저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
이학 석사학위 논문
Bacterial infection-mimicking
three-dimensional phagocytosis and chemotaxis
in nanofibrous scaffold
아 주 대 학 교 대 학 원
의생명과학과 / 약리학전공
Bacterial infection-mimicking
three-dimensional phagocytosis and chemotaxis
in nanofibrous scaffold
지도교수 곽 종 영
이 논문을 이학 석사학위 논문으로 제출함.
2017 년 8 월
아 주 대 학 교 대 학 원
의생명과학과 / 약리학전공
이 승 준
이승준의 이학 석사학위 논문을 인준함
심사위원장
곽 종 영
심 사 위 원 이 광
심 사 위 원 양 시 영
아 주 대 학 교 대 학 원
2017년 7월 14일
Abstract
Professional phagocytes such as neutrophils, macrophages, and dendritic cells (DCs) actively engulf microbes. In this study, we developed a three-dimensional (3D) in vitro infection model for investigating the cross-talk between phagocytes and microbes in inflammation. A culture system using a nanofibrous scaffold (NFS) having multi-planar pores in a 3D structure was used for coculture of Staphylococcus aureus (S.aureus) and phagocytes in the same as well as different compartments. Surface-seeded S. aureus and phagocytes were able to adhere to nanofibers inside the NFS and phagocytes migrated to and engulfed S. aureus in a 3D manner. The addition of formyl peptide receptor antagonists decreased the phagocytic rate in 3D NFS but not in two-dimensional (2D) culture dishes. The migration of phagocytes to S. aureus was evaluated in an NFS-based layer-by-layer culture system. Neutrophils, macrophages, and DCs cultured in an upper NFS migrated to the lower NFS containing bacteria. Cytokine and chemokine secretion patterns by neutrophils cultured with S. aureus differed between 2D and 3D culture conditions. DCs migrated to neutrophils phagocytosing bacteria and then engulfed neutrophils in 3D in vitro culture. In addition, neutrophils and macrophages in the upper NFS migrated to bacteria-infected lung epithelial cells in the lower NFS of the layer-by-layer system. S. aureus-infected lung epithelial cells stimulated the secretion of tumor necrosis factor (TNF)-α and IL-1ain 3D culture condition, but not in 2D culture. Therefore, NFS-based 3D culture system with phagocytes and bacteria mimics the inflammatory response to microbes in vivo.
TABLE OF CONTENTS
Abstract ………Ⅰ TABLE OF CONTENTS ……….………Ⅱ LIST OF FIGURES ……….Ⅳ
Ⅰ. INTRODUCTION ……….………..1
Ⅱ. MATERIAL AND METHODS ……….………..4
A. Materials …..……….…………4
B. Electrospinning and fabrication of the PCL nanofibers ……….4
C. Preparation of phagocytes from bone marrow ………...…………5
D. Preparation of peritoneal neutrophils and macrophages ……….6
E. MLE-12 cell culture ………...…………6
F. Bacterial culture and adhesion assay ……….………….6
G. Laser confocal microscopy ………7
H. Scanning electron microscopy (SEM) ………..………….7
I. FACS analysis of phagocytosis ……….………..7
J. Live imaging of phagocytosis ………....……..………...8
K. Migration assay in NFS-based layer-by-layer system ………..………….9.
L. Cytokine array analysis ………..………9
M. Statistical analysis ………..10
Ⅲ. RESULTS ……….……….11
B. Engulfment of S. aureus by phagocytes in the NFS ……….13
C. Differential rate of phagocytosis in 2D and 3D culture conditions …………..………..15
D. 3D migration of phagocytes to S. aureus in NFS-based layer-by-layer culture system ……….……….20
E. NFS-based horizontal system ………...………23
F. Neutrophil-induced recruitment of more neutrophils to S. aureus in NFS-based layer-by-layer culture system ………25
G. Engulfment of neutrophils by DCs in the presence of S. aureus in 2D and 3D culture conditions ……….30
H. Differential secretion of cytokines and chemokines by neutrophils cultured with S. aureus in 2D versus 3D culture conditions ……….…………..32
I. Migration of phagocytes to S. aureus-infected epithelial cells in NFS-based layer-by-layer system ……….………….35
Ⅳ. DISCUSSION ………39
Ⅴ. CONCLUSION ………..………43
REFERENCE ………...……..……….44
LIST OF FIGURES
Fig. 1A,B. Adhesion of S. aureus to the nanofibrous surface ……….12 Fig. 2A. Intracellular localization of bacteria in BM-DCs cultured in the NFS ……...…..14 Fig. 2B,C. Phagocytosis of S. aureus by neutrophils, macrophages and DCs in the 3D NFS ………..17 Fig. 2D. Effect of FPR inhibitors on phagocytosis ………..…..18 Fig. 3. Workflow for setup of a migration assay with the layer-by-layer assembly of NFS ...19 Fig. 4A,B. Migration of phagocytes to S. aureus in 3D NFS-based layer-by-layer culture system ……….……….21 Fig. 5A,B. Migration of phagocytes to S. aureus in horizontal assay system ………...…..24 Fig. 6A. Effect of FPR inhibitors on migration ……….……….26 Fig. 6B. More neutrophils are recruited when S. aureus are cultured with neutrophils than
when the bacteria are cultured alone ……….27 Fig. 6,C,D. Expression of IL-8 secreted by neutrophils cultured with bacteria in the NFS…29 Fig. 7A,B. Engulfment of neutrophils by DCs in 2D and 3D culture conditions …...…….31 Fig. 8A,B. Secretion of cytokines and chemokines by neutrophils cultured with S. aureus in 2D and 3D conditions ………...………33
Fig. 9A,B. Migration of phagocytes to S. aureus-infected epithelial cells in 3D NFS-based layer-by-layer culture system ………...………36 Fig. 9C,D. Secretion of cytokines and chemokines by MLE-12 cell cultured with S. aureus in 2D and 3D conditions ………...………38
Ⅰ. INTRODUCTION
The recognition of pathogenic microorganisms is the first step of host defense. Components derived from bacteria, such as N-formylmethionine-leucyl-phenylalanine (fMLP), trigger chemotaxis and activation of phagocytes such as neutrophils and macrophages in infectious tissues (1). Early-recruited neutrophils phagocytose the bacteria, and subsequent chemoattractant signals from the activated and necrotic neutrophils can directly and indirectly recruit more neutrophils and other phagocytes, including macrophages and dendritic cells (DCs) (2). In addition, a variety of infected host cells, such as fibroblasts, keratinocytes, and endothelial and epithelial cells, secrete phagocyte chemoattractants (2, 3).
Observing the migration of phagocytes in infected tissue is technically challenging. Numerous in vitro studies of host immune responses to bacteria have been carried out under tow-dimensional (2D) coculture of phagocytes and bacteria, although in tissues, these exist and interact under 3D conditions. Investigating cell functions such as phagocytosis and cell migration requires accurate mimicry of the in vivo microenvironment. The migration rate significantly relies on the chemoattractant gradient in tissue. The transwell-based (Boyden chamber) assay is widely used to assess cell migration; however, it provides a bulk end-point measurement and does not measure directionality of cell migration (4). The assay is not appropriate for assessing migration of cells to a variety of bacteria because non-adherent bacteria grow as a suspension culture in the bottom chamber and can float, adhere, or migrate freely to the upper chamber through the permeable filter of transwell, thus inhibiting chemoattractant gradient formation. While microfluidic devices have been used to study
phagocyte migration in stable chemokine gradients, they still provide 2D rather than 3D environments (5). To accurately mimic 3D migration conditions in vivo, the development of an appropriate 3D microenvironment in vitro is essential.
Phagocytes continue to migrate through the extracellular matrix (ECM), a 3D fiber mesh, until they reach an infected or inflamed tissue. Nanofibrous scaffold (NFS) mimics the configuration of native ECM of tissues (6). We previously reported a direct-write electrospinning process and apparatus with improved focusing and scanning functionalities for generating various nanofibrous membranes (7). For optimal cellular infiltration, we developed a hybrid electrospun poly-ε-caprolactone (PCL) NFS consisting of nano- as well as submicron-scale fibers, and we used the hybrid NFS to mimic the naturally occurring cross-talk between immune and cancer cells (8). Additionally, we demonstrated that neutrophils migrate against interleukin (IL)-8 in an electrospun PCL nanofibrous membrane (9). PCL is an FDA-approved biocompatible and biodegradable aliphatic polyester (10). Furthermore, DCs cultured on PCL nanofibers show a baseline inactive form (8), indicating that PCL is a suitable material for immune cell culture. Thus, we used electrospun PCL hybrid NFS to mimic the 3D fibrillar environment in studying the inflammatory response to bacterial infection.
S. aureus is a leading cause of human bacterial diseases, including skin and soft tissue infections, food-borne illness, toxic shock syndrome, and sepsis. In the present study, we used the in vitro infection system to analyze the early events associated with S. aureus infection of phagocytes. Cells in 3D cell culture form multilayers of cells, whereas cells grown in two dimensions form a monolayer. In this study, we designed a chemotaxis assay
system using NFS-based layer-by-layer culture, attempting to mimic inflammatory microenvironment, to evaluate early recruitment of phagocytes induced by S. aureus. The 3D coculture system can be used to evaluate in vivo-mimicking bacteria-host interactions and immune responses.
Ⅱ. MATERIAL AND METHODS
A. Materials
PCL (Mn = 700,000 - 900,000), chloroform, PKH26, PKH67,
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), lipopolysaccharide (LPS), thioglycollate, and 4',6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), RPMI-1640 medium, fetal bovine serum (FBS), penicillin/streptomycin, glutamine, and 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco (Rockville, MD, USA). Granulocyte macrophage-colony stimulating factor (GM-CSF), M-CSF, IL-4, and antibodies against CD11, Ly6G, and F4/80 were obtained from R&D Systems Inc. (Minneapolis, MN, USA). CellTracker Red CMTPX dye was purchased from Molecular Probes, Inc. (Eugene, OR, USA). Polydimethylsiloxane (PDMS) was purchased from Dow-Corning Korea, Inc. (Seoul, Korea).
B. Electrospinning and fabrication of the PCL nanofibers
Porous PCL nanofibers were prepared according to the procedure published earlier (8). Briefly, the polymer for electrospinning was dissolved in 99.5% pure chloroform at a concentration of 8.8 wt% and stirred for 5 h to obtain a homogeneous solution. Nanofiber mats were fabricated by electrospinning. Four-nozzle spinnerets with 12-mm nozzle spacing were used with an average flow rate for the PCL-chloroform solution approximately 0.125
ml/h. To produce flat nanofiber mats, the collector plates were given reciprocating 2D planar motion at a speed of 20 mm/s by means of a 2D linear stage (LPP-LM11&12, DCT Corp., Suwon, Korea), so that the nanofibers covered most of the collector plate. The nozzle tip-to-collector distance was set at 100 mm, with an electrical potential of approximately 11 kV from the grounded collector plate. An aluminum plate was used for fabricating conventionally electrospun nanofiber mats, while a stainless steel mesh, which aided in deposition guidance, was used for fabricating nanofiber mats with larger pores (11). The mesh was covered with a 2-mm-thick glass plate to weaken the electrical field strength and to allow easy removal of the nanofibers. The electrospun scaffolds were sterilized by soaking in a solution of 70% ethanol for 12 h and dried under UV exposure for 12 h.
C. Preparation of phagocytes from bone marrow
BALB/c mice were maintained in accordance with institutional animal care and use guidelines. Bone marrow (BM) cells were harvested from the femurs and tibias of the mice and BM-derived neutrophils were purified using anti-Ly6G antibody-bound microbeads (12). BM-derived DCs (BM-DCs) were obtained from BM precursors,as described previously (13). Briefly, BM cells platedin complete RPMI 1640 containing recombinant murine GM-CSF (20 ng/ml) plus recombinant murine IL-4 (20 ng/ml). On day 7 of culture, immature BM-DCs, which appeared as non-adherent proliferatingaggregates, were collected. BM-derived macrophages were obtained by culturing of mouse BM cells with recombinant murine M-CSF (20 ng/ml) for 7 days (14). BM-derived DCs and macrophages were purified using anti-CD11c and anti-F4/80 antibody-bound microbeads, respectively. The purity of
neutrophils, BM-DCs, and macrophages was verified to be >90% by flow cytometry using surface markers, Ly6G, CD11c, and F4/80, respectively.
D. Preparation of peritoneal neutrophils and macrophages
BALB/c mice were treated with 3% thioglycollate in distilled water (intraperitoneal injection) for 6 h and 3 days to purify peritoneal neutrophils and macrophages, respectively, which were isolated by lavage with phosphate-buffered saline (PBS) and purified with anti-Ly6G- and anti-F4/80 antibody-bound microbeads, respectively.
E. MLE-12 cell culture
MLE-12 mouse lung epithelial type II cells were purchased from ATCC (Manassas, VA, USA) and cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 20 mM HEPES, and 2 mM L-glutamine at 37oC in 5% CO
2 incubator.
F. Bacterial culture and adhesion assay
S. aureus strain RN4220 was grown to mid-log phase in Luria-Bertani broth, washed, and resuspended in PBS (15). Bacterial concentration was estimated by determining the optical density at 600 nm (OD600) with a spectrophotometer. The bacterial suspension was diluted
to an OD600 of 0.1. Bacteria were stained with DAPI (1 μg/ml) and washed with PBS for
on the surface of wet nanofibrous mats (1 cm × 1 cm) that were placed in an 8-well cell culture slide (SPL, Seoul, Korea). After incubation at 37°C and under 5% CO2 for the
indicated times to allow the bacteria to settle and attach to the nanofibers, the mats were gently rinsed thrice with PBS to remove not-adherent bacteria.
G. Laser confocal microscopy
Fluorescence-labeled phagocytes and S. aureus that had infiltrated the NFS was observed using a K1 confocal microscope (Nanoscope, Daejeon, Korea). Images was analyzed using the ImageJ software.
H. Scanning electron microscopy (SEM)
Phagocytes and/or bacteria cultured in NFS were affixed to aluminum mounts with double-sided carbon tape (SPI Supplies Inc.) and coated with gold-sputter. Cell structure and NFS morphology were observed using a field-emission scanning electron microscope (FESEM) (JSM-6700F, JEOL, Japan).
I. FACS analysis of phagocytosis
Neutrophils, macrophages, and BM-DCs were labeled with the PKH67 Green Fluorescent Cell Linker kit and S. aureus were labeled with the PKH26 Red Fluorescent Cell Linker kit. The cells were washed thrice with PBS. The labeled phagocytes (1×105/100 μl) were mixed
with 10 μl of labeled S. aureus suspension at OD600 = 0.1 in RPMI 1640 medium in the
presence of 5% FBS at 37°C in a humidified atmosphere containing 5% CO2. For 3D
phagocytic assay, labeled neutrophils and macrophages were top-seeded on the NFS surface and incubated for 1 h, and then, the bacterial suspension was added. Phagocytes and bacteria were detached from the NFS by soaking in trypsin-EDTA solution for 5 min. The percentage of PKH26-stained cells among total PKH67-stained phagocytes was analyzed using flow cytometry.
J. Live imaging of phagocytosis
The dynamic interactions between S. aureus and phagocytes were visualized using K1 confocal microscopy. Neutrophils, macrophages, and BM-DCs were labeled with CellTracker and S. aureus cells were labeled with PKH67 fluorescent dye. Labeled phagocytes were top-seeded on nanofiber mats in 3D culture and on culture dishes in 2D culture. After a 4-h incubation of phagocytes for cell attachment to the culture dish or NFS, 10 μl of S. aureus suspension in RPMI-1640 culture media was added to the labeled phagocytes (1×105/100 μl). Time-lapse videos of phagocytes engulfing bacteria were
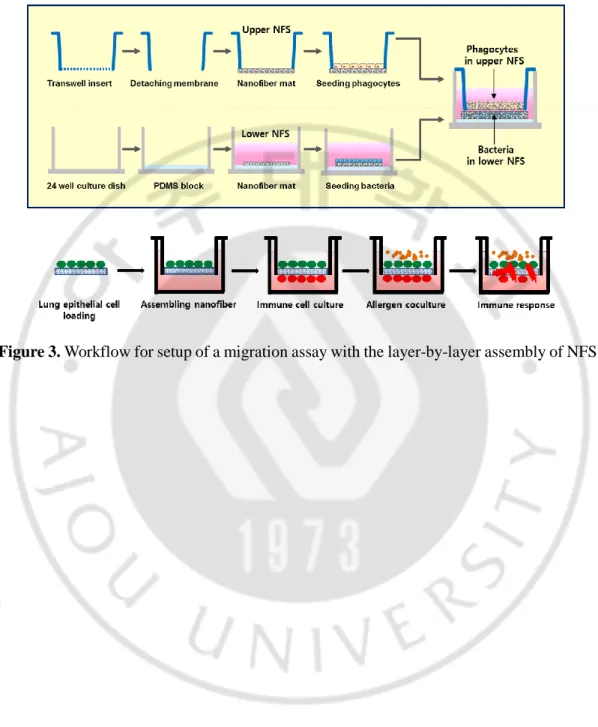
K. Migration assay in NFS-based layer-by-layer system
We assembled a layer-by-layer system of two nanofibrous mats for cell migration, as shown in Figure 3, in a modified Transwell chamber. To construct the upper NFS in the apical chamber, the polycarbonate filter of a Transwell insert was removed and replaced with a 5-mm-diameter circular section of nanofiber mat, attached with PDMS. Neutrophils, macrophages, and BM-DCs were labeled with PKH26 dye and 10 μl of cell suspension (1 × 105) was seeded onto the NFS in the apical chamber. After 4 h, the scaffold was washed
with PBS. For the lower NFS layer, PDMS was poured in the lower cultivation well to allow close contact with the upper nanofibrous mat, and a nanofibrous mat was attached on top of the surface of the gel-state PDMS. S. aureus suspension was added to the surface of the NFS in the basal chamber and bacteria were allowed to adhere for 2 h. The phagocyte- and S. aureus-seeded scaffolds were then assembled layer-by-layer by placing the insert in the well. The chamber contained 600 μl of medium and phagocytes were allowed to migrate at 37oC
in a humidified CO2 atmosphere. Migrated PKH26-labeled cells in the lower NFS were
counted under a light microscope in three random fields at a magnification of 100×. A lower NFS without bacteria served as a control to evaluate leakage of the phagocytes.
L. Cytokine array analysis
Secretion of cytokines and chemokines was determined using a Proteome Profiler Mouse Cytokine Array Kit (R&D Systems). Neutrophils and lung epithelial cells in the presence or absence of S. aureus were cultured in 2D culture dishes and 3D NFS. After culture for the indicated times, supernatants were taken and assayed as described in the user manual.
Chemiluminescence signal density was quantified with ImageJ. IL-8 levels were measured by ELISA (R&D Systems) according to manufacturer’s instruction.
M. Statistical analysis
Results are presented as means ± SDs. Student's t-testwas used to compare the means of paired or unpaired samples. A P-value of <0.05 was considered significant
Ⅲ. RESULTS
A. 3D culture of S. aureus in electrospun PCL NFS
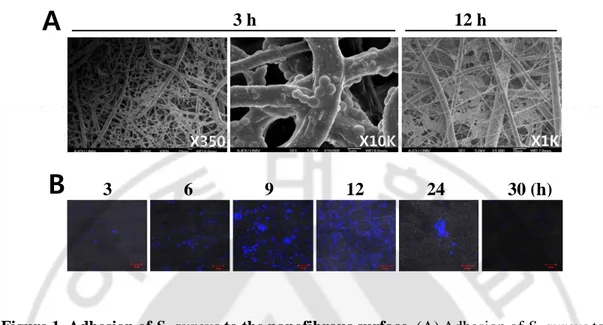
The large pores in the nano-submicron NFS were expected to allow phagocytes to infiltrate deep into the scaffold (8). First, we evaluated the attachment of the bacteria to the nanofibers by SEM in an experiment using bacteria alone. S. aureus were top-seeded on an NFS and allowed to infiltrate. S. aureus are Gram-positive round cells of 0.5 - 1 μm diameter. As shown in Figure 1A, the bacteria were well distributed throughout the scaffold and attached along the nanofiber surfaces. Small bacterial agglomerates were seen between two or more fibers in areas of fiber cross over. Bacteria cultured for 12 h formed aggregates inside the NFS. Fluorescence from DAPI-labeled bacteria in the NFS increased in density up to 12 h, but then decreased and was no longer apparent at 30 h (Figure 1B). This result indicated that the bacteria can proliferate on the fibers, producing agglomerates, but are not able to maintain large colonies in the NFS after prolonged culture because of the small diameter (400 nm - 2 μm) of the nanofibers.
Figure 1. Adhesion of S. aureus to the nanofibrous surface. (A) Adhesion of S. aureus to
the nanofibers after seeding onto a nanofibrous mat was evaluated by SEM. (B) The proliferation of DAPI-stained S. aureus after seeding onto nanofibrous mat was evaluated by confocal microscopy. Images are representative of three independent experiments.
3 6 9 12 24 30 (h)
B
A
3 h 12 h
X10K X1K
B. Engulfment of S. aureus by phagocytes in the NFS
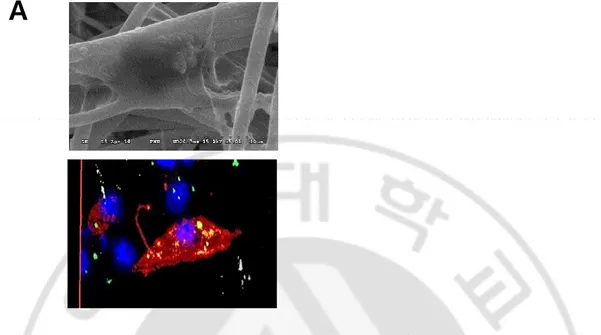
Intracellular localization of bacteria in BM-DCs cultured in the NFS was confirmed by SEM and confocal microscopy (Figure 2A). In live cell imaging analysis, PKH67-labeled S. aureus were caught and ingested by CellTracker red CMTPX-labeled neutrophils, macrophages, and BM-DCs in 2D as well as 3D culture.
Figure 2A. Intracellular localization of bacteria in BM-DCs cultured in the NFS. (A)
The engulfment of S. aureus by BM-DCs was evaluated by SEM and confocal microscopy. Data are representative of three independent experiments.
C. Differential rate of phagocytosis in 2D and 3D culture conditions
It has been demonstrated that there is a significant difference in phagocytosis of S. aureus between adherent and suspended neutrophils; the phagocytosis by adherent neutrophils is largely unaffected by opsonization with serum (16). We compared the rate of phagocytosis of live bacteria between phagocytes cultured in 2D and 3D conditions. Neutrophils, macrophages, and BM-DCs were stained with PKH67 and then cultured with the PKH26-labeled S. aureus for 2 h. Double-positive-stained cells correspond to phagocytes that have internalized bacteria (Figure 2B). The uptake of bacteria by BM-derived and peritoneal phagocytes occurred in a time-dependent manner (Figure 2C). Half of total peritoneal neutrophils (50.3 ± 5.1%) and the majority of peritoneal neutrophils (79.5 ± 6.5%) were double positively stained after 1 h and 2 h of 2D culture, respectively. In contrast, in the NFS, the number of BM-derived and peritoneal neutrophils that had phagocytosed bacteria was lower than that in 2D culture. BM-derived macrophages and DCs rapidly phagocytosed bacteria, but the phagocytic rate and ability in 3D culture were lower than those in 2D culture.
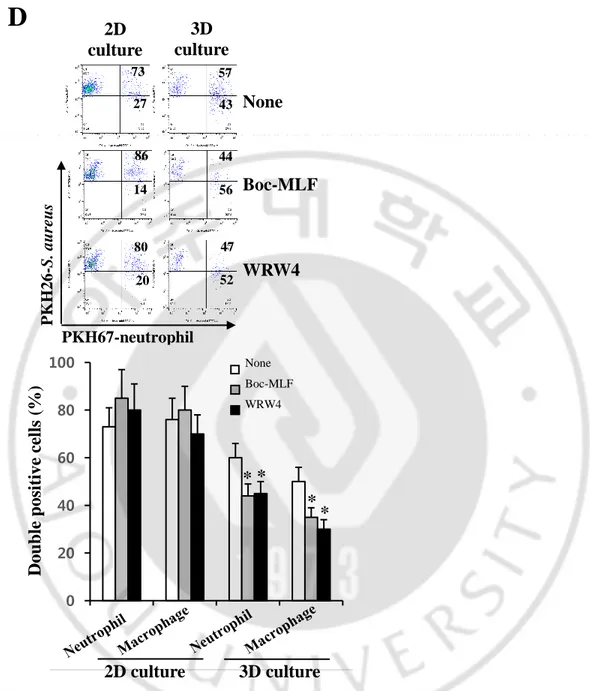
Phagocytes sense chemotactic gradients produced by bacteria, triggering phagocytosis. The bacterial peptide, fMLP is a highly potent leukocyte chemoattractant and chemotactic activity of phagocytes is mediated by formyl peptide receptor (FPR) (17). We next compared the effect of Boc-MLF and WRW4, selective FPR1 and FPR2 inhibitors in murine neutrophils (18), on phagocytosis by neutrophils and macrophages in 2D and 3D culture conditions. Boc-MLF and WRW4 decreased the phagocytic rate of neutrophils in the NFS when compared to neutrophils grown in the absence of inhibitors (44 ± 5% and 45 ± 6% vs. 60 ± 8%, respectively, p < 0.05) (Figure 2D). Similar results were obtained for macrophages
treated with Boc-MLF and WRW4 or not (35 ± 4% and 30 ± 3% versus 50 ± 7%, respectively, p < 0.05). In contrast, no inhibition of phagocytosis was observed when FPR inhibitors were added to coculture of S. aureus and neutrophils or macrophages in 2D culture. These results suggested that phagocyte migration plays a role in phagocytosis in 3D, but not in 2D culture.
Figure 2B,C. Phagocytosis of S. aureus by neutrophils, macrophages, and DCs in the 3D NFS. (B) PKH26-labeled S. aureus were cultured with PKH67-labeled BM-derived
neutrophils, and double-positive cells among PKH67-positive cells were detected by flow cytometry. (C) Phagocytosis rate was evaluated by counting of double-positive cells among PKH67-labeled phagocytes.
B
21 79 46 54 0 0.5 1 2 (h)2D
Culture
54 46 P KH 26 -S. au reu s 34 66 36 64 4 96 PKH67-neutrophil 22 78 9 913D
Culture
C
Incubation time (h)
0 20 40 60 80 100 BM-neutrophil in 2D BM-macrophage in 2D BM-DC in 2D BM-neutrophil in 3D BM-macrophage in 3D BM-DC in 3DDoub
le pos
itiv
e c
ell
s (%
)
0 0.5 1 2 0 20 40 60 80 100 0 0.5 1 2Doub
le pos
itive
c
ell
s
(%
)
Peritoneal-neutrophil in 2D Peritoneal-macrophage in 2D Peritoneal-neutrophil in 3D Peritoneal-macrophage in 3DIncubation time (h)
Figure 2D. Effects of FPR inhibitors on phagocytosis. (D) Phagocytosis of S. aureus by
BM-derived neutrophils and macrophages in the NFS was measured in the presence or absence of Boc-MLF (20 μM) and WRW4 (4 μM). Data are shown as means ± SDs (n = 4). Asterisks (*) denote significant differences compared to no treatment (P < 0.05).
D
2D
culture
57 43 44 56 86 14 73 27 80 20 47 52None
Boc-MLF
WRW4
3D
culture
PKH 26 -S. aureu s PKH67-neutrophil 0 20 40 60 80 100Doub
le pos
itive
c
ell
s (%
)
2D culture 3D culture
* *
*
*
None Boc-MLF WRW4D. 3D migration of phagocytes to S. aureus in NFS-based layer-by-layer culture system
3D migration of phagocytes to bacteria was assessed quantitatively using two NFS in layer-by-layer assembly in a Transwell chamber. PKH26-labeled phagocytes and PKH67-labeled bacteria were cultured in the upper and lower NFS, respectively. After indicated times of incubation, the numbers of phagocytes that had migrated from the upper to the lower NFS were counted. As shown in Figure 4A, increasing numbers of neutrophils and BM-DCs were detected in the lower NFS over time, while the numbers of phagocytes in the upper NFS gradually decreased. Neutrophils migrated rapidly in response to S. aureus, when compared to BM-DCs. Next, we performed a similar assay, in which equal numbers of PKH26-labeled neutrophils and PKH67-labeled macrophages were top-seeded on the upper NFS, and bacteria on the lower NFS. While both macrophages and neutrophils migrated to the lower NFS, the numbers and rates of migration of macrophages were lower than those of neutrophils (Figure 4B).
Figure 4A,B. Migration of phagocytes to S. aureus in 3D NFS-based layer-by-layer culture system. (A) PKH26-labeled phagocytes and PKH67-labeled S. aureus were seeded
and cultured in the upper and lower NFS, respectively, for the indicated times. Red-fluorescent spots were detected in the lower NFS. (B) PKH26-labeled neutrophils and PKH67-labeled macrophages were cultured in the upper NFS. DAPI-stained S. aureus were cultured in the lower NFS. Red- and green-fluorescent spots were detected in the lower NFS. The graphs depict numbers of fluorescent spots detected in the upper and lower NFS. Images are representative of three independent experiments. Data are shown as means ± SDs (n = 4).
E. NFS-based horizontal system
In addition to the layer-by-layer system, an NFS-based horizontal system was used to measure the migration of phagocytes toward bacteria. Phagocytes and bacteria were top-seeded on different regions of a nanofibrous mat, which was compartmentalized by using a plastic barrier, and then incubated for 2 h for the cells and bacteria to adhere to and infiltrate in the NFS (Figure 5A). After the plastic barrier was carefully removed, cells were cultured for the indicated times and cell migration was measured by counting the fluorescent phagocytes in the compartment of bacterial culture. As shown in Figure 5B, phagocytes were found in the compartment containing bacteria. Similar to the migration rates in the layer-by-layer system, neutrophils exhibited higher migration rates than DCs and macrophages in the horizontal system.
Figure 5A,B. Migration of phagocytes to S. aureus in horizontal assay system.
(A) Fluorescence-labeled phagocytes and DAPI-stained S. aureus were top-seeded in different compartments on the nanofibrous mat, which were separated by plastic barrier. The cells were incubated for 2 h to adhere on nanofibers and plastic barrier was removed. (B) PKH26-labeled neutrophils and PKH67-labeled BM-DCs or macrophages were cultured in the left and right compartment of S. aureus, respectively, and the cells were cultured for the indicated times. The fluorescent spots in the compartment containing S. aureus were counted.
0 30 60 90 120 150 1 2 3 4 5 0 2 6 12 18 Culture time (h) N umber s o f m igrat ed c el ls Neutrophils DCs Neutrophils BM-DCs
B
Neutrophils Macrophages Macrophages 0 30 60 90 120 150 1 2 3 4 5 Neutrophils 0 2 6 12 18 Culture time (h) N umber s o f m igrat ed c el ls PKH67-BM-DCs or PKH67-Macrophages PKH26-Neutrophils S. aureusA
F. Neutrophil-induced recruitment of more neutrophils to S. aureus in NFS-based layer-by-layer culture system.
Neutrophil migration is mediated by chemokines derived from bacteria and phagocytes. When PKH67-labeled neutrophils were pretreated with FPR inhibitors and then cultured with PKH26-stained bacteria in the presence of the inhibitors in the NFS-based layer-by-layer system, the numbers of PKH67-positive neutrophil in the lower NFS were significantly decreased when compared to those of untreated neutrophils (Figure 6A). The effects of FPR inhibitors on migration was only obvious in the first hour after coculture of neutrophils in the upper NFS and bacteria in the lower NFS, indicating that the early phase of migration is mediated by bacteria-derived chemoattractants, such as fMLP. It is possible that the secretion of chemokines by neutrophils migrated from the upper to the lower NFS may trigger a later, second phase of neutrophil migration. Thus, we measured the migration rate of PKH26-labeled neutrophils in the upper NFS using cultures of PKH67-PKH26-labeled neutrophils and/or DAPI-stained bacteria in the lower NFS. More PKH26-labeled neutrophils were observed in the lower NFS containing both S. aureus and neutrophils than in NFS containing S. aureus or neutrophils alone (Figure 6B).
Neutrophils cultured with bacteria in 3D NFS as well as in 2D culture dish secreted IL-8 into the medium (Figure 6C). The concentrations of IL-8 secreted by neutrophils cultured with bacteria in the NFS were lower than those in 2D culture, but increased in a time-dependent manner (Figure 6D). These results indicated that neutrophil-derived chemokines in the lower compartment, such as IL-8, enhance the migration of neutrophils from the upper to the lower NFS.
Figure 6A. Effects of FPR inhibitors on migration. (A) PKH67-labeled neutrophils were
top-seeded and pretreated with or without Boc-MLF (20 μM) and WRW4 (4 μM) for 1 h in the upper NFS. The migration of untreated and FRP inhibitor-pretreated neutrophils to the lower NFS containing PKH26-labeled S. aureus were measured in the presence or absence of Boc-MLF and WRW4. Green-fluorescent spots in the lower NFS were counted. Data are shown as means ± SDs (n = 4). Asterisks (*) denote significant differences compared to no treatment (P < 0.05).
A
30 min
10 min
None Boc-MLF WRW4 0 10 20 30 40 50M
igrat
ed
n
eu
tr
op
h
il
s p
er
f
ield
None Boc-MLF WRW4 10 min 30 min*
*
PKH26 neutrophils Migrated PKH26-neutrophils Upper NFS Lower NFS
B
PKH67-neutrophils DAPI-S. aureus-+
-+
-+
+
0 20 40 60 80 100 120 140 160 180 1 2 3 4M
igrat
ed
n
eu
tr
op
h
il
s p
er
f
ield
+Neutrophils+S. aureus*
*
*
-Neutrophils+S. aureus -Neutrophils-S. aureus +Neutrophils-S. aureus 0 1 2 4 Culture time (h)Figure 6B. More neutrophils are recruited when S. aureus are cultured with neutrophils than when the bacteria are cultured alone. (B) PKH26-labeled neutrophils
in the upper NFS were cultured with PKH67-labeled neutrophils and/or DAPI-stained S. aureus in the lower NFS. Red-fluorescent spots were detected in the lower NFS. The graphs depict numbers of red-fluorescent spots detected in the lower NFS. Data are shown as means ± SDs (n = 4). Asterisks (*) denote significant differences compared to no treatment (P < 0.05).
Figure 6C,D. Expression of IL-8 secreted by neutrophils cultured with bacteria in the NFS. (C) Neutrophils were cultured in the presence or absence of S. aureus and LPS for 12 h. (D) Neutrophils were cultured with S. aureus for the indicated times in the NFS. IL-8 concentrations in culture media were measured by ELISA. Images are representative of three independent experiments. Data are shown as means ± SDs (n = 4). Asterisks (*) denote significant differences compared to no treatment (P < 0.05). 0 50 100 150
IL
-8 (pg/
m
l)
None
S. aureus LPS
C
2D culture 3D culture*
*
0 5 10 15 20 25D
*
*
*
IL
-8 (
pg
/ml
)
0 1 6 12 Culture time (h)G. Engulfment of neutrophils by DCs in the presence of S. aureus in 2D and 3D culture conditions
Coculture of DCs and neutrophils showed internalization of live neutrophils by DCs (19). This study confirmed that BM-DCs engulfed live neutrophils as well as bacteria when neutrophils, BM-DCs, and bacteria were cocultured in 2D culture (Figure 7A). However, it is unknown whether the engulfment of viable neutrophils by DCs occurs in bacteria-infected tissues in vivo. Moreover, we cannot rule out the possibility of experimental artifact during coculture of different types of phagocytes in 2D culture. Thus, we investigated whether BM-DCs migrate to and are able to uptake bacteria-engulfing neutrophils in 3D culture condition. More BM-DCs migrated to the lower NFS containing both neutrophils and bacteria than to the NFS harboring neutrophils alone (Figure 7B-a). In Z-stack analysis of confocal microscopic images, the red fluorescence of neutrophils colocalized with green fluorescence of BM-DCs, indicating internalization of the neutrophils by the DCs (Figure 7B-b and –c). This result indicated that DCs migrate to neutrophils phagocytosing bacteria and then engulf neutrophils in 3D in vitro culture.
Figure 7A,B. Engulfment of neutrophils by DCs in 2D and 3D culture conditions. (A)
BM-derived DCs and/or neutrophils were cultured with S. aureus for 6 h and stained with Giemsa solution. (B) PKH67-labeled BM-DCs in the upper NFS were culture for 6 h with PKH26-labeled neutrophils and DAPI-stained S. aureus in the lower NFS. Figure ⓐ depicts green- fluorescent spots in the lower NFS. Figure ⓑ depicts focus stacking images of the lower NFS. Figure ⓒ shows red-fluorescent spots inside of green-fluorescent areas in the lower NFS. Images are representative of three independent experiments.
H. Differential secretion of cytokines and chemokines by neutrophils cultured with S. aureus in 2D versus 3D culture conditions
After phagocytosis of microorganisms, activated neutrophils secrete various cytokines and chemokines. We measured the secretion of proinflammatory cytokines and chemokines using membrane arrays to evaluate possible differences in their production between in 2D and 3D in vitro cultures. Cytokine and chemokine array analysis of conditioned medium after coculture of neutrophils and S. aureus in culture dishes revealed a time-dependent increase in the secretion of CCL2 (monocyte chemoattractant protein 1), CCL3 (macrophage inflammatory protein 1- α), CCL4 (MIP-1β, CXCL1, and CXCL2 (MIP-2α) (Figure 8A). In comparison, there was an increased secretion of CCL3, CXCL1, and CXCL2 after coculture of neutrophils and S. aureus in the NFS, whereas CCL2 and CCL4 were not detected in this condition. Among the detected chemokines, the intensity of CCL3 signal was highest in 2D culture condition but lowest in 3D culture condition. Similarly, neutrophils secreted G-CSF in the presence of S. aureus in 2D but not in 3D culture condition. IL-1rα showed different time-course patterns in 2D and 3D culture conditions. In an analogous experiment, neutrophils in 2D and 3D culture conditions were stimulated with LPS instead of bacteria. As shown in Figure 8B, LPS-stimulated neutrophils showed almost nearly similar expression patterns of cytokines and chemokines under 2D and 3D culture conditions.
Figure 8A,B. Secretion of cytokines and chemokines by neutrophils cultured with S. aureus in 2D and 3D culture conditions. (A) Neutrophils were incubated with or without
S. aureus for the indicated times on culture dishes and in NFS. (B) Neutrophils were incubated with or without LPS (1 μg/ml) for 12 h on culture dishes and in NFS. Cytokines and chemokines secreted in the culture media were detected using cytokine and chemokine arrays (n = 3). Spot intensities were measured using ImageJ software.
I. Migration of phagocytes to S. aureus–infected epithelial cells in NFS-based layer-by-layer system
Fibroblasts, epithelial cells, and endothelial cells are potentially important targets for S. aureus infection (20). PKH67-labeled MLE-12 cells and DAPI-stained S. aureus were cocultured for 24 h in the lower NFS, and then, PKH26-labeled phagocytes were top-seeded on the upper NFS. The migration of phagocytes to infected epithelial cells was measured. As shown in Figure 9A, the neutrophils and macrophages migrated toward the lung epithelial cells, and more so when these were infected with S. aureus. Immunofluorescence analysis of the upper NFS showed that the numbers of red-fluorescent neutrophils decreased but those of green-fluorescent epithelial cells from the lower NFS increased time-dependently (Figure 9B). In contrast, S. aureus-infected epithelial cells were not detected in the upper NFS when it had not been seeded with neutrophils (data not shown). These results indicated that neutrophils in the upper NFS migrated to the lower NFS, and damaged epithelial cells in the lower NFS floated up to the upper nanofibrous mat. On the basis of these observations, we tested the production of chemokines by lung epithelial cells treated with S. aureus in 2D and 3D culture conditions.
IL-8 secretion by MLE-12 cells was increased in cells cultured with S. aureus in both 2D and 3D culture conditions (Figure 9C). MLE-12 cells treated with S. aureus in the NFS, but not in culture dishes, stimulated the secretion of tumor necrosis factor (TNF)-α and IL-1α (Figure 9D). This result indicated that S. aureus infection of epithelial cells in 3D culture condition induces proinflammatory response.
0 20 40 60 80 10 2 4 2 3 Culture time (h)
Ne
u
tr
op
h
il
s p
er
f
ield
-S. aureus +S. aureus*
*
0 10 20 30 1 2 3 4M
ac
rop
h
age
s p
er
f
ield
0 2 4 12 Culture time (h)*
*
*
-S. aureus
2 h
4 h
12 h
+S. aureus
Neutrophils Epithelial cells Neutrophils Epithelial cells
B
4 h
2 h
Neutrophil Epithelial cell Merge Neutrophil Epithelial cell Merge
-S. aureus +S. aureus
Figure 9A,B. Migration of phagocytes to S. aureus-infected epithelial cells in 3D NFS-based layer-by-layer culture system. (A) PKH67-labeled MLE-12 cells were incubated
with DAPI-stained S. aureus for 24 h in NFS and washed with culture media. PKH26-labeled neutrophils and macrophages were seeded on the upper NFS and cultured for the indicated times with bacteria-infected MLE-12 in the lower NFS. Red-fluorescent spots in the lower NFS were counted. (B) PKH67-labeled MLE-12 cells were detected on the upper NFS. (n = 3). Data are shown as means ± SDs (n = 3).
Figure 9C,D. Secretion of cytokines and chemokines by MLE-12 cell cultured with S. aureus in 2D and 3D conditions. (C and D) MLE-12 cells were cultured with or without S.
aureus for 12 h on culture dishes and in NFS. The secretion of cytokine and chemokine was measured using ELISA and a cytokine array kit (n = 3). Data are shown as means ± SDs (n = 3). 0 50 100 150 200
IL
-8 (pg/
m
l)
C
S. aureus None LPS 2D culture 3D culture* *
-S. aureus +S. aureus
2D culture
TNF-α IL-1a CCL2D
3D culture
Ⅳ. DISCUSSION
The ability of phagocytes to uptake bacteria in vitro may depend on culture conditions and methodology. To date, no method for evaluating phagocytosis of bacteria in 3D coculture system, which may mimic bacterial infection in vivo, has been reported. Phagocytosis of S. aureus by human neutrophils in suspension has been shown to depend on opsonization, while adherent neutrophils do readily internalize bacteria, independent of opsonization (16). In addition, persistence of free extracellular bacteria in suspension culture has been shown to the poor killing (16). Adherence to ECM or plasma proteins of host cells is mediated by bacterial surface proteins. In addition, fibronectin-binding proteins of S. aureus bind to fibronectin on the surface of epithelial cells (20). Nanofibers with an average diameter close to bacterial sizes were found to offer support for bacterial adhesion (21), although it is unknown which protein mediates the binding. In this study, S. aureus and phagocytes adhered onto the surface of electrospun PCL nanofibers and infiltrated the NFS, allowing 3D evaluation of phagocytosis in NFS-based in vitro culture system.
Inflammation is characterized by an influx of phagocytes to the site of infection. While neutrophils are very rapidly recruited into infection site, macrophages constitute the majority of immune cells residing in the tissue at steady state. In a mouse infection model, DCs were actively recruited to infected tissue and phagocytosed S. aureus, but they did not actively kill the bacteria (22). Phagocytes reach the site of infection through directed migration along an increasing gradient of chemoattractants, which are derived from bacteria as well as
secreted by activated cells including leukocytes and epithelial cells. In vitro studies have been crucial in understanding cell migration, but the direct exposure of phagocytes to bacteria is in most cases poorly justified. Methods such as Transwell and microfluidic-based assays have been used to evaluate the migration of immune cells in response to chemokines, and the migration of phagocytes in vitro is usually measured by chemotaxis through a filter in Transwell chambers. When compared to Transwell assay, the 3D NFS system has some advantages. First, it is easy to observe the movement of phagocytes over time in the 3D NFS. Second, 3D migratory behavior of neutrophils, macrophages, and DCs can be evaluated. We found that neutrophils and BM-DCs on nanofibers become elongated and motile in the vicinity of bacteria, and travel along the nanofiber to reach the nearest bacteria.
Cell components released from S. aureus may act as a phagocyte chemoattractant, and bacteria-activated phagocytes secrete cytokines and chemokines, thereby sustaining and prolonging inflammation (23, 24). It has been demonstrated that FPR is required for neutrophil migration to and killing of bacteria, but not for bacterial phagocytosis (25). This study showed that FPR inhibitors partially inhibited phagocytosis of S. aureus by neutrophils and macrophages in 3D, but not in 2D culture, suggesting that FPR agonist released from bacteria may be involved in the detection of bacteria by phagocytes only in 3D culture. Host-derived chemotactic factors, such as IL-8 have been shown to recruit additional neutrophils to areas of infection (26). These chemokines may be crucial during the early phase of bacterial infection. Thus, the first wave of neutrophil migration is dependent on bacterial chemokines and the second wave of migration of neutrophils, macrophages, and DCs is dependent on chemokines secreted from already migrated neutrophils in the infected regions. In this study, the migration of phagocytes through the microstructure of the NFS should be
correlated to chemotactic gradients. In this aspect, there was increased secretion of chemokines in the NFS, indicating that phagocytes were further recruited to chemokine-secreting cells via a chemoattractant gradient in the 3D NFS-based layer-by-layer system. Protein levels of CXCL1 and CXCL2, neutrophil specific chemokines, were increased in the presence of bacteria in both 2D and 3D culture conditions. It has been demonstrated that genes encoding CXCL2 in neutrophils are strongly induced by phagocytosis of bacterial pathogens (27). This study also revealed a distinct chemokine secretion pattern in 3D culture when compared to 2D culture; CCL2 was secreted by neutrophils in the presence of S. aureus in 2D, but not in 3D culture condition. It is known that CCL2 recruits monocytes, macrophages, and DCs, but not neutrophils to the sites of inflammation produced by infection (28). Therefore, the compartmentalization and directional orientation of the layer-by-layer system can be applied for the analysis of migration of phagocytes in response to a chemotactic gradient.
Neutrophils and macrophages migrated to S. aureus-infected lung epithelial cells in the NFS-based layer-by-layer system. By using this system, we can compare the capacity of various bacteria in addition to S. aureus to cause phagocyte influx into an infected area. The secretion of TNF-α and IL-1α was increased in lung epithelial cells cultured with S. aureus in 3D, but not in 2D culture. TNF-α functions as an initiator of the inflammatory response. Thus, an in vivo inflammation-mimicking response occurred in 3D culture condition. In addition to neutrophil migration to the lower NFS containing epithelial cells and bacteria, epithelial cells were also detected in the upper NFS. The detachment of S. aureus-infected epithelial cells was observed only in the presence of migrating neutrophils from the upper to the lower NFS. It is possible that activated neutrophils produce reactive oxygen species and
release cytoplasmic granules, which results in significant host cell damage or death as well as the killing of bacteria. This finding suggests that epithelial integrity is lost in 3D culture condition, as observed in infected lung tissue.
Ⅴ. CONCLUSION
The NFS-based culture system provides a 3D space for cell attachment and migration. In the 3D NFS, cell-derived products can diffuse easily and tight cell-to-cell contact can be avoided. Whereas hydrogels can mimic the native 3D microenvironment, limited diffusion of chemokines and migration of cells hampers their use in 3D coculture of bacteria and phagocytes. Technically, the NFS can be easily assembled into the layer-by-layer system, which mimics multilayers of cells in tissue. Moreover, PCL nanofibers are inert and biocompatible in immune cell culture because the adhesion of BM-DCs to PCL nanofibers does not affect their activation status (8, 9). Finally, 3D migration to surrounding bacteria and phagocytosis processes in the NFS can be visualized though a live cell-imaging setup. Thus, our NFS-based 3D culture system is widely applicable to the biomimetic study of various microbe infections.
REFERENCE
1. Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A 72:1059-62, 1975.
2. Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev 16:561-80, 2005.
3. Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol 36:547-55, 2015.
4. Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453-66, 1962.
5. Sackmann EK, Berthier E, Young EW, Shelef MA, Wernimont SA, Huttenlocher A, et al. Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays. Blood 120:e45-53, 2012.
6. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng 12:1197-211, 2006.
7. Lee J, Lee SY, Jang J, Jeong YH, Cho D-W. Fabrication of patterned nanofibrous mats using direct-write electrospinning. Langmuir 28:7267-75, 2012.
8. Kim TE, Kim CG, Kim JS, Jin S, Yoon S, Bae HR, et al. Three-dimensional culture and interaction of cancer cells and dendritic cells in an electrospun nano-submicron hybrid fibrous scaffold. Int J Nanomedicine 11:823-35, 2016.
9. Jin S, Park TM, Kim CH, Kim JS, Le BD, Jeong YH, et al. Three-dimensional migration of neutrophils through an electrospun nanofibrous membrane. Biotechniques 58:285-92, 2015.
10. Woodruff MA, Hutmacher DW. The return of a forgotten polymer-polycaprolactone in
the 21st century. Prog Polym Sci 35:1217-56, 2010.
11. Wang Y, Wang G, Chen L, Li H, Yin T, Wang B, et al. Electrospun nanofiber meshes
with tailored architectures and patterns as potential tissue-engineering scaffolds. Biofabrication 1:015001, 2009.
12. Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr
Protoc Immunol 110:3.20.1-15, 2015.
13. Song MG, Hwang SY, Park JI, Yoon S, Bae HR, Kwak JY. Role of aquaporin 3 in
development, subtypes and activation of dendritic cells. Mol Immunol 49:28-37, 2011.
14. Pineda-Torra I, Gage M, de Juan A, Pello OM. Isolation, culture, and polarization of
murine bone marrow-derived and peritoneal macrophages. Methods Mol Biol 1339:101-9, 2015.
15. Park KH, Kurokawa K, Zheng L, Jung DJ, Tateishi K, Jin JO, et al. Human serum
mannose-binding lectin senses wall teichoic acid glycopolymer of Staphylococcus aureus, which is restricted in infancy. J Biol Chem 285:27167-75, 2010.
16. Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. Phagocytosis and killing of
Staphylococcus aureus by human neutrophils. J Innate Immun 6:639-49, 2014.
17. Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, et al. International
union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 61:119-61, 2009.
18. Skovbakke SL, Winther M, Gabl M, Holdfeldt A, Linden S, Wang JM, et al. The
peptidomimetic Lau-(Lys-βNSpe)6-NH2 antagonizes formyl peptide receptor 2
expressed in mouse neutrophils. Biochem Pharmacol 119:56-65, 2016.
19. Alfaro C, Suarez N, Oñate C, Perez-Gracia JL, Martinez-Forero I, Hervas-Stubbs S, et
al. Dendritic cells take up and present antigens from viable and apoptotic polymorphonuclear leukocytes. PLoS One 6:e29300, 2011.
20. Sinha B, François PP, Nüsse O, Foti M, Hartford OM, Vaudaux P, et al.
Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin a5b1. Cell Microbiol 1:101-17, 1999.
21. Abrigo M, Kingshott P, McArthur SL. Electrospun polystyrene fiber diameter
influencing bacterial attachment, proliferation, and growth. ACS Appl Mater Interfaces 7:7644-52, 2015.
22. Schindler D, Gutierrez MG, Beineke A, Rauter Y, Rohde M, Foster S, et al. Dendritic
cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection. Am J Pathol 181:1327-37, 2012.
23. Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, et al.
Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1β secretion. Cell Host Microbe 7:38-49, 2010.
24. Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus
25. Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A, et al. Formylpeptide receptors
are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep (2012) 2:786.
26. Sadik CD. Kim ND, Luster AD. Neutrophils cascading their way to inflammation.
Trends Immunol (2011) 32:452-60.
27. Kobayashi SD, Braughton KR, Whitney AR, Voyich JM, Schwan TG, Musser JM, et
al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A (2003) 100:10948-53.
28. Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1).
국문요약 호중구, 대식세포, 수지상 세포와 같은 전문적인 포식 세포들은 미생물을 활발히 에워싼다. 본 연구에서는, 염증 상태일 때 포식세포와 미생물간에 연관을 조사하기 위하여 생체 외 조건에서 3 차원적인 감염 모델을 개발하였다. 황색포도상구균과 포식세포들을 같이 배양하기 위해서 3 차원적 구조 내에서 다층 구조인 나노 섬유를 이용한 배양 시스템을 사용하였다. 표면에 부착된 황색포도상구균과 포식 세포들은 나노 섬유 안으로 이동이 가능하며, 포식세포들은 3 차원적 조건에서 포도상구균으로 이동하고 에워싼다. 또한 포밀 펩타이드 수용체 억제제를 처리하면 식균 작용의 비율이 3 차원적 조건에서는 감소를 하지만 2 차원적 조건에서는 감소를 하지 않았다. 황색포도상구균으로의 포식 세포의 이동은 나노 섬유 기반 다층 구조 배양 시스템을 이용하여 측정하였다. 상위의 나노 섬유 지지체 내 포식 세포들은 아래 층의 지지체로 이동을 하였다. 또한 황색포도상구균과 같이 배양한 포식 세포들에 의한 사이토카인과 케모카인은 분비 양상을 2 차원적과 3 차원적 조건일 때 비교를 하였다. 그리고 3 차원적인 3 차원 조건일 때 수지상 세포는 박테리아를
잡아먹은 호중구로 이동을 한 후, 호중구를 에워쌋다. 게다가 상위의 지지체에 있는 호중구와 대식 세포들은 다층 구조 지지체 배양 시스템에서 아래쪽의 지지체 내의 박테리아에 감염된 폐 상피세포로 이동을 확인했다. 그리고 박테리아에 감염된 폐의 상피세포에 박테리아를 감염시키면 3 차원적 조건에서만 종양괴사인자-알파와 인터류킨-1α 가 분비가 되는 것을 확인했다. 이것을 통해 나노 섬유를 이용한 3 차원적 배양 시스템은 생체 내 미생물에 대한 반응처럼 염증 반응을 흉내 낼 수 있다는 것을 증명하였다.