저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The effect of cutaneous vasculature on skin
pigmentation
by
Ji-Youn Park
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
The effect of cutaneous vasculature on skin
pigmentation
by
Ji-Youn Park
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Ph.D. in Medicine
Supervised by
Hee Young Kang, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Ji-Youn Park is approved.
SUPERVISORY COMMITTEE
Hee Young Kang
Tae Jun Park
Sang Myun Park
Jang-Hee Kim
Eunsun Jung
The Graduate School, Ajou University
June, 19th, 2015
- ABSTRACT-
The effect of cutaneous vasculature on skin pigmentation
Previously, it was demonstrated that a hyperpigmented melasma lesion is characterized by increased vasculature, which may play a role in the development of hyperpigmentation. Herein, I report the in vitro evidence of the vascular modulation of skin pigmentation. In the presence of endothelial cells, the pigmentation of melanocytes and of ex vivo cultured human skin was reduced. Contrary to non-stimulated cells, UV-irradiated endothelial cells showed stimulatory action on skin pigmentation. However, other stimulation, such as VEGF treatment and hypoxic condition, did not shown any significant effect. Gene expression profiling was undertaken to identify melanogenic factors from UV-irradiated endothelial cells. Endothelin-1 (ET-1) was found to be up-regulated, and the increased pigmentation elicited by UV-irradiated endothelial cell-derived conditioned medium was prevented by inhibiting the ET-1 receptor. Our study suggests that endothelial cells have a negative effect on pigmentation. However, their effect differs after they are irradiated with UV, as they then positively influence the induction of melanogenesis. This may play a role in the development of hyperpigmentary disorders.
Keywords: Co-culture, Cutaneous vasculature, Endothelial cells, Hyperpigmentary disorder, Melanocytes, Melasma, Skin pigmentation
TABLE OF CONTENTS
ABSTARCT ··· i
TABLE OF CONTENTS ··· ii
LIST OF FIGURES ··· iv
LIST OF TABLES ··· vii
I. INTRODUCTION ··· 1
II. MATERIALS AND METHODS ··· 3
A. Cell cultures and treatment ··· 3
B. Co-culture using transwell system ··· 4
C. Preparation of the bEnd.3/HUVECs conditioned medium ··· 4
D. Ultraviolet (UV) irradiation of endothelial cells ··· 5
E. Endothelial tube formation assay model using GeltrexTM Matrix ··· 5
F. Hypoxia treatments ··· 5
G. Melanin content and tyrosinase activity ··· 5
H. Western blot analysis ··· 6
I. Real-time PCR and RT-PCR ··· 6
J. RNA sequence analysis ··· 7
K. Ex vivo skin organ culture and pigmentation assay of cultured skin ··· 7
L. Statistical analysis ··· 8
III. RESULTS ··· 9
1. Effect of HUVECs on the proliferation of fibroblasts ··· 9
B. The presence of endothelial cells has a negative effect on pigmentation ··· 10
1. The effect of murine endothelial cells on pigmentation ··· 10
2. The verification of the effect of endothelial cells with human cells ··· 12
C. Additional modification of endothelial cells ··· 15
1. UV irradiation: Contrary to non-stimulated cells, UV-irradiated endothelial cells induce pigmentation ··· 15
2. VEGF treatment ··· 19
3. Endothelial tube formation assay using GeltrexTM Matrix ··· 20
4. Endothelial cell-conditioned media with hypoxic condition ··· 22
5. Triple co-culture study with NHMs, HUVECs, and fibroblasts ··· 23
D. Identification of possible melanogenic factors secreted by endothelial cells ··· 24
1. Identification of endothelin-1 as one of melanogenic factors secreted by UV-irradiated endothelial cells ··· 24
2. Identification of candidate cytokines derived from HUVECs using angiogenesis-related cytokine antibody array ··· 28
IV. DISCUSSION ··· 31
V. CONCLUSION ··· 36
REFERENCES ··· 37
LIST OF FIGURES
Fig. 1. Effect of HUVECs on the proliferation of fibroblasts. ··· 9
Fig. 2. Effects of endothelial cells on the pigmentation of B16 melanoma cells ··· 11
Fig. 3. Effects of endothelial cells on the pigmentation of normal human melanocytes (NHMs) and of ex vivo human skin ··· 14
Fig. 4. Effect of UV-irradiated endothelial cells on pigmentation··· 17
Fig. 5. VEGF treatment on NHMs – HUVECs co-culture model ··· 20
Fig. 6. Endothelial tube formation assay using GeltrexTM Matrix ··· 21
Fig. 7. Using hypoxic condition as one of the activating conditions for endothelial cells 22 Fig. 8. Triple co-culture study with NHMs, HUVECs, and fibroblasts using transwell plates. ··· 23
Fig. 9. The study using HUVECs-, fibroblasts-, or both HUVECs/fibroblasts-conditioned medium. ··· 24
Fig. 10. Identification of endothelin-1 as a melanogenic factor secreted by UV-irradiated endothelial cells ··· 27
LIST OF TABLES
Table 1. Primer sequences (5′–3′) used in real-time PCR ··· 6
Table 2. Different expression of angiogenic-related cytokines in HUVECs-conditioned medium compared with control group ··· 30
I. INTRODUCTION
Epidermal melanocytes synthesize melanin pigments. A crucial role of skin pigmentation is to absorb ultraviolet (UV) radiation, thus serving as a natural sunscreen. However, the excessive production of melanin results in hyperpigmentation, which is represented as melasma or solar lentigo. Skin pigmentation by UV radiation is regulated by interactions between melanocytes and neighboring cutaneous cells, such as keratinocytes, fibroblasts, or inflammatory cells by complex regulatory signals (Schallreuter et al., 2008; Yamaguchi and Hearing, 2009; Abdel-Malek et al., 2010). Recently, increasing evidence of this type of interaction has highlighted the contribution of dermal components in the regulation of pigmentation. For example, dermal fibroblasts were demonstrated to exert a regulatory function with regard to the determination of human constitutive skin color through the secretion of soluble factors, specifically Dickkopf-related protein-1 (DKK1) and neuregulin-1 (Yamaguchi et al., 2009; Choi et al., 2010). The role of UV-irradiated fibroblasts in the development of hyperpigmentary disorders such as solar lentigo or melasma has also been highlighted (Kang et al., 2006; Kovacs et al., 2010; Choi et al., 2015). Melasma is a commonly acquired hyperpigmentary disorder of the face, but its pathogenesis is poorly understood. Previously, it was reported that lesional melasma skin is characterized by increased vasculature as well as increased pigmentation (Kim et al., 2007; Kang et al., 2010; Kang et al., 2011b). The increased vasculature in melasma was thought to be one of the consequences of UV irradiation considering the increased expression of VEGF, which is known to be a major angiogenic factor of UV-irradiated skin. Interestingly, a
positive correlation exists between pigmentation and the degree of vasculature. Indeed, in clinical settings, vascular targeted treatments have shown considerable therapeutic effects for melasma (Lee et al., 2010; Passeron et al., 2011; Na et al., 2013). It was suggested that vascular reduction decreases melanocyte stimulation and that skin vascularization plays a role in pigmentation processes. A long-lasting effect of a vascular-targeted treatment in melasma was also demonstrated (Passeron, 2013). Furthermore, the upregulation of several angiogenesis-related genes, such as angiopoietin-like 1 and 2, heparanase, and MMP-2 in the lesional skin of melasma were demonstrated by transcriptional study (Kang et al., 2011b). Taken together, these findings suggest a possible biological role of cutaneous vasculature in the regulation of melanogenesis and in the development of UV-induced hyperpigmentary disorders.
Although the previous clinical investigation showed the possibility of the involvement of cutaneous vasculature on melanogenesis, yet there is little in vitro study to prove. Therefore, in the present study, I investigated the role of endothelial cells in regulation of skin pigmentation. Also, I tried to demonstrate that their effects could be affected by UV irradiation.
II. MATERIALS AND METHODS
A. Cell cultures and treatment
Normal human melanocytes (NHMs) and fibroblasts were isolated from human foreskin during circumcision surgery through a modification of a previously described method (Tsuji and Karasek, 1983). NHMs were cultured in the F12 medium or MCDB153 medium. The F12 medium was supplemented with 10% fetal bovine serum (FBS, Gibco-BRL, Bethesda, MD), 24 µg/ml 3-isobutyl-1-methylxanthine, 80 nM 12-O-tetradecanoyl phorbor 13-acetate (TPA), 1.2 ng/ml basic fibroblast growth factor (bFGF), and 0.1 µg/ml cholera toxin (all from Sigma, St. Louis, MO). The MCDB153 (Welgene, Daegu, Korea) medium contained 4% FBS, 0.6 ng/ml bFGF, 5 µg/ml insulin, 0.1 µg/ml vitamin E, and 1 µg/ml transferrin (all from Sigma). In the experiments, the NHMs were used at passages from 2 to 7. Human fibroblasts at passage 2 or 3 were maintained in RPMI1640 medium supplemented with 10% FBS. Human umbilical vein endothelial cells (HUVECs, Lifeline Cell Technology, Frederick, MD) were cultured in HUVECs growth medium (HGM, Lifeline Cell Technology). The HUVECs were used until passage 5. Human dermal microvascular endothelial cells (HDMECs, Lonza, Basel, Switzerland) were cultured in HDMECs growth medium (EGM, Lonza). Murine B16 melanoma cells and bEnd.3 (murine endothelial cell line) were purchased from ATCC (Manassas, VA) or kindly provided by Prof. Jung YS (College of Pharmacy, Ajou University), respectively, and were cultured in DMEM containing 10% FBS. For experiments, HUVECs were treated with 10 ng of human recombinant vascular endothelial growth factor (VEGF, R&D systems Inc., Minneapolis, MN) and B16 melanoma
cells were treated with 1 µM BQ-123 (Sigma) in conjunction with a UV-irradiated conditioned medium for 3 days.
B. Co-culture using transwell system
For the co-culture of B16 melanoma cells and bEnd.3 cells, bEnd.3 cells were seeded onto inserts of six-well transwell plates (Corning, Tewksbury, MA) in DMEM. After 24 hr of stabilization, the inserts were placed in wells overlying B16 melanoma cells. After a 3-day co-culture period, the B16 melanoma cells were harvested.
For the co-culture of NHMs and HUVECs, pre-selection of the most adequate medium was performed. Several types of commercial media were tested, specifically MCDB153, F12, HGM, and a mixture 1:1 and 1:4 of these last two media. The cell morphology was examined under an inverted microscope (Axiovert 200, Zeiss, Oberkochen, Germany), and the cytotoxicity was analyzed using a MTT assay. After the selection of the medium, NHMs and HUVECs were co-cultured using transwell plates for 5 days. The insert with HUVECs was changed to a fresh one every 2 days.
C. Preparation of the bEnd.3/HUVECs conditioned medium
To measure the cellular effects of the conditioned medium, bEnd.3 cells were cultured in DMEM for 24 hrs. HUVECs were cultured in the MCDB153 medium with the aforementioned supplements for 24 hrs. The media were collected and filtered through 0.2-μm filters (Millipore, Billerica, MA) to remove cellular components and debris. The medium obtained from the bEnd.3 cells were further concentrated by 60-fold with protein concentrators (pore size 3kDa, Thermo, Rockford, IL). Fifty µl of the concentrated conditioned medium in total1.5 ml media volume was used to treat.
D. Ultraviolet (UV) irradiation of endothelial cells
The bEnd.3 and HUVECs were washed with phosphate-buffered saline (PBS) once and placed in fresh PBS. The cells were irradiated with 50 mJ/cm2 of UVB using TL 20W/12 RS
UV lamps (Philips, Eindhoven, Netherlands). After irradiation, the cells were incubated in DMEM or HGM medium (for the bEnd.3 or HUVECs, respectively) for 24 hrs.
E. Endothelial tube formation assay model using GeltrexTM Matrix
Endothelial tube formation assay was performed using GeltrexTM Matrix (Invitrogen).
GeltrexTM Matrix was thawed during overnight in refrigerator (4°C) and placed 50 µL per
cm2 onto the growth surface of 6-well plate. The coated 6-well plate was incubated at 37°C
for 30 minutes. Endothelial cells were seeded on the coated 6-well plate and incubated for 16 hr, until the endothelial tube formation.
F. Hypoxia treatments
Hypoxic condition was obtained as follow: Briefly, basal medium was preequilibrated with a hypoxic gas mixture (1% O2, 5% CO2, and 94% N2). After the equilibration of medium, the
growth factors of medium were added. Monolayer cultures were replenished with the hypoxic medium, placed in the hypoxia incubator and maintained for 24 h at 37°C. Control monolayer cultures were maintained in a normoxic environment, which refers to standard cell culture in a humidified incubator (20% O2, 5% CO2, and 75% N2).
G. Melanin content and tyrosinase activity assays
The cells were lysed with a 0.1 M phosphate buffer (pH 6.8) containing 1% Triton X-100 with a protease inhibitor cocktail (Roche, Basel, Switzerland). The supernatants were measured to determine the protein concentration using the Lowry assay system. Pellets were
solubilized in 100 µl of 1 N NaOH for 3 hrs at 60°C and the absorbance was measured at 490 nm to analyze the melanin content. The melanin content was calculated from a standard curve using synthetic melanin (Sigma). For the assay of the tyrosinase activity, each sample was incubated with 2 mM L-DOPA (sigma) in a 0.1 M phosphate buffer (pH 6.8) for 90 min at 37°C. After incubation, the tyrosinase activity was measured at 490 nm.
H. Western blot analysis
Cells were lysed in a RIPA buffer with Protease Inhibitor Cocktail (Roche). The lysates were separated by SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked with 5% skimmed milk for 1 hour and incubated with anti-MITF (Abcam Cambridge UK) and anti-tyrosinase (Santa Cruz Biotechnology TX) monoclonal antibodies for 24 hours at 4°C. Development of the membrane was realized performed using enhanced chemiluminescence kits (Millipore).
I. Real-time PCR and RT-PCR
Total cellular RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA), and the cDNA was obtained using a SuperScriptTM III Reverse Transcriptase (Invitrogen). The primer sequences were noted on Table 1.
Table 1. Primer sequences (5′–3′) used in real-time PCR.
Target Sense (5′-3′) Anti-sense(3′-5′)
Mouse tyrosinase TTACAGCTGACAAGGGTCTT ACATACCTTGAACCGCTAGA Mouse MITF TCTGGATCTCACGGACGGTA CGGTGACTCCAACAGGTGAG Mouse GAPDH GGAGCCAAAAGGGTCATCAT GTGATGGCATGGACTGTGGT
Mouse ET-1 GGAAACTACGAAGGTTGGAGGC CTGTAGAAGCCACACAGATGGTCT Human tyrosinase CACCACTTGGGCCTCAATTTC AAAGCCAAACTTGCAGTTTCCAC Human MITF AGAACAGCAACGCGCAAAAGAAC TGATGATCCGATTCACCAAATCTG Human VEGF-A TCTTCAAGCCATCCTGTGTG TCTGTCGATGGTGATGGTGT Human GAPDH GCACCGTCAAGGCTGAGAAC TGGTGAAGACGCCAGTGGA
PCR amplification was performed under the following conditions: 27 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 35 s for ET-1; 27 cycles at 94°C for 30 s, 52°C for 30 s, and 72°C for 35 s for GAPDH.
J. RNA sequence analysis
Total cellular RNA of the UV-irradiated or non-irradiated bEnd.3 cells was extracted using the RNeasy Mini Kit (Qiagen). The quantity and quality of the RNA were measured using a nano-photometer (Implen GmbH, Munich, Germany) and with agarose gel electrophoresis. The total RNA was enriched for mRNA using poly(A) selection. Standard Illumina protocols (Illumina, San Diego, CA) were used to generate 2x100 bp paired-end read libraries that were sequenced on the Illumina HiSeq 2500 platform. The R package DESeq was utilized to identify differentially expressed genes (Anders and Huber, 2010). The difference was considered significant if the adjusted p-value did not exceed 0.05.
K. Ex vivo skin organ culture and pigmentation assay of cultured skin
Human skin samples were obtained during plastic surgery after receiving consent and were cultured as previously described (Moll et al., 1998). Briefly, a sterilized stainless steel grid was placed on a culture dish containing DMEM supplemented with 4% FBS. The skin
specimens were placed on the stainless steel grid and maintained in an incubator at 37°C with 5% CO2. After 3 days, the specimens were fixed in 10% formalin and embedded in paraffin sections. Melanin pigments were visualized by Fontana–Masson staining. Image analysis was performed using Image Pro Plus Version 4.5 software (Media Cybernetics Co., Rockville, MD) and the pigmented area per epidermal area (PA/EA) was measured.
L. Statistical analysis
Statistical significance was tested with student’s t tests. A p-value of <0.05 was considered significant.
III. RESULTS
A. Preliminary study
1. Effect of HUVECs on the proliferation of fibroblast
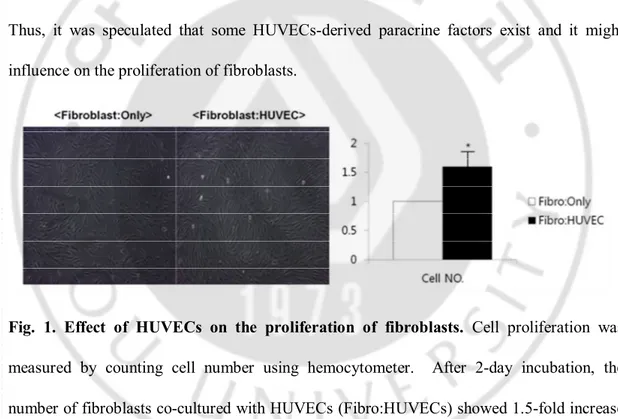
In order to determine the paracrine effect of HUVECs-derived factors, fibroblasts were co-cultured with HUVECs using transwell plates as a preliminary study. After 2-day incubation, the number of fibroblasts showed 1.5-fold increase compared with that of the control (Fig. 1). Thus, it was speculated that some HUVECs-derived paracrine factors exist and it might influence on the proliferation of fibroblasts.
Fig. 1. Effect of HUVECs on the proliferation of fibroblasts. Cell proliferation was
measured by counting cell number using hemocytometer. After 2-day incubation, the number of fibroblasts co-cultured with HUVECs (Fibro:HUVECs) showed 1.5-fold increase compared with that of the control (Fibro:only). All values indicate the mean of three independent experiments ± SD.
B. The presence of endothelial cells has a negative effect on pigmentation 1. The effect of murine endothelial cells on pigmentation
To evaluate whether endothelial cells play a role in the regulation of melanogenesis, B16 cells, a murine melanoma cell line, were co-cultured with bEnd.3 cells, a murine (brain) endothelial cell line, using transwell plates. The melanin contents of the B16 cells were significantly reduced in the presence of bEnd.3 cells (Fig. 2A, right panel). Tyrosinase is a critical enzyme in melanogenesis. Consistent with the changes in the melanin content, there was a significant reduction in the level of tyrosinase activity (Fig. 2A, right panel). The expression levels of melanogenesis-associated proteins, microphthalmia-associated transcription factor (MITF), and tyrosinase were also significantly down-regulated in the cells (Fig. 2A, left panel). For further clarification of the paracrine role of the endothelial cells in the pigmentation process, B16 cells were treated with a conditioned medium obtained from bEnd.3 cells. These results showed that the melanin content and tyrosinase activity of the cells were significantly reduced (Fig. 2B, right panel). The protein levels of MITF and tyrosinase were also significantly decreased (Fig. 2B, left panel). To exclude the metabolic effect of the conditioned medium on melanogenesis with regard to the
consumption of glucose and the production of lactates, the effect of a two-fold concentrated conditioned medium was evaluated. An inhibitory effect of the bEnd.3-derived concentrated conditioned medium on melanogenesis was consistently reproduced (Fig. 2C).
Fig. 2. Effects of endothelial cells on the pigmentation of B16 melanoma cells. (A) B16
melanoma cells were co-cultured with bEnd.3 (murine endothelial cell line) cells using transwell plates for 3 days. The melanin content, tyrosinase activity and expression levels of MITF and tyrosinase were analyzed. (B) B16 melanoma cells were treated with the bEnd.3-derived conditioned medium (CM) for 3 days and the melanin content, tyrosinase activity, and MITF/tyrosinase protein expression levels were analyzed. (C) The concentrated conditioned medium (CCM) was used to treat B16 melanoma cells for 3 days and the melanin content, tyrosinase activity and MITF/tyrosinase protein expression were analyzed. All values indicate the mean of three independent experiments ± SD.
2. The verification of the effect of endothelial cells with human cells
The results obtained with murine cell lines were verified with normal human melanocytes (NHMs) and human umbilical vein endothelial cells (HUVECs). Generally, NHMs are cultured in the F12 medium or MCDB153 medium, whereas HUVECs are cultured in HGM. Therefore, initially, I established a co-culture system and then selected the optimal medium for the successful co-culturing of both types of cells. The cell morphology and cytotoxicity were analyzed in several different types of culture media, including MCDB153, F12, and HGM. HUVECs cultured in F12 medium lost their morphological characteristics (no cobblestone morphology) and they became elongated cells, therefore in vitro behavior of these cells could not be investigated (Fig. 3A) (Iacoviello et al., 1995; Bala et al., 2011). HUVECs grown in MCDB153 media retained a cobblestone-like appearance up to 5 days. Hence, the MCDB153 media was selected for further study. NHMs were co-cultured with
HUVECs using transwell plates in the MCDB153 medium. In the presence of HUVECs, there was a significant reduction in the melanin content and tyrosinase activity (Fig. 3B). The mRNA and protein levels of MITF and tyrosinase were also significantly down-regulated (Fig. 3C). The conditioned medium obtained from HUVECs also inhibited pigmentation in NHMs (Fig. 3D). For a closer investigation of the role of HUVECs in pigmentation, ex vivo human skin was maintained in the presence of a HUVECs-derived conditioned medium. After 3 days, in the presence of the conditioned medium, the skin showed significantly less pigmentation than the control skin according to Fontana–Masson staining results (Fig. 3E). Taken together, these results indicate that the presence of endothelial cells has a negative effect on pigmentation.
Fig.3. Effects of endothelial cells on the pigmentation of normal human melanocytes (NHMs) and of ex vivo human skin
(A) To select the optimal co-culture medium of HUVECs and NHMs, HUVECs and NHMs were cultured in HGM, F12 or MCDB153 for 3 days. The cell morphology (left panel) was examined and cytotoxicity was analyzed using MTT assay (right panel). (B) NHMs were co-cultured with HUVECs using transwell plates in the MCDB153 medium for 5 days. The melanin content and tyrosinase activity levels were measured. (C) The expression levels of mRNA and protein of MITF and tyrosinase were analyzed. (D) NHMs were treated with the HUVEC-derived CM for 5 days and the melanin content and tyrosinase activity levels were measured. (E) Ex vivo human skin was maintained with HUVECs-derived CM for 3 days. The value of the pigmented area/epidermal area (PA/EA) ratio was measured (Fontana-Masson stain). All values indicate the mean of three independent experiments ± SD.
C. Additional modification of endothelial cells
1. UV irradiation: Contrary to non-stimulated cells, UV-irradiated endothelial cells induce pigmentation
UV is the most important extrinsic factor that directly and/or indirectly induces pigmentation (Abdel-Malek et al., 2010), and published results all seem to indicate that skin cells, including keratinocytes and fibroblasts, play a pivotal role in the regulation of pigmentation, especially when these cells are stimulated with UV (Kumar et al., 2012; Adini et al., 2014; Duval et al., 2014; Salducci et al., 2014; Soodgupta et al., 2014). Therefore, I investigated the effect of UV-irradiated endothelial cells on the regulation of pigmentation. Endothelial
cells were exposed to UVB at 20~100 mJ/cm2, and 50 mJ/cm2 was determined as the
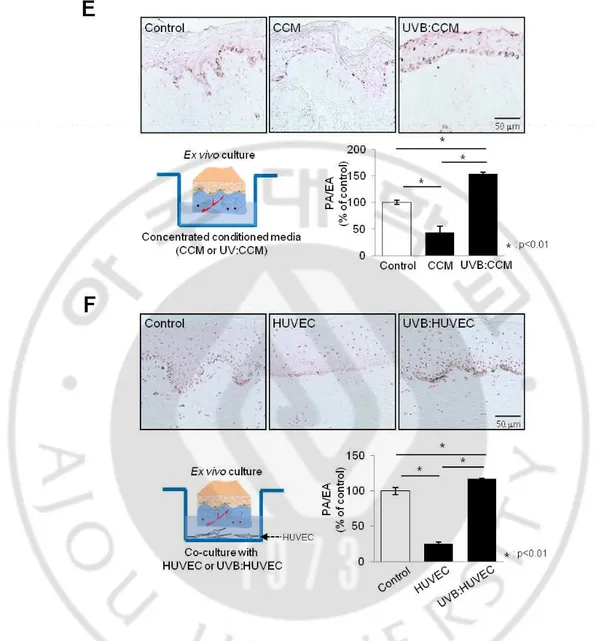
non-cytotoxic energy level according to cell morphology (Fig. 4A). The concentrated conditioned medium obtained from the UV-irradiated bEnd.3 cells were then treated in B16 cells. Interestingly, the concentrated conditioned medium obtained from UV-irradiated cells increased the melanin content and tyrosinase activity as compared to non-irradiated cells (Figs. 4B, 4C). The protein levels of MITF and tyrosinase in the cells were also increased (Fig. 4C, right panel). Stimulatory effects of the concentrated conditioned medium in pigmentation were consistently found in the NHMs. The melanin content and tyrosinase activity were significantly increased in the cells treated with the concentrated conditioned medium derived from UV-irradiated HUVECs (Fig. 4D). These observations were further evaluated in ex vivo cultured skin. A Fontana-Masson analysis revealed that both skin incubated with a concentrated conditioned medium obtained from UV-irradiated HUVECs (Fig. 4E) and in the presence of UV-irradiated HUVECs (Fig. 4F) showed the accumulation of melanin in the epidermis. Taken together, these data indicate a positive role of UV-irradiated endothelial cells in the regulation of melanogenesis, in contrast to non-UV-irradiated cells.
Fig. 4. Effect of UV-irradiated endothelial cells on pigmentation. (A) bEnd.3 cells were
irradiated with 50 mJ/cm2 of UVB and the cell morphology was examined. B16 melanoma
cells were treated with CCM obtained from UV-irradiated or non-irradiated bEnd.3 cells for 3 days. (B) Then, cell pellet assay was performed. (C) The melanin content and tyrosinase activity levels (left panel) and the protein expressions of MITF and tyrosinase (right panel) were analyzed in the above cell pellet. (D) NHMs were treated with CCM derived from
UV-irradiated HUVECs for 5 days and the melanin content and tyrosinase activity levels were measured. Ex vivo human skin was maintained (E) in CCM derived from UV-irradiated HUVECs and (F) in the presence of UV-irradiated HUVECs for 3 days. The value of the pigmented area/epidermal area (PA/EA) ratio was measured (Fontana-Masson stain). All values indicate the mean of three independent experiments ± SD.
2. VEGF treatment
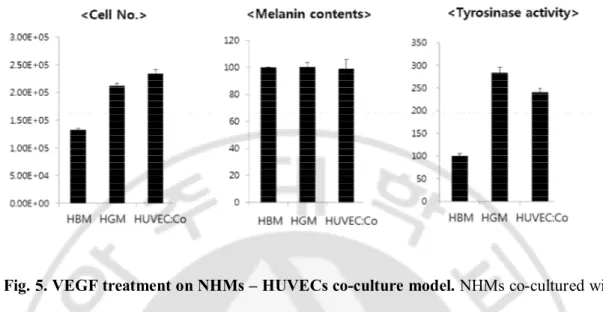
VEGF is known as the major growth factor for angiogenesis of endothelial cells (Kim et al., 2007). Also the presence of VEGF receptor on NHMs was previously demonstrated (Kim et al., 2005). Therefore, I tried to demonstrate the effect of VEGF-treated endothelial cells on pigmentation. NHMs co-cultured with HUVECs were treated with 10ng of recombinant human VEGF and incubated for 5 days. There was minimal difference on the melanin content and tyrosinase activity compared with VEGF non-treated control (Fig. 5). This result was correlated with the previous report that VEGF could not induce any melanogenic change in NHMs (Kim et al., 2005).
Fig. 5. VEGF treatment on NHMs – HUVECs co-culture model. NHMs co-cultured with
HUVECs (HUVEC:Co) were treated with 10ng of recombinant human VEGF and incubated for 5 days. There was minimal difference on the melanin content and tyrosinase activity compared with NHMs cultured with HUVECs basal medium (HBM) or HUVECs growth media (HGM) only, as VEGF non-treated control.
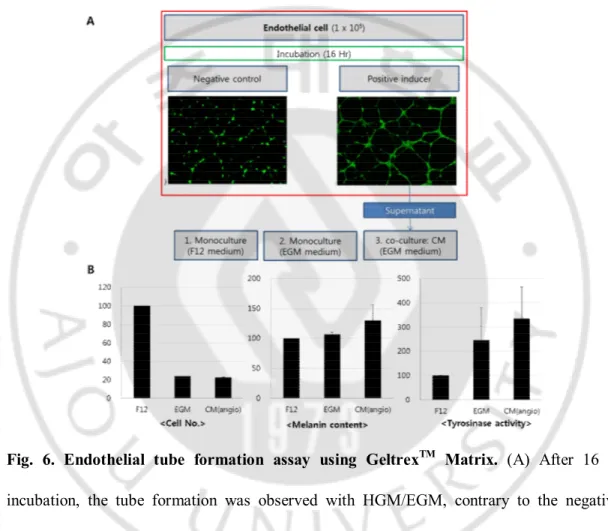
3. Endothelial tube formation assay using GeltrexTM Matrix
I assumed that endothelial cells would be changed to activate melanocytes when they are in the phase of endothelial tube formation. So, the tube formation assay was performed using both HUVECs and human dermal microvascular endothelial cells (HDMECs). After 16 hr incubation, the tube formation was observed with HGM or HDMECs growth medium (EGM), contrary to the negative control which showed no tube formation with HUVECs/HDMECs basal medium (HBM/EBM). The supernatant (as endothelial tube-conditioned media) was obtained and treated to NHMs (Fig. 6A). After 5 day-incubation, the
number of NHMs significantly decreased compared to the control (F12). The melanin contents and tyrosinase activity increased in NHMs with endothelial tube-conditioned media compared with both control (F12) and EGM media only (Fig. 6B). However, the result was not reliable because of the significant reduction of cell viability.
Fig. 6. Endothelial tube formation assay using GeltrexTM Matrix. (A) After 16 hr
incubation, the tube formation was observed with HGM/EGM, contrary to the negative control which showed no tube formation with HUVECs/HDMECs basal medium (HBM/EBM). The supernatant was obtained and treated to NHMs and incubated during 5 days. (B) The result of HDMECs with EGM was provided. Melanin content and tyrosinase activity increased in NHMs with endothelial tube-conditioned media compared with both
control (F12) and EGM media only group. However, the result was not reliable because of the significant reduction of cell viability.
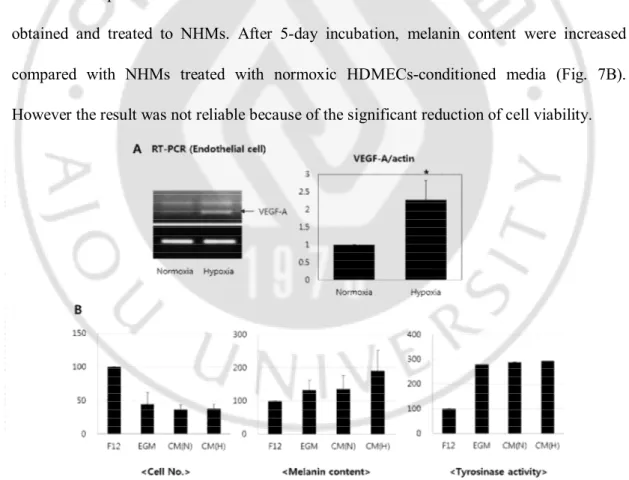
4. Endothelial cell-conditioned media with hypoxic condition
The hypoxic condition has been widely used as one of the activating conditions for endothelial cells. The activation of endothelial cell was confirmed by VEGF-A level of RT-PCR analysis (Fig. 7A). After 24-hr incubation, the level of VEGF-A of hypoxic HDMECs was induced up to 2.5-fold of that of normoxic cells. The HDMECs-conditioned media was obtained and treated to NHMs. After 5-day incubation, melanin content were increased compared with NHMs treated with normoxic HDMECs-conditioned media (Fig. 7B). However the result was not reliable because of the significant reduction of cell viability.
Fig. 7. Using hypoxic condition as one of the activating conditions for endothelial cells.
The level of VEGF-A of hypoxic HDMECs was induced up to 2.5-fold of that of normoxic cells. (B) After 5-day incubation, melanin content were increased (CM(H)) compared with NHMs treated with normoxic HDMECs-conditioned media (CM(N)). However the result was not reliable because of the significant reduction of cell viability.
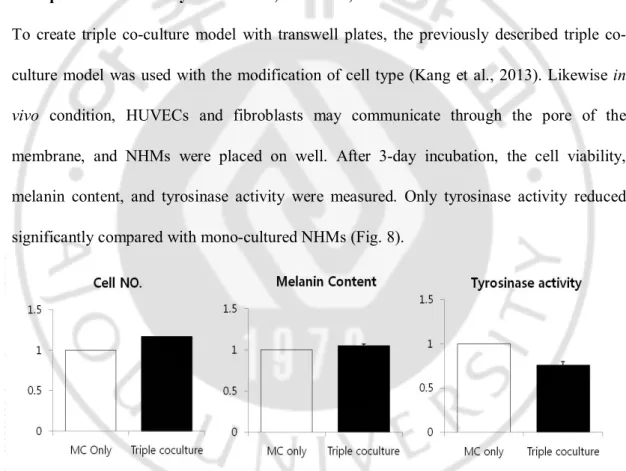
5. Triple co-culture study with NHMs, HUVECs, and fibroblasts
To create triple culture model with transwell plates, the previously described triple co-culture model was used with the modification of cell type (Kang et al., 2013). Likewise in vivo condition, HUVECs and fibroblasts may communicate through the pore of the membrane, and NHMs were placed on well. After 3-day incubation, the cell viability, melanin content, and tyrosinase activity were measured. Only tyrosinase activity reduced significantly compared with mono-cultured NHMs (Fig. 8).
Fig. 8. Triple co-culture study with NHMs, HUVECs, and fibroblasts using transwell plates. After 3-day incubation, only tyrosinase activity reduced significantly compared with
mono-cultured NHMs. The values indicate the mean of five independent experiments ± SD. *P < 0.01.
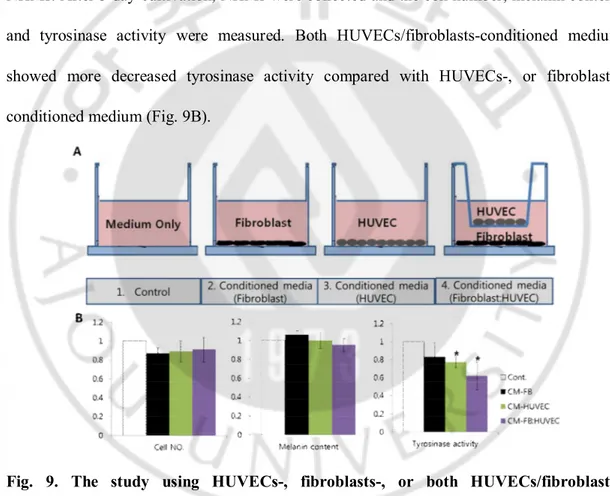
Also, the HUVECs-, fibroblasts-, or both HUVECs/fibroblasts-conditioned medium was used to determine the effect of secreted factors from HUVECs, fibroblasts, or both HUVECs/fibroblasts on melanogenesis (Fig. 9A). MCDB153 was used as basal medium for conditioned medium. After 2-day incubation, the conditioned medium was collected and concentrated using centrifuge. After concentration, each conditioned medium was treated to NHMs. After 5 day-cultivation, NHMs were collected and the cell number, melanin content, and tyrosinase activity were measured. Both HUVECs/fibroblasts-conditioned medium showed more decreased tyrosinase activity compared with HUVECs-, or fibroblasts-conditioned medium (Fig. 9B).
Fig. 9. The study using HUVECs-, fibroblasts-, or both HUVECs/fibroblasts-conditioned medium. (A) Co-culture model using HUVECs/fibroblasts-conditioned medium from each cell was
established. After 2-day incubation, the conditioned medium was collected and concentrated using centrifuge, and treated to NHMs. (B) Both HUVECs/fibroblasts-conditioned medium showed more decreased tyrosinase activity compared with HUVECs-, or
fibroblasts-conditioned medium. The values indicate the mean of three independent experiments ± SD. *P < 0.01.
D. Identification of possible melanogenic factors secreted by endothelial cells 1. Identification of endothelin-1 as one of melanogenic factors secreted by UV-irradiated endothelial cells
To investigate the differences noted between UV-irradiated and non-irradiated endothelial cells in terms of their ability to modulate cutaneous pigmentation, we analyzed the gene expression profiling of the cells. A biostatistical analysis showed that only 396 genes (254 up-regulation and 142 down-regulation) were differentially expressed in UV-irradiated and non-irradiated bEnd.3 cells (p < 0.05). The majority of the up-regulation genes included those in the DNA damage response gene family (the p53 signaling pathway). We examined the expression levels of various known secreted factors that influence the melanocyte function (Fig. 10A). Interestingly, endothelin-1 (ET-1) showed more than 2.98-fold up-regulation (log2 ratio: 1.57) on average after UV exposure, while none of the rest of the genes encoding known paracrine melanogenic factors, such as stem cell factor (SCF), showed a significant increase after UV exposure, although some factors, such as tissue-type plasminogen activator (tPA) or bone morphogenetic protein-2 (BMP-2), showed a slight increase after UV exposure. Consistent with gene expression profiling, the mRNA of ET-1 were significantly upregulated in UV-irradiated bEnd.3 cells compared with non-irradiated cells (Fig. 10B). Furthermore, ELISA data showed that the ET-1 level in the concentrated conditioned medium obtained from UV-irradiated endothelial cell was significantly higher
than that of the control (1.5-fold up-regulation, p < 0.05). To verify the role of ET-1 in modulating the skin pigmentation of UV-irradiated endothelial cells, B16 cells were treated with 1 µM of ET-1 receptor antagonist (BQ-123) in conjunction with the UV-irradiated concentrated conditioned medium. The increased pigmentation elicited by the UV-irradiated endothelial cell-derived concentrated conditioned medium was abrogated by ET-1 receptor inhibition (Fig. 10C). These data suggest that ET-1 is one of the key melanogenic factors secreted from UV-irradiated endothelial cells.
Fig. 10. Identification of endothelin-1 as a melanogenic factor secreted by UV-irradiated endothelial cells
(A) Differential gene expression profiling of UV-irradiated and non-irradiated bEnd.3 cells with an RNA sequencing analysis. (B) The expression levels of mRNA of ET-1 of UV-irradiated or non-UV-irradiated bEnd.3 cells were analyzed by real-time PCR and RT-PCR. (C) B16 melanoma cells were treated with/without BQ-123 (1 µM) in conjunction with UV-irradiated CCM for 3 days. The melanin content and tyrosinase activity levels were measured. The values indicate the mean of three independent experiments ± SD.
2. Identification of candidate cytokines derived from HUVECs using angiogenesis-related cytokine antibody array
The candidate cytokines derived from HUVECs was evaluated using a protein array method, angiogenesis-related cytokine antibody array (RayBio® Human Angiogenesis Antibody Array, RayBiotech C Series 1000, RayBiotech, Inc Norgross, GA) (Fig. 11). Conditioned medium was obtained after 1 day incubation at 37℃ and 5% CO2. HGM was used as
control. Each array was incubated with 1.2 mL of medium at 4℃ overnight, and bound antigens were detected according to the manufacturer’s instructions. Several cytokines showed different expression between HUVECs-conditioned medium and control (HGM) (Table 2).
Fig. 11. Angiogenesis-related cytokine antibody array. The secretion of angiogenic
cytokines from HUVECs was evaluated using two kinds of antibody array kits (RayBio® Human Angiogenesis Antibody Array C1 and C2, POS; positive control, NEG; negative control).
Table 2. Different expression of angiogenic-related cytokines in HUVECs-conditioned medium compared with control group
Array C1 Array C2
No change EGF, ENA-78, bFGF, IGF-1, RANTES, TGF-beta 1, THPO, VEGF-D
Endostatin, G-CSF, GM-CSF, I-309, IL-10, IL-1 alpha, IL-1 beta, IL-2, IL-4, MCP-3, Tie-2, TNF-alpha, VEGFR3
Increased expression ANG, IFN gamma, IL-6, IL-8, Leptin, MCP-1, PDGF-BB, PLGF, TIMP-1, TIMP-2, GRO
ANGPT1, ANGPT2, PLG, I-TAC, MCP-4, MMP-9, uPAR, VEGFR2, MMP-1, PECAM-1 Reduced expression VEGF
ANG; angiogenin, ANGPT; angiopoietin, bFGF; basic fibroblast growth factor, EGF; epidermal growth factor, ENA-78; CXCL5, G-CSF; granulocyte colony stimulating factor, GM-CSF; granulocyte-macrophage colony stimulating factor, GRO; growth-related protein, I-TAC; tachykinin, I-309; CCL1, IFN gamma; interferon gamma, IGF-1;insulin growth factor-1, IL; interleukin, MCP; monocyte chemotactic protein, MMP; matrix metalloproteinases, PDGF-BB; platelet derived growth factor-BB, PECAM-1; platelet endothelial cell adhesion molecule-1, PLG; plasminogen, PLGF; placental growth factor, RANTES; CCL5, TGF-beta 1;
transforming growth factor beta 1, THPO; thrombopoietin, TIMP; a tissue inhibitor of
metalloproteinases, TNF-alpha; tumor necrosis factor alpha, uPAR; urokinase receptor, VEGF; vascular endothelial growth factor
IV. DISCUSSION
In this study, it was reported for the first time that endothelial cells, when they are stimulated with UV irradiation, are responsible for stimulatory action upon skin pigmentation. UV-irradiated endothelial cells were clearly involved in the regulation of melanogenesis in melanocytes, and there was increased epidermal pigmentation of human skin in the presence of UV-irradiated endothelial cells. Our results indicate that endothelial cell-derived soluble factors mediate the effects of endothelial cells on melanocytes. According to the results of this study, ET-1 at least is assumed to play a role. ET-1, which has been considered to be secreted from keratinocytes, is a well-known melanogenic factor which induces pigmentation and the tanning response upon UV irradiation (Imokawa et al., 1995; Rees, 2004; Denat et al., 2014). Previous studies reported that HUVECs and endothelial cells of other origins express ET-1 (Yanagisawa et al., 1988; Delerive et al., 1999). We confirmed these findings by showing that ET-1 is expressed by endothelial cells and that UV irradiated cells in particular produce more ET-1 than do non-stimulated cells. These results are consistent with previous work which showed that stimulated endothelial cells could produce more ET-1 than non-stimulated cells (Delerive et al., 1999). Inhibition studies using an ET-1 antibody confirm the crucial role of soluble ET-1 in mediating endothelial cell-dependent melanocyte differentiation (Rouzaud et al., 2005). On previous studies showing increased vasculature in UV-induced hyperpigmentary skin of melasma and solar lentigo (Kim et al., 2007; Kang et al., 2010; Kang et al., 2011a), these promoting effects of UV-irradiated endothelial cells on melanogenesis are consistent with in vivo
findings. It is speculated that the interaction between melanocytes and endothelial cells during sun exposure plays a role in the activation of the melanocyte function and in the consequent cutaneous pigmentation process, resulting in the development of hyperpigmentary disorders.
This report also provides new evidence that non-UV-irradiated endothelial cells instead play an inhibitory role during skin pigmentation. In our co-culture system and in the ex vivo skin culture experiments conducted here, the presence of endothelial cells had a negative effect on melanogenesis and inhibited tyrosinase expression through MITF down-regulation. In fact, these findings were unexpected based on our initial hypothesis in this study; however, they are consistent with epidemiological data showing that light-skinned individuals show much more prominent telangiectasia than dark-skinned persons (Berg, 1989; Halder et al., 2003; Chosidow and Cribier, 2011). Very recently, it was also reported that low levels of melanin are correlated with the development of angiogenic ocular and skin diseases (Adini et al., 2014). Indeed, human fibroblasts, unlike UV-irradiated fibroblasts, can also have an inhibitory influence on human epidermal pigmentation, although they secrete melanogenic factors such as HGF and SCF (Hedley et al., 2002; Lee et al., 2003). These findings were clearly observed in in vitro full-thickness pigmented skin models, which allow physiological interactions between the melanocyte-containing epidermis and the dermal compartment with living fibroblasts (Cario-Andre et al., 2006; Duval et al., 2014). Recently, it was shown that fibroblast-derived DKK1 is responsible for the hypopigmentation of palmo-plantar skin (Yamaguchi et al., 2009). How endothelial cells down-regulate pigmentation remains unclear, though the process may be evoked by yet unknown endothelial factors and/or an issue not
addressed in the present study.
In the preliminary phase of our study, the appropriate medium for the co-culture system using HUVECs and NHMs was established. There were dramatic influences of the batch of numerous supplements in the cell culture medium on each cell behavior. The tested media included those routinely used to culture the two cell types in a monoculture (MCDB153 and F12 for NHMs and HGM for HUVECs, respectively) as well as various mixtures (1:1 and 1:4) of F12 and HGM. Here, the medium eventually selected was the MCDB153 medium. Because the cell viability of HUVECs was significantly decreased after 4 days of incubation in MCDB153 medium, we replaced the HUVECs with fresh ones every 2 days up to 5 days, which is mandatory to observe their effect on melanin production in NHMs (Tomita et al., 1987; Carsberg et al., 1994). The cell performance in this culture condition was closer to that obtained with HGM for HUVECs. Interesting observations included TPA, a crucial supplement for melanocyte growth, which showed a remarkable effect on HUVECs. It changed the cell morphology, creating elongated cells with an irregular cell boundary. The HGM medium itself had stimulatory effects on melanogenesis in NHMs according to our preliminary data.
Several studies have reported increased expressions of several paracrine factors, including α-MSH, ET-1, KGF, bFGF, and SCF in UV-exposed skin and hyperpigmented skin, showing the effects of these cytokines on melanocyte differentiation (Costin and Hearing, 2007; Abdel-Malek et al., 2010). However, none of these paracrine factors except 1 were significantly changed from gene expression profiling in our study. However, ET-1 may not be the only factor involved in melanocyte-endothelial cell interaction. In our study,
among other up-regulated genes identified in UV-irradiated bEnd.3 cells, tissue-type plasminogen activator (tPA) was another soluble factor (showing a 2.66-fold increase, log2 ratio: 1.41, p=0.0025). It was previously reported that UV irradiation induces tPA synthesis and plasmin activity in cultured keratinocytes (Takashima et al., 1992) and that the tPA generated by keratinocytes increases the activity of melanocytes in vitro (Maeda K, 2007). Up-regulation of bone morphogenetic protein-2 (BMP-2) was also noted in UV-irradiated endothelial cells, although the levels were not significant (p=0.06). BMP-2 is produced by HUVECs and fibroblasts (Csiszar et al., 2005); this was shown to have a stimulatory effect on tyrosinase activity and melanocyte proliferation (Bilodeau et al., 2001). Further studies to elucidate the roles of BMP-2, tPA, and/or yet unknown factors in the interaction between endothelial cells and melanocytes are necessary.
One of the limitations of this study was that we should reproduce the consistent results with HDMECs on the melanogenesis of NHMs. Also, the additional gene expression profiling with human endothelial cells should be performed in future to find other candidate genes besides ET-1. In terms of UV irradiation, UVA irradiation should be performed additionally to evaluate the effects of different wavelength on melanogenesis.
In summary, this study provides valuable insights into the role of endothelial cells in the regulation of skin pigmentation. Our study demonstrates that endothelial cells have a negative effect on pigmentation. However, their effect differs after they are irradiated with UV, as they then positively influence the induction of melanogenesis. The UV-irradiated endothelial cells may produce secreted factors, in this case ET-1 mediates the stimulatory effect on pigmentation which may play a role in the development of hyperpigmentary
disorders. Future studies are required to identify many other regulators of melanogenesis from endothelial cells based on our gene expression profiling.
V. CONCLUSION
In conclusion, this study provides valuable insights into the role of endothelial cells in the regulation of skin pigmentation. It demonstrates that endothelial cells have a negative effect on pigmentation. However, their effect differs after they are irradiated with UV, as they then positively influence the induction of melanogenesis. The UV-irradiated endothelial cells may produce secreted factors, in this case ET-1 mediates the stimulatory effect on pigmentation which may play a role in the development of hyperpigmentary disorders. Future studies are required to identify many other regulators of melanogenesis from endothelial cells based on our gene expression profiling.
REFERENCES
1. Abdel-Malek ZA, Kadekaro AL, Swope VB: Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res 23: 171-186, 2010
2. Adini I, Ghosh K, Adini A, Chi ZL, Yoshimura T, Benny O, Connor KM, Rogers MS, Bazinet L, Birsner AE, Bielenberg DR, D'Amato RJ: Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment. J Clin Invest 124: 425-436, 2014
3. Bala K, Ambwani K, Gohil NK: Effect of different mitogens and serum concentration on HUVEC morphology and characteristics: implication on use of higher passage cells. Tissue Cell 43: 216-222, 2011
4. Berg M: Epidemiological studies of the influence of sunlight on the skin. Photodermatol 6: 80-84, 1989
5. Bilodeau ML, Greulich JD, Hullinger RL, Bertolotto C, Ballotti R, Andrisani OM: BMP-2 stimulates tyrosinase gene expression and melanogenesis in differentiated melanocytes. Pigment Cell Res 14: 328-336, 2001
6. Cario-Andre M, Pain C, Gauthier Y, Casoli V, Taieb A: In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res 19: 434-442, 2006
7. Carsberg CJ, Warenius HM, Friedmann PS: Ultraviolet radiation-induced melanogenesis in human melanocytes. Effects of modulating protein kinase C. J Cell Sci 107 ( Pt 9): 2591-2597, 1994
8. Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, Hearing VJ: The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci 123: 3102-3111, 2010
9. Choi WJ, Kim M, Park JY, Park TJ, Kang HY: Pleiotrophin inhibits melanogenesis via Erk1/2-MITF signaling in normal human melanocytes. Pigment Cell Melanoma Res 28: 51-60, 2015
10. Chosidow O, Cribier B: Epidemiology of rosacea: updated data. Ann Dermatol Venereol 138 Suppl 3: S179-183, 2011
11. Costin GE, Hearing VJ: Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 21: 976-994, 2007
12. Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z: Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364-2372, 2005
13. Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B: Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 274: 32048-32054, 1999
14. Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA: Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol 134: 1512-1518, 2014
15. Duval C, Cohen C, Chagnoleau C, Flouret V, Bourreau E, Bernerd F: Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: impact of photo-aging. PLoS One 9: e114182, 2014 16. Halder RM, Brooks HL, Callender VD: Acne in ethnic skin. Dermatol Clin 21:
609-615, vii, 2003
17. Hedley SJ, Layton C, Heaton M, Chakrabarty KH, Dawson RA, Gawkrodger DJ, MacNeil S: Fibroblasts play a regulatory role in the control of pigmentation in reconstructed human skin from skin types I and II. Pigment Cell Res 15: 49-56, 2002 18. Iacoviello L, Kolpakov V, Salvatore L, Amore C, Pintucci G, de Gaetano G, Donati MB: Human endothelial cell damage by neutrophil-derived cathepsin G. Role of cytoskeleton rearrangement and matrix-bound plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol 15: 2037-2046, 1995
19. Imokawa G, Miyagishi M, Yada Y: Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol 105: 32-37, 1995
20. Kang HS, Okamoto K, Takeda Y, Beak JY, Gerrish K, Bortner CD, DeGraff LM, Wada T, Xie W, Jetten AM: Transcriptional profiling reveals a role for RORalpha in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiol Genomics 43: 818-828, 2011a
21. Kang HY, Bahadoran P, Suzuki I, Zugaj D, Khemis A, Passeron T, Andres P, Ortonne JP: In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol 19: e228-233, 2010
22. Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, Kang WH: The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol 154: 1094-1099, 2006
23. Kang HY, Suzuki I, Lee DJ, Ha J, Reiniche P, Aubert J, Deret S, Zugaj D, Voegel JJ, Ortonne JP: Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol 131: 1692-1700, 2011b
24. Kang YB, Rawat S, Cirillo J, Bouchard M, Noh HM: Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: toward engineering the liver sinusoid. Biofabrication 5: 045008, 2013
25. Kim EH, Kim YC, Lee ES, Kang HY: The vascular characteristics of melasma. J Dermatol Sci 46: 111-116, 2007
26. Kim EJ, Park HY, Yaar M, Gilchrest BA: Modulation of vascular endothelial growth factor receptors in melanocytes. Exp Dermatol 14: 625-633, 2005
27. Kovacs D, Cardinali G, Aspite N, Cota C, Luzi F, Bellei B, Briganti S, Amantea A, Torrisi MR, Picardo M: Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br J Dermatol 163: 1020-1027, 2010
28. Kumar R, Parsad D, Kanwar AJ, Kaul D: Altered levels of LXR-alpha: crucial implications in the pathogenesis of vitiligo. Exp Dermatol 21: 853-858, 2012
29. Lee DY, Lee JH, Lee ES, Cho KH, Yang JM: Fibroblasts play a stimulatory role in keratinocyte proliferation but an inhibitory role in melanocyte growth and pigmentation in a skin equivalent system from skin type IV. Arch Dermatol Res 294:
444-446, 2003
30. Lee HI, Lim YY, Kim BJ, Kim MN, Min HJ, Hwang JH, Song KY: Clinicopathologic efficacy of copper bromide plus/yellow laser (578 nm with 511 nm) for treatment of melasma in Asian patients. Dermatol Surg 36: 885-893, 2010 31. Maeda K TY: Mechanism of the inhibitory effect of tranexamic acid on
melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci 53: 389-396, 2007
32. Moll I, Houdek P, Schmidt H, Moll R: Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes. J Invest Dermatol 111: 251-258, 1998
33. Na JI, Choi SY, Yang SH, Choi HR, Kang HY, Park KC: Effect of tranexamic acid on melasma: a clinical trial with histological evaluation. J Eur Acad Dermatol Venereol 27: 1035-1039, 2013
34. Passeron T: Long-lasting effect of vascular targeted therapy of melasma. J Am Acad Dermatol 69: e141-142, 2013
35. Passeron T, Fontas E, Kang HY, Bahadoran P, Lacour JP, Ortonne JP: Melasma treatment with pulsed-dye laser and triple combination cream: a prospective, randomized, single-blind, split-face study. Arch Dermatol 147: 1106-1108, 2011 36. Rees JL: The genetics of sun sensitivity in humans. Am J Hum Genet 75: 739-751,
2004
37. Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ: MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res 571: 133-152, 2005
38. Salducci M, Andre N, Guere C, Martin M, Fitoussi R, Vie K, Cario-Andre M: Factors secreted by irradiated aged fibroblasts induce solar lentigo in pigmented reconstructed epidermis. Pigment Cell Melanoma Res 27: 502-504, 2014
39. Schallreuter KU, Kothari S, Chavan B, Spencer JD: Regulation of melanogenesis--controversies and new concepts. Exp Dermatol 17: 395-404, 2008
40. Soodgupta D, Kaul D, Kanwar AJ, Parsad D: Modulation of LXR-alpha and the effector genes by Ascorbic acid and Statins in psoriatic keratinocytes. Mol Cell Biochem 397: 1-6, 2014
41. Takashima A, Yasuda S, Mizuno N: Determination of the action spectrum for UV-induced plasminogen activator synthesis in mouse keratinocytes in vitro. J Dermatol Sci 4: 11-17, 1992
42. Tomita Y, Iwamoto M, Masuda T, Tagami H: Stimulatory effect of prostaglandin E2 on the configuration of normal human melanocytes in vitro. J Invest Dermatol 89: 299-301, 1987
43. Tsuji T, Karasek M: A procedure for the isolation of primary cultures of melanocytes from newborn and adult human skin. J Invest Dermatol 81: 179-180, 1983
44. Yamaguchi Y, Hearing VJ: Physiological factors that regulate skin pigmentation. Biofactors 35: 193-199, 2009
45. Yamaguchi Y, Morita A, Maeda A, Hearing VJ: Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J Investig Dermatol Symp Proc 14: 73-75, 2009
46. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411-415, 1988
Acknowledgement
- 국문요약 -
피부 혈관이 피부 색소변화에 끼치는 영향
최근 기미의 과색소 침착 병변에서 혈관이 증가되어 있음을 확인하였고, 이것이 과색소 발생에 중요한 역할을 할 수 있다는 주장이 제기되어 왔다. 본 연구에서는 피부 혈관이 색소 형성에 있어서 어떤 역할을 하는지에 대한 in vitro 연구를 시행해보고자 하였다. 혈관내피세포가 같이 존재하게 되면, 멜라닌세포와 ex vivo skin culture 에서의 색소가 모두 감소하는 것을 확인하였다. 하지만, 이런 비자극 혈관내피세포와는 다르게, UV 를 조사한 혈관내피세포는 피부 색소를 오히려 자극하여 증가시켰다. UV 외의 다른 자극 (VEGF, hypoxia 등)을 주었을 경우에는 크게 변화를 관찰하기 어려웠다. UV 조사된 혈관내피세포에서 유래하는 색소 유발 인자를 발견하기 위해 gene expression profiling 을 시행하였고, endothelin-1 (ET-1)의 발현이 증가되어있음을 확인하였다. 또한, UV 를 조사한 혈관내피세포에서 유래한 conditioned medium 에서 증가되었던 색소 변화는 ET-1 receptor 를 처리하였을 때 다시 상쇄됨을 관찰하였다. 이 연구에서, 혈관내피세포 자체는 색소 형성을 감소시켰지만, UV 를 조사하였을 때 색소에 대한 효과가 바뀜을 알 수 있었다. 결론적으로, UV 조사된 혈관내피세포가 과색소 질환에 중요한 역할을 할 수 있을 것이라 생각된다.핵심어 : 과색소 질환, 기미, 공동배양, 멜라닌세포, 피부 색소변화, 피부 혈관, 혈관내피세포