemic insult, a strong signal from FITC is shown in the ipsilateral hemisphere (B). When hypothermia was applied, FITC signal is not strong compared with normothermic ischemia group (C). BBB: blood brain barrier, FITC:
Fluorescein isothiocyanate.
A B C
A
C
B Con 37 33 I
II
III
IV
V
VI
*
**
††
†
Con 37 33 Figure 3. Six regions in the ipsilateral
hemisphere were selected to measure EBA expression (A). Representative im- ages of EBA expression in the normal brain (Con), brain exposed to the normo- thermic ischemia (37), and hypothermic ischemia (33) in ipsilateral hemisphere were demonstrated (B). By counting the number of EBA positive vessels from the ipsilateral cortical (dark bars) and subcortical (light bars), effect of ische- mic insult at normal temperature (37) and hypothermic condition (33) was e- valuated (C). *(†) p<0.05 vs. con and
**(††) p<0.05 vs normothermic ische- mia. 400× magnification. EBA: endo- thelial barrier antigen.
and this was shown at 6 hour only in normothermic MCAO.
The increase of ZO-1 phosphorylation stayed at high level until 24 hours in hypothermic MCAO brain (Fig. 5 upper panel). Phosphorylated occludin was also increased by MC- AO and there was no difference between hypothermia and normothermia (Fig. 5 lower panel).
Interaction between occludin and ZO-1
Since the interaction between occludin and ZO-1 was well
known in the TJ complex, we observed whether there is any change of this interaction during MCAO or hypothermia.
We first immunoprecipitated the proteins from brain tissue with occludin specific antibody and then the precipitant was reacted with ZO-1 antibody for Western blot analysis. In the normal brain, the binding between occluding and ZO-1 is tight as shown in the dense ZO-1 band. After ischemic injury, ZO-1 band density is reduced markedly at 6 and 24 hours. In contrast to our expectation, decrease of occluding bound ZO- 1 level was not reversed by hypothermia (Fig. 6).
Discussion
In this study, we demonstrated focal cerebral ischemia in- duced dysfunction of BBB and hypothermic protection. Is- chemia induced BBB disruption is accompanied by increas- ed phosphorylation of occludin and ZO-1 and decreased in-
Figure 4. Immunoblot analysis of occl- udin, ZO-1 and claudin-1 in the ischemic hemisphere. There were no significant differences between control group and other experimental groups in the pro- tein levels of occludin, ZO-1 and clau- din-1. Tissues are taken from the cortex (CI) or subcortex (SI) under normal (dark bars at 0 hour), normothermic ischemia (dark bars at 2, 6 & 24 hour) and hy- pothermic ischemia (light bars at 6 & 24 hour). Values are mean±SD.
1.4 1.2 1 0.8 0.6 0.4 0.2 0
CI Occludin
1.4 1.2 1 0.8 0.6 0.4 0.2 0
SI
ZO-1 1.6 1.4 1.2 1 0.80.6 0.4 0.2 0 1.81.6
1.41.2 1 0.80.6 0.4 0.20
Claudin-1 1.4 1.2 1 0.8 0.6 0.4 0.2 0 1.2
1 0.8 0.6 0.4 0.2 0
0 2 6 24 0 2 6 24
Figure 5. Detection of phosphorylated occludin and ZO-1 was done using immunoprecipitation with phosphorylated tyrosine (p- Tyr) antibody and then western blot analysis with occludin and ZO-1 antibodies. Phosphorylation of occludin and ZO-1 was in- creased by ischemic insult and there were no differences between normothermic and hypothermic ischemic brains. Tissues were taken from ipsilateral cortex of normal, normothermic ischemia (37 C) or mild hypothermia groups (33 C). IP: immunoprecipitation, WB:
Western Blot.
IP: p-Try WB: Zo-1 Total Zo-1
IP: p-Try WB: occludin Total occludin
0 6 h 24 h 6 h 24 h 37 C 33 C
Figure 6. Interaction between occludin and ZO-1. After ischemic injury, occluding bound ZO-1 protein level was reduced and pro- minent at 24 hour groups. There were no differences in the diffe- rent temperature groups. Tissues are from ischemic cortex under normothermic ischemia (37 C) or mild hypothermia (33 C). IP:
immunoprecipitation, WB: Western Blot.
WB: Zo-1 IP: occludin
0 6 h 24 h 6 h 24 h 37 C 33 C
teraction between occludin and ZO-1. But these changes of occludin and ZO-1 were not reduced by hypothermia.
BBB dysfunction is known as a detrimental event in many acute brain diseases such as stroke and traumatic brain injury.
With the acute disruption of BBB, brain edema is induced and consequently leads to increase of intracranial pressure and death sometimes. Therefore control of BBB function in acute brain injury is critical to many clinicians. From the ac- cumulated data, hypothermia has been known as one of the robust protective therapeutic tool against acute brain injury.20-22 But there are few data supporting the hypothermic effect on BBB function and especially from the point of TJ proteins.
And there is no definitive explanation on the BBB preserving mechanisms of hypothermia.
In this study, protective effect of mild hypothermia was re- peated in a focal cerebral ischemic injury model. Since BBB dysfunction can easily observed by measuring the permea- bility of high molecular weight substance through BBB, we used FITC-labeled dextran. Disruption of the BBB caused extravasation of albumin and other high molecular weight
compounds,28-30 and passage of low molecular weight sub- stances is increased as well.31 Extravasation of proteins into
extracellular spaces is correlated with the development of vasogenic edema,32 which causes increased intracranial pres- sure, one of the main life-threatening complications of is- chemia/reperfusion injury. In addition, Dietrich et al.33 found a spatial correlation between increased vascular permeability and neuronal death showing the close interrelationship bet- ween neuronal injury and microvascular defects. Since in- creased BBB permeability could worsen the outcome after ischemia/reperfusion in several ways. In addition, EBA ex- pression is well known marker for BBB intactness, we also counted EBA expressing endothelial cells. EBA is known to be expressed normally in rat cerebral microvessels possessing BBB properties.34 Both experiments showed hypothermia is effective in maintaining BBB function. Since the tightness of BBB is mainly controlled by TJ, we investigated the changes of major TJ proteins such as occludin, ZO-1 and claudin-1.
When we measured the total amount of these proteins, MCAO caused no significant changes at all suggesting there is no loss or increase of these proteins in our model. Then we tried to determine the status of the TJ proteins. Phosphorylation is most common type of protein modification and there are known phosphorylation sites in occluding and ZO-1, we ob- served the changes of phosphorylated occludin and ZO-1 level in the ischemic brain. Even though ischemia increased phosphorylation of these two proteins, the amount of phos- phorylated proteins was not affected by hypothermia. Occlu- din physically interacts with several structural proteins at TJs.
The C-terminal cytoplasmic domain of occludin binds to the
GuK region of ZO-1 in vitro, and this interaction may serve to link occludin to the actin cytoskeleton.35,36 Similarly, ZO-2 and ZO-3 bind to the C-terminus of occludin in vitro.5,37 In- creased occludin phosphorylation correlates with enhanced permeability in several models, although decreased occludin phosphorylation in association with increased epithelial per- meability has also been described. Therefore, we examined whether phosphorylation and interaction of TJ proteins is affected by MCAO. Although phosphorylation of occludin occurs predominantly on serine and threonine residues, several studies indicate that occludin tyrosine phosphorylation may also play a role in both TJ assembly and disassembly. ATP depletion of MDCK cells decreased transendothelial electrical resistance (TER) and mediated the internalization of occlu- din from cell borders to a punctate intracellular pool.38 Oc- cludin was tyrosine phosphorylated when it was localized to TJ, however calcium depletion rapidly mediated the loss of tyrosine phosphorylation and caused TJ disassembly. Occlu- din phosphorylation on serine, threonine, and tyrosine resi- dues contributes to both occludin cellular localization and TJ permeability, and it is possible that multiple, distinct signal- ing pathways mediate occludin phosphorylation. Phosphory- lation of ZO-1 may also affect barrier properties that result in enhanced permeability. For instance, ZO-1 phosphorylation was enhanced in MDCK cells with low TER compared to MDCK cells with high TER.6 Furthermore, MDCK cells grown in low calcium conditions to prevent junction forma- tion possessed hypo-phosphorylated ZO-1 compared with cells grown in normal calcium conditions. The biological function of ZO-1 may also be regulated by tyrosine phosph- orylation. For example, epidermal growth factor stimulated ZO-1 tyrosine phosphorylation in association with ZO-1 rear- rangement from the lateral membrane to the apical cell bor- ders in A431 cells.
Our result showed that phophorylation of occluding and ZO-1 was increased and the interaction between occludin and ZO-2 was weakened after MCAO. However there was no hypothermic effect on TJ proteins even though BBB per- meability was reduced by hypothermia. Here we suggest two hypotheses. First, phosphorylation and interaction between TJ proteins are not directly related with BBB regulation me- chanism. Second, there is another pathway of hypothermic effect on BBB permeability not through TJ system.
Acknowledgments
This work was supported by Han’s grant No. RTI04-01-01 from the Re- gional Technology Innovation Program of the Ministry of Knowledge Eco- nomy (MKE) & Brain Korea 21 Project in 2006.
REFERENCES
1. Furuse M, Hirase T, Itoh M, NagafuchiA, Yonemura S, Tsukita S,
Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993;123:1777-1788.
2. Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998;141:1539-1550.
3. Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998;
142:117-127.
4. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junc- tions. Nat Rev Mol Celll Biol 2001;2:285-293.
5. Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junc- tion, interacts with ZO-1 and occludin. J Cell Biol 1998;141:199-208.
6. Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Iden- tification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelial. J Cell Biol 1986;103:755-766.
7. Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol 1994;
124:949-961.
8. Ilzecka J. The structure and function of blood-brain barrier in is- chaemic brain stroke process. Ann Univ Mariae Curie Sklodowska 1996;51:123-127.
9. Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflam- matory response in acute traumatic brain injury: a double-edged sword.
Curr Opin Crit Care 2002;8:101-105.
10. del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysi- ology of ischemic stroke. Thromb Res 2000;98:73-81.
11. Kempski O. Cerebral edema. Semin Nephrol 2001;21:303-307.
12. Petty MA, Wettstein JG. Elements of cerebral microvascular ischae- mia. Brain Res Brain Res Rev 2001;36:23-34.
13. Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia com- promises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther 1999;289:668-675.
14. Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-in- duced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1.
Microvasc Res 2002;63:70-80.
15. Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 2002;282:H1485-H1494.
16. Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neu- rochem 1997;68:874-877.
17. Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci 2004;9:777-785.
18. Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, et al. Regulation of tight junction permeability and occludin phosph- orylation by Rhoa-p160ROCK-dependent and -independent mecha- nisms. J Biol Chem 2001;276:10423-10431.
19. Ward PD, Klein RR, Troutman MD, Desai S, Thakker DR. Phospho- lipase C-gamma modulates epithelial tight junction permeability through hyperphosphorylation of tight junction proteins. J Biol Chem 2002;
277:35760-35765.
20. The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest.
N Engl J Med 2002;346:549-556.
21. Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia a critical appraisal with implications for clinical treatment. Mol Neu- robiol 1997;14:171-201.
22. Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK.
Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke 1998;29:2171-2180.
23. Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postische- mic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994;14:620-627.
24. Huang F, Zhou L. Effect of mild hypothermia on the changes of cere- bral blood flow, brain blood barrier and neuronal injuries following reperfusion of focal cerebral ischemia in rats. Chin Med J (Engl) 1998;111:368-372.
25. Smith SL, Hall ED. Mild pre- and posttraumatic hypothermia attenu- ates blood-brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma 1996;13:1-9.
26. Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. In- fluence of mild hypothermia on inducible nitric oxide synthase ex- pression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci 2002;22:3921-3928.
27. Velloso LA, Carneiro EM, Crepaldi SC, Boschero AC, Saad MJ.
Glucose- and insulin-induced phosphorylation of the insulin receptor and its primary substrates IRS-1 and IRS-2 in rat pancreatic islets.
FEBS Lett 1995;337:353-357.
28. Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke 2004;35:2220- 2225.
29. Kitagawa K, Matsumoto M, Tagaya M, Ueda H, Oku N, Kuwabara K, et al. Temporal profile of serum albumin extravasation following cerebral ischemia in a newly established reproducible gerbil model for vasogenic brain edema: a combined immunohistochemical and dye tracer analysis. Acta Neuropathol 1991;82:164- 171.
30. Maeda M, Akai F, Nishida S, Yanagihara T. Intracerebral distribution of albumin after transient cerebral ischemia: light and electron micro- scopic immunocytochemical investigation. Acta Neuropathol 1992;
84:59-66.
31. Nakagawa Y, Fujimoto N, Matsumoto K, Cervos-Navarro J. Morph- ological changes in acute cerebral ischemia after occlusion and re- perfusion in the rat. Adv Neurol 1990;52:21-27.
32. Kuroiwa T, Cahn R, Juhler M, Goping G, Campbell G, Klatzo I. Role of extracellular proteins in the dynamics of vasogenic brain edema.
Acta Neuropathol 1985;66:3 -11.
33. Dietrich WD, Halley M, Valdes I, Busto R. Interrelationships between increased vascular permeability and acute neuronal damage following temperature-controlled brain ischemia in rats. Acta Neuropathol 1991;
81:615-625.
34. Sternberger NH, Sternberger LA. Blood-brain barrier protein recog- nized by monoclonal antibody. Proc Natl Acad Sci USA 1987;84:
8169-8173.
35. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junc- tion protein ZO-1 establishes a link between the transmembrane pro- tein occludin and the actin cytoskeleton. J Biol Chem 1998;273:
29745-29753.
36. Furuse M, Itoh M, Hirase T, Nagafuchi S, Yonemura S, Tsukita S, et al. Direct association of occludin with ZO-1 and its possible involve- ment in the localization of occludin at tight junctions. J Cell Biol 1994;127:1617-1626.
37. Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem 1999;
274: 5981-5986.
38. Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reas- sembly of occludin and other tight junction proteins. Am J Physiol 1999;276:F737-F750.