This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/
by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Outcome of Robotic Gastrectomy in Gastric Cancer in Comparison with Laparoscopic Gastrectomy:
A Case-Control Study
Sang-Yong Son, M.D., Chang Min Lee, M.D., Sang-Hoon Ahn, M.D., Ju Hee Lee, M.D., Do Joong Park, M.D., Ph.D., Hyung-Ho Kim, M.D., Ph.D.
Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
Purpose: The aim of this study was to compare the outcomes of robotic gastrectomy (RG) and laparoscopic gastrectomy (LG) for surgical treatment of patients with gastric cancer.
Methods: Patients who underwent RG (study group) between December 2007 and December 2011 were enrolled in the study, and patients who underwent LG (control group) during the same period were matched for age, gender, and co-morbidity. Analysis of clinicopathological data, operative data, postoperative morbidity, and pathologic data was performed.
Results: Twenty one RG patients (study group) and 42 LG patients (control group) were enrolled in the study. The mean age of patients in the RG group was 52.3 years, and the ratio of male to female was 14 : 7. Nineteen distal gastrectomies (90.4%), one total gastrectomy (4.8%), and one proximal gastrectomy (4.8%) were performed in the RG group. A longer mean operative time (267.2 [range, 170∼360] vs. 166.7
[range, 95∼275] min, p<0.001) and more estimated blood loss (173.2 vs. 116.6 ml, p=0.014) were observed in the RG group; however, no difference in the mean numbers of harvested lymph nodes (39.7 vs. 46.5, p=0.063), duration of hospital stay (6.4 vs. 5.9 day, p=0.508), and early complication rate (9.6% vs. 4.8%, p=0.416) was observed between the two groups.
Conclusion: In our experience, RG with lymphadenectomy for treatment of early gastric cancer is technically feasible.
However, compared with LG, longer operative time and more estimated blood loss was observed with RG, while no difference was observed in numbers of harvested lymph nodes and length of hospital stay.
Key words: Robotic gastrectomy, Gastric cancer, Minimally invasive surgery
Received May 21, 2012, Revised May 29, 2012, Accepted May 30, 2012
※ Corresponding author:Hyung-Ho Kim
Department of Surgery, Seoul National University Bundang Hospital, 166, Gumi-ro, Bundang-gu, Seongnam 463-707, Korea Tel:+82-31-787-7095, Fax:+82-31-787-4055
E-mail:hhkim@snubh.org
INTRODUCTION
Gastric cancer is one of the leading causes of death due to cancer worldwide.1 Although chemotherapy has steadily impro- ved in the past decade, surgery still remains the primary mode of treatment. The prevalence of early gastric cancer (EGC) is growing with advance in diagnostics and screening programs, and minimally invasive surgery has become increasingly frequent. Laparoscopic surgery is a well-established minimally invasive surgical alternative to open surgery and has advantages ofreduced postoperative pain, shorter hospital stays, and less complications.2,3
Robotic surgery has recently emerged as minimally invasive option, and indications for this procedure have become more widespread. In gastric surgery, robot-assisted gastrectomy for
gastric adenocarcinoma was first reported in 2003.4 Since this report, several other studies reported short-term outcomes of robotic gastrectomy (RG) in gastric cancer. However, most studies had limitations mostly due to the nature of a small sample size and the retrospective design.5-8 To date, there has been no randomized controlled trial or long-term outcome study of RG, so its advantages in gastric cancer remain unclear.
The aim of this study was to evaluate the technical feasibility and oncological adequacy of RG in gastric cancer versus LG.
MATERIALS AND METHODS 1) Patients
We retrospectively identified 21 consecutive patients who underwent RG with lymphadenectomy for gastric cancer at the Seoul National University Bundang Hospital between Decem- ber 2007 and December 2011. LG patients (control group) were matched to RG patients (study group) in the proportion of 2 : 1 for age, gender, comorbidity, tumor stage, and operation methods during the same period.
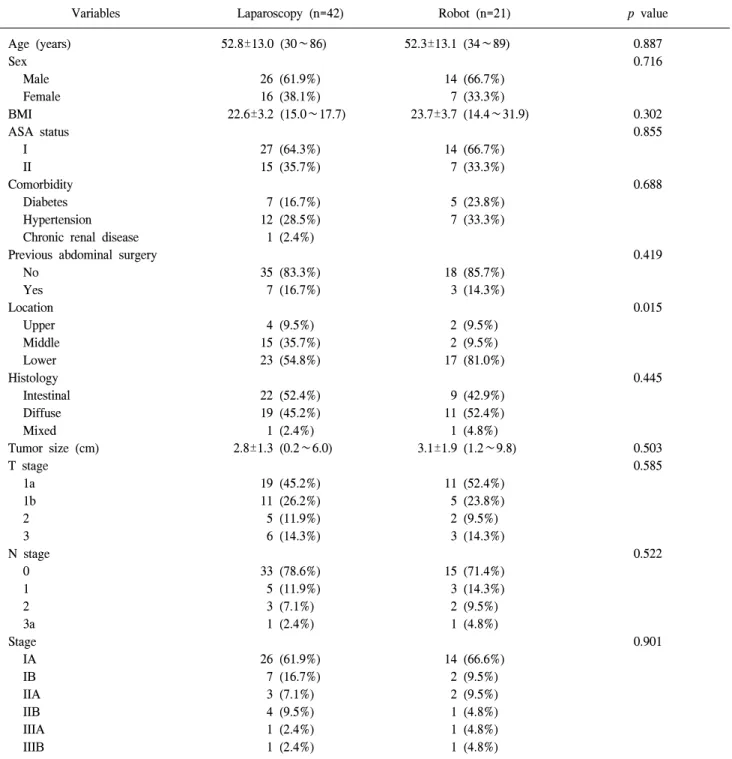
Table 1. Clinicopathological characteristics of patients
Variables Laparoscopy (n=42) Robot (n=21) p value
Age (years) Sex
Male Female BMI ASA status
I II Comorbidity
Diabetes Hypertension Chronic renal disease Previous abdominal surgery
No Yes Location
Upper Middle Lower Histology
Intestinal Diffuse Mixed Tumor size (cm) T stage
1a 1b 2 3 N stage
0 1 2 3a Stage IA IB IIA IIB IIIA IIIB
52.8±13.0 (30∼86)
26 (61.9%) 16 (38.1%) 22.6±3.2 (15.0∼17.7)
27 (64.3%) 15 (35.7%)
7 (16.7%) 12 (28.5%) 1 (2.4%)
35 (83.3%) 7 (16.7%)
4 (9.5%) 15 (35.7%) 23 (54.8%)
22 (52.4%) 19 (45.2%) 1 (2.4%) 2.8±1.3 (0.2∼6.0)
19 (45.2%) 11 (26.2%) 5 (11.9%) 6 (14.3%)
33 (78.6%) 5 (11.9%) 3 (7.1%) 1 (2.4%)
26 (61.9%) 7 (16.7%) 3 (7.1%) 4 (9.5%) 1 (2.4%) 1 (2.4%)
52.3±13.1 (34∼89)
14 (66.7%) 7 (33.3%) 23.7±3.7 (14.4∼31.9)
14 (66.7%) 7 (33.3%)
5 (23.8%) 7 (33.3%)
18 (85.7%) 3 (14.3%)
2 (9.5%) 2 (9.5%) 17 (81.0%)
9 (42.9%) 11 (52.4%) 1 (4.8%) 3.1±1.9 (1.2∼9.8)
11 (52.4%) 5 (23.8%) 2 (9.5%) 3 (14.3%)
15 (71.4%) 3 (14.3%) 2 (9.5%) 1 (4.8%)
14 (66.6%) 2 (9.5%) 2 (9.5%) 1 (4.8%) 1 (4.8%) 1 (4.8%)
0.887 0.716
0.302 0.855
0.688
0.419
0.015
0.445
0.503 0.585
0.522
0.901
Data was presented as n (%) or mean±SD (range). BMI = body mass index; ASA = American Society of Anesthesiologists.
2) Surgical procedure
All patients underwent subtotal or total or proximal gastrectomy with D1+β or greater lymph node dissection.
Reconstruction after subtotal gastrectomy was gastroduodeno- stomy or uncut Roux-en-Y gastrojejunostomy, and Roux-en-Y esophagojejunostomywas performed after total gastrectomy.
Esophagogastrostomy was performed in the case of proximal gastrectomy.
Cancers were staged according to the tumor node metastasis (TNM) classification system (7th edition) as reported by the American Joint Committee on Cancer (AJCC). A potentially curative resection was defined as R0 resection using the AJCC residual tumor classification.
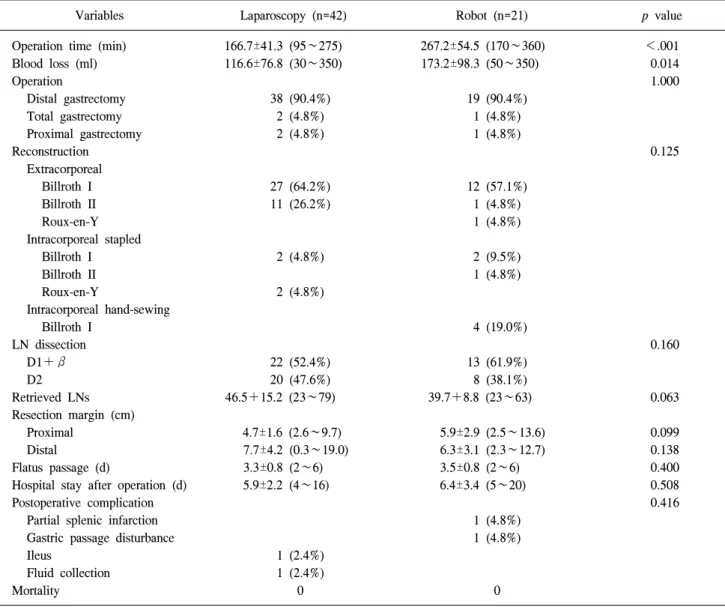
Table 2. Operative and short term outcomes
Variables Laparoscopy (n=42) Robot (n=21) p value Operation time (min)
Blood loss (ml) Operation
Distal gastrectomy Total gastrectomy Proximal gastrectomy Reconstruction
Extracorporeal Billroth I Billroth II Roux-en-Y Intracorporeal stapled
Billroth I Billroth II Roux-en-Y
Intracorporeal hand-sewing Billroth I
LN dissection D1+β D2 Retrieved LNs Resection margin (cm)
Proximal Distal
Flatus passage (d)
Hospital stay after operation (d) Postoperative complication
Partial splenic infarction Gastric passage disturbance Ileus
Fluid collection Mortality
166.7±41.3 (95∼275) 116.6±76.8 (30∼350)
38 (90.4%) 2 (4.8%) 2 (4.8%)
27 (64.2%) 11 (26.2%)
2 (4.8%) 2 (4.8%)
22 (52.4%) 20 (47.6%) 46.5+15.2 (23∼79)
4.7±1.6 (2.6∼9.7) 7.7±4.2 (0.3∼19.0) 3.3±0.8 (2∼6) 5.9±2.2 (4∼16)
1 (2.4%) 1 (2.4%)
0
267.2±54.5 (170∼360) 173.2±98.3 (50∼350)
19 (90.4%) 1 (4.8%) 1 (4.8%)
12 (57.1%) 1 (4.8%) 1 (4.8%)
2 (9.5%) 1 (4.8%)
4 (19.0%)
13 (61.9%) 8 (38.1%) 39.7+8.8 (23∼63)
5.9±2.9 (2.5∼13.6) 6.3±3.1 (2.3∼12.7) 3.5±0.8 (2∼6) 6.4±3.4 (5∼20)
1 (4.8%) 1 (4.8%)
0
<.001 0.014 1.000
0.125
0.160
0.063
0.099 0.138 0.400 0.508 0.416
Data was presented as n (%) or mean±SD (range). LN = lymph node.
All RG with lymphadenectomy was performed using the Da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA) by two advanced laparoscopic surgeons.
3) Statistical analysis
All statistical analyses were carried out using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The χ2 test and Fisher’s exact test, and an independent Student’s test were used for comparisons between the two groups. p values of less than 0.05 were considered statistically significant.
RESULTS
Details of clinical and pathological features of 21 RG
patients and 42 LG patients are shown in Table 1. The mean age of the RG group was 52.3 years, and the ratio of male to female was 14 : 7. 19 distal gastrectomies (90.4%), 1 total gastrectomy (4.8%), and 1 proximal gastrectomy (4.8%) were performed in the RG group. Extracorporeal anastomosis was performed via mini-laparotomy in 14 patients (66.7%), and 7 intracorporeal anastomoses were performed in the RG group, including 4 hand-sewn gastroduodenostomies (33.3%) (Table 2). The RG group showed longer mean operative time (267.2 vs. 166.7 min, p<0.001) and more estimated blood loss (173.2 vs. 116.6 ml, p=0.014), but there was no difference in the mean numberof the harvested lymph nodes (39.7 vs. 46.5, p=0.063), length of hospital stay (6.4 vs. 5.9 day, p=0.508), and early complication rate (9.6% vs. 4.8%, p=0.416) between the two
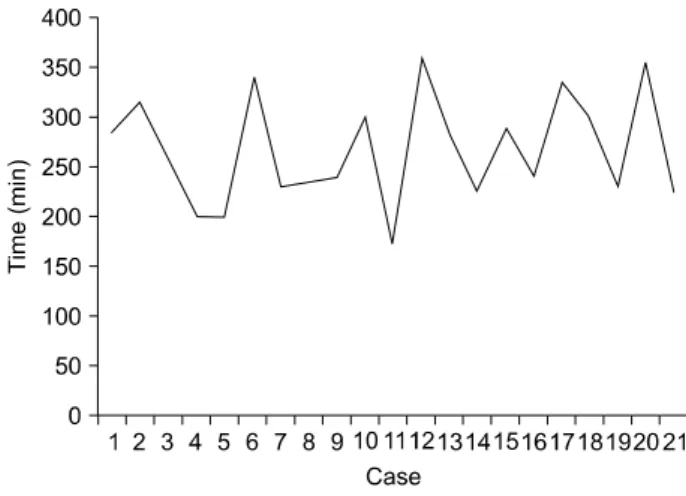
Fig. 1. Operation times.
groups. One gastric passage disturbance due to anastomotic stenosis was observed in a patient who underwent robotic distal gastrectomy with hand-sewn Billroth I anastomosis using robotic arms, but the patient’s condition was improved after conservative management and the patientswas discharged at postoperative day 20 without further complications.
DISCUSSION
The clinical advantage of the da Vinci system still remains controversial due to its high cost, even though it may offer surgeons the technical solutions to limitations of conventional laparoscopic surgery. These solutions include the following: a steady camera platform with a 3D magnified imaging, surgical instrument with a high freedom of degrees (EndoWrist), filtration of resting tremor, and anergonomical design for increased comfort forsurgeons.9,10 For these reasons, robotic gastrectomyappears to have most advantages in fine operations, such as D2 lymph node dissection and intracorporeal anasto- mosis.
Since the first robot-assisted gastrectomy for adenocarci- noma, more than 20 reports related to RG in gastric cancer were published until 2011. Some studies reported the promising results of RG in gastric cancer in terms of less intraoperative bleeding and shorter hospital stay, in comparison to conven- tional LG.5,6,11
In the present study, there were no differences in the number of harvested lymph nodes and length of hospital stay between the RG and LG groups. However, the RG group showed longer operative time and higher estimated blood loss. It is well-kno- wn that the operation time for RG is longer than for LG due to longer operation set-up time and the added learning curve.7,12,13 However, there was no learning curve on the operative time in the present study (Fig. 1). We speculate two reasons to explain this. First, it may be because intracorporeal anastomoses were performed in the late period. Three intracor- poreal anastomoses using staplers or 4 hand-sewn anastomoses using robotic arms were performed within the late 10 cases, and the mean operation time was longer than that of the early 11 cases (284.5 [range, 225∼360] vs. 251.5 min [range, 170∼
340], p=0.172). In particular, the mean operation time of 4 hand-sewn cases was relatively longer, with an average of 322.5 min [range, 240∼355]. Second,the surgeons who per- formed the operation were highly experienced in LG. Our medical center is one of the large-volume LG centers in South Korea, and more than 70% gastrectomies in gastric cancer are performed with laparoscopy. In fact, one of the surgeons
performed more than 40 robotic gastrectomies up to 2011, including the experience from other institute. For these reasons, we might not have observed a learning curve in this study.
In contrast to other studies, our results showed a highere- stimated blood loss in the RG group compared to the LG group.
We postulate that this finding us due to the limitations of the present study, mostly stemming from its small sample size or surgeon’s competence in laparoscopy. Robotic surgery still has several shortcomings, including the absence of tactile sense, lack of various robotic instruments such as a suction-irrigator and endostaplers. And its macroscopic manipulation speeds and shift of scene in the da Vinci system are not quick enough, and it also needs more time to change instruments rather than the laparoscopic surgery. For these shortcomings, it cannot be excluded that unfamiliar circumstance to surgeons, who are very accustomed in laparoscopic surgery, might affect the result of this study.
The best advantage of robot surgery may be the free movement with its articulated robotic arms, which could allow a robot-sewn anastomosis intracorporeally. A recent study reported that a robot-sewn anastomosis in gastric cancer is technically feasible.14 In this study, 4 robot-sewn gastroduode- nostomies were performed, and the mean operation time of these operations were relatively longer than other operations.
Although one gastric passage disturbance occurred among 4 robot-sewn anastomoses, we still presume that robot-sewn anastomosis is technically feasible.
Recent advances in laparoscopic instruments and skills have allowed the development of the single-incision laparoscopic approach. Ozdemir et al.15 first reported the single-port laparo- scopic subtotal gastrectomy in gastric cancer. However, unre- solved issues still exist relating to single-port laparoscopic
surgery (SPLS). The major problem of SPLS is the clashing of instruments during the operation.16 The robotic system may be the solution to this SPLS limitation in the near future.
Flexible multichannel robotic systems, various robotic instru- ments, and intracorporeal mobile devices are likely to be developed soon.
In conclusion, this study provided evidence that RG with lymphadenectomy for the surgical treatment of gastric cancer is safe, technically feasible, and oncologically effective in the aspect of number of harvested lymph nodesand the adequacy of lymphadenectomy compared with conventional LG.
Prospective studies and long-term oncologic outcomes are needed to further confirm the clinical advantages of RG in the treatment of gastric cancer.
ACKNOWLEDGEMENTS
This work was supported by grant (SNUBH 02-2008-015) from the Seoul National University Bundang Hospital, Republic of Korea.
REFERENCES
1) Parkin DM. International variation. Oncogene 2004;23:6329- 6340.
2) Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M.
Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int 2006;97:1190-1193.
3) Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC.
Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg 2006;202:
874-880.
4) Hashizume M, Sugimachi K. Robot-assisted gastric surgery.
Surg Clin North Am 2003;83:1429-1444.
5) Caruso S, Patriti A, Marrelli D, et al. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot
2011;7:452-458.
6) Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 2010;24:610-615.
7) Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 2012;26:
1377-1381.
8) Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH.
Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 2009;23:1204-1211.
9) Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Buchler MW. Robot-assisted abdominal surgery. Br J Surg 2004;91:
1390-1397.
10) Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-784.
11) Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086-1092.
12) Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH.
Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-932.
13) D'Annibale A, Pende V, Pernazza G, et al. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res 2011;166:e113-120.
14) Hur H, Kim JY, Cho YK, Han SU. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer.
J Laparoendosc Adv Surgical Tech A 2010;20:693-697.
15) Ozdemir BA, Thomas RL, Soon Y. Single-port laparoscopic subtotal gastrectomy with DIalpha lymphadenectomy. Surg Innov 2011;18:NP1-4.
16) Lee JH, Lee MS, Kim HH, et al. Comparison of single- incision laparoscopic distal gastrectomy and laparoscopic distal gastrectomy for gastric cancer in a porcine model. J Laparoendosc Adv Surgical Tech A 2011;21:935-940.