저작자표시-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Characterization of an nf-actin gene
in the pathogenic Naegleria fowleri
by
Hae-Jin Sohn
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Characterization
of an nf-actin gene
in the pathogenic Naegleria fowleri
by
Hae-Jin Sohn
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for the
Degree of Doctor of Philosophy of Biomedical Sciences
Supervised by
Ho-Joon Shin, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
i
-ABSTRACT-
Characterization of an nf-actin gene in
the pathogenic Naegleria fowleri
Naegleria fowleri, a pathogenic free-living amoeba has been isolated from samples
obtatined from soil,polluted water and chlorinated swimming pool waster and existed as a virulent pathogen which causes fatal primary amoebic meningoencephalitis (PAM) in experimental animal or humans. The cytotoxicity of N. fowleri on target cells requires the contact-dependent mechanism such as a phagocytosis and the contact-independent mechanism such as a secretion of proteases. The phagocytosis mechanisms involve a process of piecemeal ingestion of target cells by food-cups (amoebastomes). Phagocytosis is an actin-dependent process that includes polymerization of monomeric G-actin into filamentous F-actin. Despite the numerous studies concerning with phagocytosis, the detailed role of actin in the N. fowleri food-cup formation has been poorly reported. In this study, we cloned and characterized an nf-actin gene for elucidation of the role of nf-actin in N. fowleri pathogenesis. The nf-actin gene is composed of 1,124 bp and the sequence identity was 82% with nonpathogenic N. gruberi. No sequence identity with other mammals or human actin gene. Immunofluorescence assay using anti-Nf-actin polyclonal antibody developed in BALB/c mice immunized with recombinant protein (rNf-actin fused with His-tag) revealed that the Nf-actin was localized in the cytoplasm and pseudopodia, especially, food-cup structure (amoebastome), of N. fowleri trophozoites. When N. fowleri were co-cultured with
ii
CHO cells, the Nf-actin was observed to localize around on phagocytic food-cups. When the Nf-actin protein was inhibited with cytochalasin D, the actin polymerization inhibitor, or
nf-actin gene knock-downed by transfection with antisense oligomers, N. fowleri trophozoites
showed reduced food-cup structures and in vitro cytotoxicity. It suggests that Nf-actin plays an important role in phagocytic activity of pathogenic N. fowleri. In addition, the nf-actin gene was inserted into C2 vector for overexpression, and then Ubi-pEGFP-C2/nf-actin was transfected to N. fowleri trophozoites. The strong GFP fluorescence was observed in N. fowleri trophozoites transfected with Ubi-pEGFP-C2/nf-actin, and the expression of EGFP-Nf-actin protein was detected by western blot. The nf-actin overexpressed transgenic N. fowleri showed significantly increasing adherence ability against extracellular matrix components such as fibronectin, collagen I and fibrinogen in comparison to wild type N. fowleri. Moreover, the nf-actin overexpressed transgenic N.
fowleri showed increasing phagocytotic ability and cytotoxicity in comparison with the wild
type N. fowleri. Finally, these results suggest that the nf-actin gene plays an important role in cell adhesion, phagocytosis and cytotoxicity of N. fowleri.
Key words: Naegleria fowleri; actin gene; adhesion; contact-dependent mechanism; cytotoxicity; food-cups; pathogenicity; phagocytosis
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF TABLE ... vi
LIST OF FIGURES ... vii
I. INTRODUCTION ... 1
A. Free-living amoeba, Naegleria fowleri ... 1
B. Primary amoebic meningoncephalitis ... 2
C. Mechanisms of pathogenicity ... 4
D. An antigenic nfa1 gene ... 5
E. Actin cytoskeleton ... 5
E. Purpose of this study ... 6
II. MATERIALS AND METHODS ... 8
A. Cultivation of N. fowleri and CHO cells ... 8
B. Gene cloning ... 8
C. Sequence analysis and homology alignment ... 10
D. Expression and purification of recombinant Nf-actin ... 10
E. Production of anti-Nf-actin polyclonal antibody ... 11
F. SDS-PAGE and immunoblotting ... 11
G. Immunofluorescence assay and confocal microscope practice... 13
iv
1) Effect of cytochalasin D treatment on Nf-actin expression ... 14
2) In vitro cytotoxicity ... 14
I. Construction of EGFP expression vector ... 15
J. Transfection and G418 selection ... 16
K. Observation of EGFP expression on N. fowleri ... 17
L. Knock-down system for inhibition of nf-actin gene ... 17
M. cDNA synthesis and RT-PCR ... 18
N. Adhesion assay ... 19
O.Phagocytosis assay ... 19
P. In vitro cytotoxicity analysis ... 20
Q. Statistical analysis ... 21
III. RESULTS ... 22
A. Cloning of an nf-actin gene ... 22
B. Homology analysis of the nf-actin gene ... 25
C. Characterization of recombinant protein (rNf-actin) and polyclonal anti-Nf-actin antibody ... 28
D. Cellular localization of the Nf-actin by IFA ... 30
E. Localization of the Nf-actin in N. fowleri trophozoites co-cultured with target cells ... 31
F. Function of the Nf-actin by treatment of actin inhibitor ... 34
v
H. Transfection of nf-actin and nfa1 gene and observation of Nf-actin and
Nfa1 expression ... 41
I. Knock-down of nf-actin or nfa1 gene using antisense oligomers ... 43
J. Expression of the Nf-actin and Nfa1in transgenic N. fowleri ... 45
K. Cytotoxicity against target cells in transgenic N. fowleri ... 47
L. Ability of adherence in transgenic N. fowleri ... 50
M. Phagocytic activity in transgenic N. fowleri ... 53
IV. DISCUSSION ... 56
V. CONCLUSION ... 62
REFERENCES ... 63
vi
LIST OF TABLES
vii
LIST OF FIGURES
Fig. 1. Complete nucleotide sequences of the nf-actin gene ... 23
Fig. 2. Amplified PCR products of the nf-actin gene from genomic DNA ... 24
Fig. 3. Multiple sequence alignments of the deduced amino acid sequence of nf-actin with those from other species ... 26
Fig. 4. SDS-PAGE and Western blot of the ITPG-induced recombinant Nf-actin ... 29
Fig. 5. Cellular localization of the Nf-actin by IFA ... 30
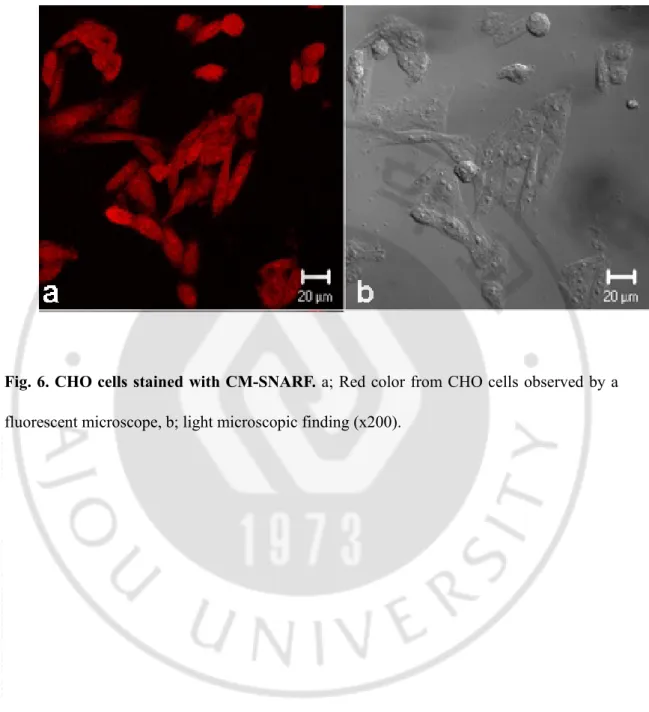
Fig. 6. CHO cells stained with CM-SNARF ... 32
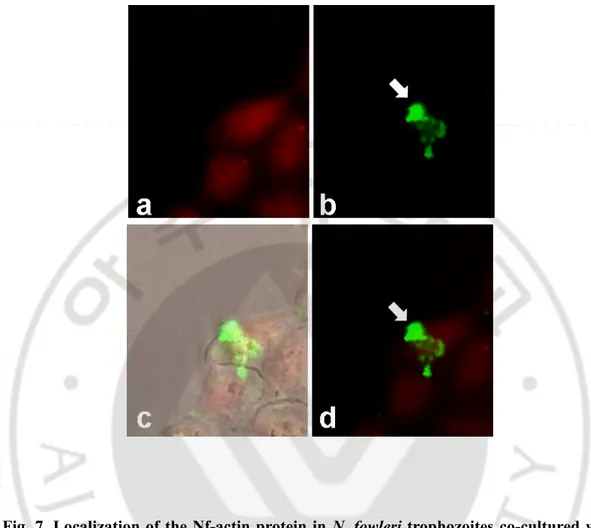
Fig. 7. Localization of the Nf-actin protein in N. fowleri trophozoites co-cultured with CHO cells ... 33
Fig. 8 Lcalization of the Nf-actin in N. fowleri treated with cytochalasin D ... 35
Fig. 9. Inhibition of the Nf-actin with cytochalasin D in N. fowleri co-cultured with CHO cells ... 36
Fig. 10. In vitro cytotoxicity in Nf-actin inhibited N. fowleri ... 38
Fig. 11. Construction of eukaryotic expression vectors ... 40
Fig. 12. Fluorescence microscope finding and Western blot analsysis in nf-actin or nfa1 overexpressed N. fowleri ... 42
Fig. 13. Knock-down of the Nf-actin expression in N. fowleri treated with antisense nf-actin oligomer ... 44
Fig. 14. Expression of the Nf-actin and Nfa1 in nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri ... 46
viii
Fig. 15. Cytotoxicity against CHO cells in nf-actin overexpressed or
knock-downed N. fowleri ... 48 Fig. 16. Cytotoxicity against CHO cells in nfa1 overexpressed or
knock-downed N. fowleri ... 49 Fig. 17. Adhesion activity to the ECM components in nf-actin overexpressed
or knock-downed N. fowleri ... 51 Fig. 18. Adhesion activity to the ECM components in nfa1overexpressed
or knock-downed N. fowleri ... 52 Fig. 19. Phagocytosis assay in nf-actin overexpressed or knock-downed N. fowleri ... 54 Fig. 20. Phagocytosis assay in nfa1 overexpressed or knock-downed N. fowleri ... 55
- 1 -
I. INTRODUCTION
A. Free-living amoeba, Naegleria fowleri
Naegleria is a free-living amoeba has been isolated from samples obtained from that
chlorinated swimming pools, freshwater lakes, hot springs domestic eater supplies, thermally polluted waters, sewage, soil, air and wetland (Culberston et al, 1970; Kollars et al., 1996).
Naegleria fowleri, only one species of Naegleria infects peoples commonly referred to
as the ‘brain-eating amoeba’. N. fowleri infects people by entering to body through the nose. This typically occurs when people go swimming or diving in warm fresh water, such as lakes and rivers. It also exists as a virulent pathogen causing fatal primary amoebic meningoencephalitis (PAM) in experimental animal, human and other mammals (Yoder et al., 2010; Visvesvara, 2010). The name “primary amoebic meningoencephalitis (PAM)” was used first by Butt, and later by Cater, to distinguish infection of the central nervous system (CNS) in human by free-living amoeba from the uncommon invasion of the brain by the intestinal amoeba Entamoeba histolytica.
Introduced amoeba via nasal cavity are able to enter the nervous system through the olfactory nerve and digest neuronal tissues and other mammalian cells by unusually effective cytolysis and phagocytosis as observed in culture or in sections infected brain tissue (Marciano-Cabral and Cabral, 2007; Yoder et al., 2010; Visvesvara, 2010).
Naegleria is an amoebaflagellate and has three stages in its life cycle: trophozoites, cyst,
- 2 -
infective and reproductive form and characterized by a nucleus with a large karyosome surrounded by a halo, and that is vegetative or feeding stage (DE Jonchkeere et al., 1976; Cursons et al., 1980).
Trophozoites exhibit food cups which are cytoplasmic extensions of surface. The food cups, that are used to ingest bacteria, yeast cells, and cellular debris, may serve as the attachment organelles. The next ameba-flagellate stage can be confirmed if the trophozoites are placed on sterile distilled water or saline. When the environment becomes worse, as the food was not supplied, within several hours the trophozoites transforms into an amoeba-flagellate. The cyst is surrounded by smooth double wall containing two or three pores, sometimes sealed with a mucoid plug. The cyst has resistance to waterlessness, but when the environment is favorable, the organism has excysted (Marciano-Cabral and Cabral, 2007; Visvesvara, 2010).
B. Primary Amoebic Meningoncephalitis (PAM)
PAM is a rare disease and rapidly fatal disease which occurs generally in previously healthy children and young adults with a history of swimming in freshwater lakes or ponds. Presumably, infection results from introduction of water containing amoeba into the nasal cavity and subsequent passage of the organisms to the CNS via the olfactory apparatus (Carter, 1968; 1970; 1972). The incubation period of this disease produced by N. fowleri may vary from 2 to 3 days to as long as 7 to 15 days. It has an acute onset with severe headache, nausea, fever ataxia, sign of meningeal irritation, encephalitis (Jonh and John,
- 3 -
1989; Jonckheere, 2011).
As of 2012 year, approximately 128 cases of amoebic meningoencephalitis had been reported throughout the U.S and PAM lead to death in most cases (Yoder et al., 2012). The mortality of patients with PAM is greater than 98 % because of the rapid progression of the disease, delayed diagnosis, and lack of effective healing agents. For the diagnosis and treatment, the antigen related gene producing the antigenic molecule has been largely unsuccessful. Most human infections with N. fowleri have been associated with swimming in warm water but other poorly reports of infects include tap water (Yoder et al., 2012) and host baths.
The final diagnosis of PAM is based on the isolation and culture of free-living amoeba from CSF (cerebrospinal fluid) or the demonstration of amoebic trophozoites in biopsied brain tissue. Antibodies may be detected in serum; however, serologic tests usually are of no value in the diagnosis of infection with free-living amoeba (Visvesvara, 2005; Tuppeny, 2011). Amphotericin B is the only agent with clinical efficacy in PAM and has been reported in some patients treated with amphotericin B alone or in combination with other drugs (Apley et al., 1970; Seidel et al., 1982; Loschiavo et al., 1993). Unfortunately, to date, there is no completely effectively therapeutic drug for PAM.
- 4 - C. Mechanisms of Pathogenicity
The elucidation of pathogenicity of N. fowleri is very important in the mechanisms of parasite-host interaction. The factors that determine the pathogenicity of N. fowleri have not clearly established. In similar free living amoeba, Acanthamoeba, cytopathogenic effects of
amoeba on host cells reported in the adhesion of amoeba to host cell, phagocytosis, and
amoebic proteolytic enzymes include serine proteases (Kim et al., 2009), contact-dependent metalloproteases (Ondarza, 2007), elastases, cysteine proteases (Hysmith and Franson, 1982; Barbour and Marciano-Cabral, 2001), and cytotoxic proteinases (Aldape et al., 1994; Serrano-Luna et al., 2007) induced by mannose-mediated adhesion.
The adhesion is one of the crucial steps for the pathogenicity of amoeba. In addition, Khan et al (2000) reported that pathogenicity would involves both contact-dependent and contact-independent pathways in order to kill host cells quickly and to reduce the degree to which defense can be induced.
In contact-dependent mechanism, Naegleria trophozoites exhibit food-cups or
amoebastomes, which are cytoplasmic extension of the surface. The food-cups, which vary in size and number depending on the species and strain if N. fowleri is used to ingest bacteria, yeast cells, and cellular debris concluded that N. fowleri injured target cells by nibbling, a
process termed “trogocytosis” (Cursons and Brown, 1978; Marciano-Cabral and John, 1984;
John et al., 1984). N. fowleri destroys target cells by trogocytosis, a process of piecemeal ingestion of target cells by food-cups. This may suggest that the cytoplasmic extensions termed food-cups may play a role in attachment of amoebae to substrates in addition to being
- 5 -
used for ingestion of particulate material (Marciano-Cabral et al., 1982; 1988).
D. An antigenic nfa1 gene
The pseudopodia-specific nfa1 gene was reported that it participates in the cytopathic activity of N. fowleri on rat microglial cells in a contact-dependent pathogenic mechanism (Oh et al., 2005; Lee et al., 2007). An nfa1 cloned from a cDNA library of N. fowleri by immunoscreening had a coding nucleotide sequence consisting of 360 bases and produced a recombinant protein of 13 kDa (Shin et al., 2001). The Nfa1 protein was mainly located on tips of the pseudopodia and especially food-cups in N. fowleri trophozoties co-cultured with target cells. And the treatment of an anti-Nfa1 antibody is able to induced the decreasing effect on the cytotoxicity of N. fowleri trophozoties (Jeong et al., 2004; Kang et al., 2005). Therefore, the nfa1 gene is the key molecule concerned with cytotoxicity against host cells in regard to contact-dependent mechanisms of the N. fowleri pathogenesis.
E. Actin cytoskeleton
The actin cytoskeleton is abundant protein in most eukaryotic cells. The fibrous actin (F-actin) consists of a helical polymer of globular polypeptide chain with G-actin (Pollard et al., 1990). Actin plays a important role in a variety of cellular process like motility (Lazarides, 1974), cytokinesis, cell-to-cell and cell-substrate interactions, intracellular transport, endocytosis (Robertson, 2009), exocytosis and phagocytosis. All these processes
- 6 -
are made possible through remodeling of diverse F-actin structures arising by interactions among actin filaments and regulatory components. Actin-binding proteins contribute to the structural organization of the actin cytoskeleton by filament cross-linking (e.g. filamin, a-actinin) or bundling (e.g. villin, fimbrin), some anchor cytoskeletal structures to the plasma membrane, nuclear envelope or cell-to-cell adhesion sites (e.g. spectrin, interaptin, plectin), others, such as the myosins, mediate movement of cargoes along F-actin tracks (Rivero and Cvrčková, 2006).
A number of infectious diseases regulate the production of actin or of it associated protein. Also the protection of actin is important to the process of pathogenic microorganism’s infection and related to the pathogenicity of intracellular pathogen such as
Salmoella, Shigella, Listeria, Toxoplasma gondii, have evolved their own actin cytoskeletal
systems (Reisler, 1993; Goode and Eck, 2007).
The expression of pathogenic activity requires a dynamic cytoskeleton that allows rapid movement, tissue penetration, and changes in parasite morphology. There are many studies reported that actin cytoskeleton plays a structural and dynamic role during phagocytosis in other parasites, Entamoeba histolytica, Acanthamoeba castellanii, Acanthamoeba healyi (Hostos, 1993; 1999; Eleonor et al., 2005; Okada et al., 2005).
F. Purpose of this study
N. fowleri destroys target cells through the contact-dependent mechanism such as a
- 7 -
actin cytoskeleton is playing a key of role in the pseudopodia formation and related with the phagocytosis in various protozoa. In D. discoideum, coronin has been found to play important roles in actin activity such as cell motility, cytokinesis, endocytosis and
phagocytosis (Hostos, 1993; 1999; Eleonor et al., 2005; Okada et al., 2005). Actin and
myosin IB was shown that localizes to the phagocytic cup and phagosomes during ingestion
of target cells in E. histolytica (Okada et al., 2005). However despite the numerous studies
concerning with pahgocytosis (food-cup formation or amoebastomes), the role of cytoskeleton protein of N. fowleri has been poorly reported.
In this study, I cloned and characterized an actin gene of N. fowleri to understand the role of actin gene in the food-cup formation and cytotoxicity against target cells of N. fowleri. And the nf-actin gene have transfected into N. fowleri in transfection system which show synergic effect of pathogenicity in N. fowleri. For the transfection of the nf-actin gene into N.
fowleri, pEGFP-C2 and pEGFP-C2/nf-actin containing a ubiquitin promoter were
constructed, and knock-down of nf-actin using antisense oligonucleotides was carried out. After transfection, expressed GFP was observed under a fluorescent microscope and nf-actin gene product was detected by PCR. GFP/Nf-actin fusion protein was detected by western blotting using GFP antibody. And then the influence of Nf-actin on phagocytosis, cytotoxicity and adhesion activity response were observed by overexpression or knock-down system.
- 8 -
II. MATERIALS AND METHODS
A. Cultivation of N. fowleri and CHO cells
N. fowleri trophozoites (Carter NF69; ATCC No. 30215) were axenically cultured at
37 °C in Nelson’s medium containing 10 % fetal bovine serum (Willaert, 1971). Chinese hamster ovary (CHO) cells were cultured with Dulbecco's modified Eagle's minimal essential medium (DMEM; Gibco BRL, Gaithersburg, MD, USA) containing 10 % fetal bovine serum at 37 °C in 5 % CO2 incubator.
B. Gene cloning
Total RNA was prepared from trophozoites of N. fowleri using an isolation kit RNeasy®Mini kit (QIAGEN, Valencia, CA. USA). Briefly presented, after 600 μl of RLT buffer was mixed with pellet of 1 x 106 trophozoites by pipetting. It was centrifuged at
13,000 rpm for 3 min at room temperature (RT). The supernatant was transferred to a new eppendorf tube and reacted with 600 μl of 70 % ethanol. The reagent was transferred to a new spin column and centrifuged at 13,000 rpm for 1 min at RT. Add 700 μl of RW1 buffer to the spin column and centrifuged at 13,000 rpm for 1 min at RT. Add 500 μl of RPE buffer to the column and centrifuged at 13,000 rpm for 1 min. RNA yield and quality were checked by using spectrophotometrically and by analytical agarose gel electrophoresis. For reverse transcription polymerase chain reaction (RT-PCR), a superscript first strand synthesis
- 9 -
System kit (Invitrogen, Carlsbad, CA, USA) was used to generate cDNA from 5 μg of total RNA.
For amplification of cDNA by PCR, degenerated oligonucleotide primers were designed on conserved region of actin gene in N. fowleri Lee strain, and Oligo dT primer was used for 3’ end.
The genomic DNA was prepared from trophozoites of N. fowleri using DNeasy Tissue kit following the manufacturer’s instruction (QIAGEN, Valencia, CA. USA). For the amplification of genomic DNA by PCR, primers were designed on actin gene of N. fowleri (Nf-actin forward primer: ATG TGT GAC GAC GTT CAA GCA CTC, Nf-actin reverse primer: AAG ATC TTT CTG TGG ACA ATA CCT GGA). The amplification PCR product was purified from the gel and subcloned for sequence analysis into pCR2.1 TOPO TA vector (Invitrogen). The nucleotide sequence of cloned cDNA fragments was determined using an ABI Perkin Elmer 373A automated DNA sequencer (Applied Biosystems, Forster City, CA, USA). The open reading frame of Nf-actin was subcloned into the pCR-T7/NT-TOPO cloning /expression vector (Invitrogen) to produce Nf-actin-(His)6 fusion protein.
The recombinant expression plasmid pCR-T7/NT-TOPO: nf-actin was sequenced to ensure that the inserts were in the correct reading frame. Briefly presented, 4 μl of fresh amplified nf-actin ORF by PCR was mixed to 1 μl of PCR-T7/NT-TOPO expression vector (Fig. 2) and incubated for 5 min at room temperature. One μl of the 6 x TOPO cloning stop solution was added and mix for 10 sec. The reaction tube was placed on ice. PCR subsequently, the E. coli strain BL21 (DE3) pLysS was transformed with pCR-T7/NT-TOPO:
- 10 - C. Sequence analysis and homology alignment
Deduced amino acid sequences were analyzed with the EditSeq.V 1.0.3 program and Clustal of the MegAlign program, a multiple-alignment program of the DNASTAR package (DNASTAR, Madison, WI, USA).
D. Expression and purification of recombinant Nf-actin
A fresh overnight culture form the E. coli cells containing pCR-T7/NT-TOPO: nf-actin was 1:100 in Luria-Bertani medium supplemented with 100 μg/ml ampicillin and 34 μg chloramphenicol, and grown at 37 °C until the OD600 reached 0.5. Expression was initiated
with 1 mM isopropyl β-D-thiogalactopyranoside. The cells were grown for additional 4 h at 37 °C before being harvested by centrifugation at 8,000 rpm for 5 min at 4 °C. Expressed recombinant antigen was purified using the probondTM Purification System (Invitrogen). Harvested cells were resuspened in native binding buffer (20 mM sodium phosphate, 500 mM sodium chloride, pH 7.8) and sonicated with two or three 10 second bursts at a medium intensity setting while holding the suspension on ice. Sonicated cells were freezed immediately in liquid nitrogen and quickly thawed at 37 °C. Final concentration of 5 μg/ml RNase and 5 μg/ml DNase was treated on ice for 15 min. Insoluble debris was removed by centrifugation at 12,000 rpm for 20 min. The recombinant gene product was eluted using ProbondTM resin column (Invitrogen) by increasing imidazol concentration. The eluted protein was dialyzed and concentrated by freeze-dry. Recombinant Nf-actin was purified
- 11 -
from supernatant by chelating chromatography on Ni-NTA agarose resin (Invitrogen) by increasing imidazol concentration. The eluted proteins concentrated with Amicon Ultra-15 (Milipore, Bedford, MA, USA).
E. Production of anti-Nf-actin polyclonal antibody
For the production of polyclonal antibody, the Nf-actin protein (50 μg) was mixed an equal volume of complete Freund’s adjuvant (Sigma), and injected intraperitioneally into 7-week-old female BALB/c mice (Korea). The mouse was boosted biweekly for another 4 weeks with the Nf-actin protein (25 μg) containing an equal volume of incomplete Freund’s adjuvant (Sigma). After the third boosting the Nf-actin protein (5 μg) was injected intravenously without the adjuvant. Four day later, a polyclonal anti-Nf-actin antibody was collected from the mouse blood by centrifuging at 2,500 x g for 30 min at 4 °C.
F. SDS-PAGE and immunoblotting
12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing condition was performed for the fraction of amoeba lysates. The lysates samples were suspended in reducing sample buffer (62.2 mM Tris, pH 6.8; 10 % glycerol; 10 % 2-mercaptoethanol; 3 % SDS and 0.1 % bromophenol blue), boiled for 5 min prior to loading onto the gel and then separated by electrophoresis. For Western blotting, proteins were transferred onto Polyvinylidene difluoride (PVDF) sheet and reacted with polyclonal
anti-- 12 anti--
Nf-actin antibody (1:1,000 dilutions) and normal sera (1:1,000 dilutions) at 4 °C for overnight. After washing with 0.05 % PBST three times, peroxidase-conjugated goat anti-mouse whole IgG (1:2,000 dilutions) (Sigma Chemical Co., St. Louis, USA) was added on each sheet. After washing with 0.05 % PBST three times, they were soaked in enhanced chemiliminescence (ECL) solution (Intron, Dajon, Korea) and exposed to an X-ray film (Konica, Tokyo, Japan).
Transgenic amoeba was extracted by cell lysis buffer (Intron, Dajon Korea) and quantified by centrifuged to remove cell pellets. The supernatants were quantified the Brafored method (Bradford, 1976). The sample was analyzed by 12 % SDS-PAGE using reducing sample buffer (62.2 mM Tris, pH 6.8; 10 % 2-mercaptoethanol; 3 % SDS; and 0.1 % bromophenol blue). For Western blotting, proteins were transfected onto PVDF membrane for 2 h at 250 mA. The membrane was blocked with 5 % skim milk for 2 h at RT and reacted with anti-Nf-actin antibody (1:1,000 dilutions with 3 % BSA), anti-Nfa1 antibody (1:1,000 dilutions with 3 % BSA) and anti-GFP antibody (1:1,000 dilutions with 3 % BSA, abcam) for overnight at 4 °C. The membrane was washed with PBS containing Tween-20 (PBST) 3 times for 15 min and reacted with secondary antibody of a goat anti-mouse IgG conjugated with horseradish peroxidase (1:2,000 dilutions with 3 % BSA) for 2 h at room temperature. After washing with PBST 3 times, they were soaked in enhanced chemical luminescence’s solution and exposed to X-ray film.
- 13 -
G. Immunofluoroscence assay and confocal microscope practice
To observe the cellular localization of Nf-actin protein in N. fowleri, immunofluroscence assay was performed according to the method in a precious paper. Amoeba trophozoites were fixed with 10 % formalin and treated with 1 % NH4OH
ammonium hydroxide), and attached to a glass slide. Polyclonal anti-Nf-actin antibody (1:100 dilutions) was added to a slide glass. After incubating the glass slide at 4 °C for 2 h, a goat anti-mouse whole immunoglobulin (1:200 dilutions) conjugated with a fluorescein isothiocyanate (FITC, Sigma. USA) was added. Fluorescent localization in intact trophozoites was observed under a fluorescence microscope.
For immunofluorescene studies, cultivating trophozoites of N. fowleri or CHO cells used as target cells were fixed in 10 % formaladehyde for 10 min at room temperature, permeabilized in 1 % NH4OH, washed in Tween 20 for 5 min and extensively washed with
0.82 % saline. N. fowleri was incubated overnight at 4 °C with an anti-Nf-actin polyclonal antibody diluted serially with 3 % BSA. This was followed by incubation with the appropriate FITC-conjugated anti-mouse antibody (Sigma) a dilution of 1:100 at 4 °C for 2 h. As the controls, N. fowleri trophozoites and CHO cells stained with the FITC-conjugated anti-mouse antibodies. For labeling the living target cells, CHO cells were incubated 30 min at 37 °C. DMSO stock solutions (100 μl in 5-(and-6)-chlormethyl seminaphthorhodafluor-1 acetate (CM-SNARF) stock) are typically diluted 1:1,000 into loading buffer should be serum-free because often contains esterase activity. Total fluorescence of cells was visualized in optical section produced by confocal microscope (Olympus FV-500). The
- 14 -
collected images were processed using Adobe Photoshop 7.0 software.
H. Inhibition assay of the Nf-actin
Cytochalasin D (Sigma) is a cell permeable and potent inhibitor of actin polymerization. It disrupts actin microfilaments and activates the p53-dependent pathways causing arrest of the cell cycle at the G1-S transition. It is believed to bind to G actin and prevent polymerization of actin monomers.
1) Effect of cytochalasin D treatment on Nf-actin expression
To determine viability of N. fowleri by cytochalasin D treatment, trophozoites of N.
fowleri was removed from a 75 T flask. After twice washing with PBS, the trophozoites were
treated at 20 μM, 100 μM concentration of cytochalasin D in 6 well plates, and incubated at 37 °C for 30 min, 1 h. Cytochalasin D was dissolved in dimethyl sulfoxide (DMSO). The same concentration of DMSO was used as a negative control.
2) In vitro cytotoxicity
As CHO cells are useful in observing in vitro cytotoxicity of Nf-actin inhibited N.
fowleri trophozoites (Shin et al., 2001). CHO cells were cultured as monolayer using 24 well
- 15 -
cells were co-cultured with N. fowleri trophozoties pretreated with cytochalasin D. Lactate dehydrogenase (LDH) release assay was practiced to measure in vitro cytotoxicity because the LDH could be released from lysed cells. For LDH assay, 50 µl of reacted supernatant in each well was transferred on 96 well assay plates (Nunc A/S, Roskilde, Denmark). After 50 µl of the reconstituted substrate mix buffer in CytoTox96® Non-radioactive Cytotoxicity Assay Kit (Promega, Madison, WI, USA) for LDH release assay was added, the plate was incubated 30 min at room temperature and then 50 µl of stop solution was added. The reactants were read at 490 nm with ELISA reader. The formula of in vitro cytotoxicity was as follows.
Cytotoxicity (%) =
experimental release - spontaneous release
X 100
maximum release - spontaneous release
I. Construction of GFP expression vectors
The eukaryotic expression vectors transfected into N. fowleri were the pEGFP-C2 vector (Clontech). The pEGFP-C2 vector containing the cytomegalovirus (CMV) promoter encodes a red-shifted variant of wild-type enhanced GFP (EGFP), which has been optimized to maximize fluorescence and expression in mammalian cells and was used as a control construct. We cloned to modify the ubiquitin promoter was inserted into the CMV promoter was deleted. The ubiquitin promoter might be able to act on nf-actin and express it more
- 16 -
efficiently than CMV promoter. The amplified ubiquitin promoter was cloned into CMV promoter-deleted pEGFP-C2 vector.
The nf-actin gene were inserted downstream of the gene encoding EGFP in the pEGFP vector. We constructed of Ubi-pEGFP-C2/nf-actin, which produced 7.7 kbp fragments.
In previously study, the nfa1 gene (GenBank Accession No. AF230370) was cloned from nfa1 gene-cloned vector, PCR-T7/NT TOPO (Shin et al., 2001). The nfa1 gene was inserted into Ubi-pEGFP-C2 vector, which produced 6.8 kbp fragments.
J. Transfection and G418 selection
The Ubi-pEGFP-C2/nf-actin or Ubi-pEGFP-C2/nfa1 were transfected to N. fowleri on a 12 well plate using transfection reagent (PEI; Polyscience, Warington, PA, USA), according to the manufacturer’s instructions. Briefly, 40 μl of transfection reagent, polyethylenimine (PEI), was added to 200 μl of serum-reduced medium (Opti-MEM; Life Technologies,
Rockville, MD, USA). After 200 μl of Opti-MEM containing 10 μg DNA (
Ubi-pEGFP-C2/nf-actin or Ubi-pEGFP-C2/nfa1) was added, the total sample was mixed by gently swirling the plate, and after incubation for 10 min at room temperature to allow formation of DNA-polyethylenimine complexes in final volume of 500 μl of Opti-M, samples were added
to each well of 6 well plate (Costar, Cambridge, MA, BK) containing 5 × 105 trophozoites.
Two days after transfection, N. fowleri were added a lethal dose the antibiotic G418 (Geneticin; Gibco BRL) (1 mg/ml) was used to select against untransfected N. fowleri. Transfected N. fowleri trophozoites were subcultured every week until 70 % confluent.
- 17 - K. Observation of EGFP expression on N. fowleri
The expression of EGFP in transfected N. fowleri was observed by fluorescent microscopy (Olympus) using standard fluorescein isothiocyanate excitation/emission filters (488 nm/500 nm). The strong EGFP fluorescence was observed in N. fowleri transfected with Ubi-pEGFP-C2/nf-actin or Ubi-pEGFP-C2/nfa1.
L. Knock-down system for inhibition of nf-actin or nfa1 gene
Expression of nf-actin was knocked down transiently by transfection with nf-actin-specific antisense oligonucleotides. Phosphorothioate antisense oligonucleotides were designed to anneal on the nf-actin ATG start codon (5′-TGC TTG AAC GTC GTA ACA CAT) or the nfa1 ATG start codon (5′-TGG TGA TGG AAT TGT GCC CAT). Oligonucleotides were introduced into N. fowleri trophozoites by using the methods of transient transfection. Briefly, 10 μl of transfection reagent, polyethylenimine (PEI), was added to 50 μl of serum-reduced medium (Opti-MEM; Life Technologies, Rockville, MD, USA). After 50 μl of Opti-MEM containing 5 μg DNA (the antisense effect was observed to be dependent on oligonucleotide; amount was 5 μg of DNA determined by repeated concentration optimization experiments) was added, the total sample was mixed by gently swirling the plate, and after incubation for 10 min at room temperature to allow formation of DNA-polyethylenimine complexes in final volume of 500 μl of Opti-M, samples were added
- 18 -
After incubation for 5 h post-transfection, 1 ml of complete Nelson's media was added to each well.
M. cDNA synthesis and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RNA of N. fowleri and nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri were isolated by RNeasy®Mini kit (QIAGEN, Valencia, CA, USA) according to instructions of manufacturer. The isolated RNA was quantitated by spectrophotometry and equalized and the purity was checked on 1 % agarose gel.
The cDNA was synthesized from 5 μg of total RNA in reaction mixture containing oligo (dT) primer. The mixtures was incubated at 42 °C for 1 h and then subsequently for 5 min at 94 °C and at 4 °C, respectively according to the instructions of the manufacturer. RT-PCR was performed using primer specific for nf-actin gene. RT-PCR condition was 95 °C for 5 min, 30 cycles at 95 °C for 45 sec, 50 °C for 45 sec, 72 °C for 45 sec and then final extension for 10 min at 72 °C.
The amplified products were separated by electrophoresis on EtBr-stained 1 % agarose gel and detected under UV light.
- 19 - N. Adhesion assay
For adhesion assay, the CytoSelect™ Cell Adhesion Assay Kit (Cell Biolabs, San Diego, CA, USA) utilizing an ECM protein-coated plate was carried out according to the manufacturer’s protocol. The ECM-plate coated with fibronectin, collagen type I, collagen type IV, laminin type I, fibrinogen and BSA as a negative control. N. fowleri and nf-actin (or
nfa1) overexpressed (or knock-downed) N. fowleri were seed 1 x 106 cells/ml incomplete
Nelson’s media to the each well (BSA-coated wells are provided as a negative control) at 37 °C for 3 h and the removal of unattached N. fowleri by PBS washes. And 200 μl of cell stain solution at RT for 10 min and removed the cell strain solution and DW washes and let the wells air dry. And 200 μl of Extraction solution per well and then incubate 10 min on an orbital shaker. Finally the extracted samples were transferred to a 96 well plate and measured the OD 560 nm in a microplate reader.
O. Phagocytosis assay
For phagocytosis assay, the CytoSelect™ 96-well Phagocytosis Assay (Cell Biolabs, San Diego, CA, USA) using prelabeled Zymosan particles as a phagocytosis pathogen was carried out. N. fowleri and nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri were cultured a monolayer using 96 well cell culture plate (Nunc A/S, Roskilde, Denmark) in Nelson's media and added 10 μl of zymosan to the each well at 37 °C for 1 h. Amoeba were washed with complete Nelson’s media to remove media and added of 100 μl of
- 20 -
Fixation solution at RT for 5 min. Amoeba was removed fixation solution and added of 1X Blocking solution at RT for 1 h on orbital shaker. Also amoeba was removed solution and added 100 μl of 1X Permeabilization solution at RT for 5 min and then removed solution and added 100 μl of 1X Detection solution at RT for 1 h on orbital shaker. Finally, amoeba was gently removed solution followed by the addition of 50 μl of Detection buffer at RT for 10 min on orbital shaker. And 100 μl of substrate buffer at 37 °C for 20 min incubation and then stop solution was added. The reactants were measure the OD 405 nm in a plate reader.
P. In vitro cytotoxicity analysis
CHO cells are useful in observing in vitro cytotoxicity of the transfected N. fowleri (Jeong et al., 2004; Oh et al 2005). CHO cells were cultured as monolayer using 24 well cell culture plate (Nunc A/S, Roskilde, Denmark) in DMEM (Gibco BRL, Gaithersburg, MD) at 37 °C.
In control group, 3 x 104 CHO cells were cultured only in 500 μl of DMEM and 3 x 104
CHO cells cultured with trophozoites of N. fowleri with the vector, nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri was experimental groups for 24 h at 37 °C. Lactate dehydrogenase (LDH) release assay was practiced to measure in vitro cytotoxicity because the LDH could be released from lysed cells. For LDH assay, 50 µl of reacted supernatant in each well was transferred on 96 well assay plates (Nunc A/S, Roskilde, Denmark). After 50 µl of the reconstituted substrate mix buffer in CytoTox96® Non-radioactive Cytotoxicity Assay Kit (Promega, Madison, WI, USA) for LDH release assay
- 21 -
was added, the plate was incubated 30 min at RT and then 50 µl of stop solution was added. The reactants were read at 490 nm with ELISA reader. The formula of in vitro cytotoxicity was as follows:
Cytotoxicity (%) =
experimental release - spontaneous release
X 100
maximum release - spontaneous release
Q. Statistical analysis
All experiments were repeated at least three times for each determination. Statistical differences between groups or samples were determined with Student′s t-test. The difference was considered significant when P was < 0.05.
- 22 -
III. RESULTS
A. Cloning of an nf-actin gene
A 1.2 kb DNA fragment from N. fowleri, tentatively identified as nf-actin, was amplified by degenerated oligonucleotide primer which was designed upon nf-actin of N.
fowleri lee strain (GenBank accession number: U37719). The open reading frame (ORF) of
nf-actin was subcloned into pEXP5-NT-TOPO cloning expression vector to produce
Nf-actin-(His)6 fusion protein. The full sequence of cDNA was contained an ORF of 1.2 kb
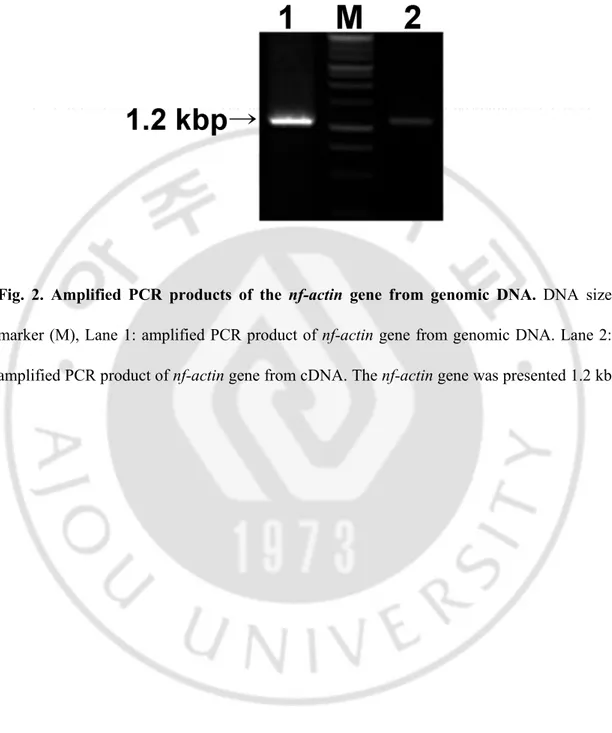
encoding a 375 amino acid protein with a deduced molecular weight of 41.3 kDa (50 kDa, fused with His tag). The nucleotide sequence of Nf-actin has been deposited in the GenBank database with the accession number EU890397 (Fig. 1). There are no introns in the nf-actin gene in genomic DNA of N. fowleri (Fig. 2).
- 23 -
Fig. 1. Complete nucleotide sequences of the nf-actin gene. The coding region was shown
- 24 -
Fig. 2. Amplified PCR products of the nf-actin gene from genomic DNA. DNA size
marker (M), Lane 1: amplified PCR product of nf-actin gene from genomic DNA. Lane 2: amplified PCR product of nf-actin gene from cDNA. The nf-actin gene was presented 1.2 kb.
- 25 - B. Homology analysis of the nf-actin gene
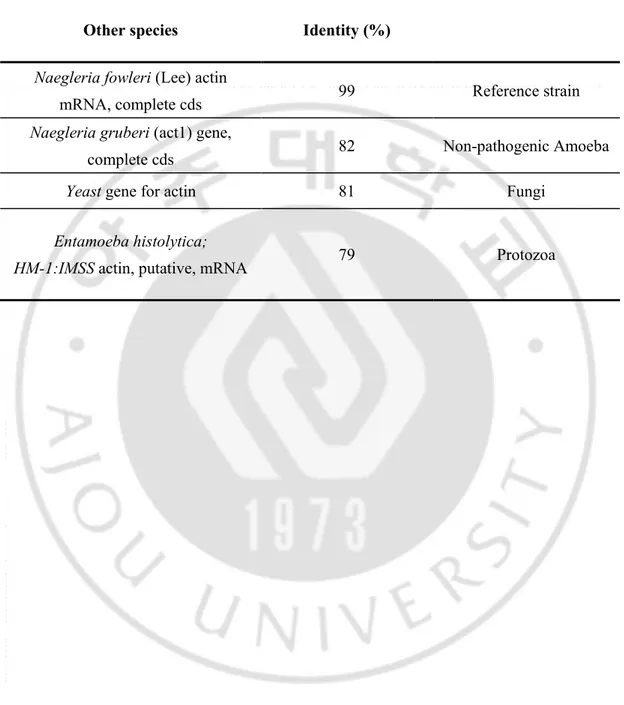
Homology search demonstrated that the amino acid sequence of the nf-actin gene is closely related to the nf-actin gene from amoeba to Yeast. The whole deduced amino acids sequences of the nf-actin gene had 82 % identity with actin gene of N. gruberi. The extent of homology of deduced amino acid sequence of the nf-actin gene ranged from 81 % with actin gene of Saccharomyces cerevisiae to 79 % with actin gene of Entamoeba histolytica (Fig. 3, Table 1).
- 26 -
Fig. 3. Multiple sequence aligments of the deduced amino acid sequence of nf-actin with those from other species. Gaps were introduced to maximize alignment. The aligned sequences were actin gene from N. fowleri (Lee; U37719), N. gruberi (AAF37002), Yeast (Saccharomyces cerevisiae; AAA34391), and Entamoeba histolytica (HM-1:IMSS; XP001913786).
- 27 -
Table 1. Identity (%) of the nf-actin gene amino acid sequence with other species
Other species Identity (%)
Naegleria fowleri (Lee) actin
mRNA, complete cds 99 Reference strain
Naegleria gruberi (act1) gene,
complete cds 82 Non-pathogenic Amoeba
Yeast gene for actin 81 Fungi
Entamoeba histolytica;
- 28 -
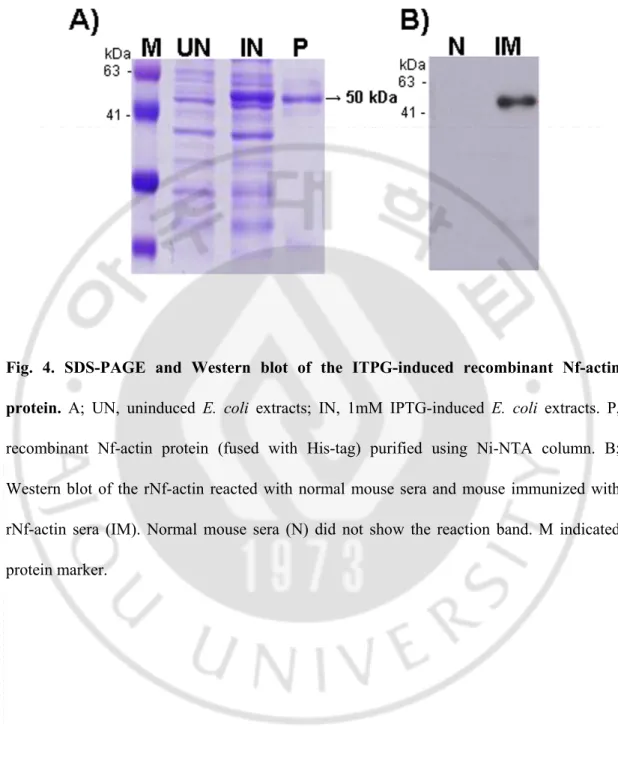
C. Characterization of recombinant protein (rNf-actin) and polyclonal anti-Nf-actin antibody
The recombinant Nf-actin-(His)6 fusion-protein expressed in E. coli containing the
nf-actin gene showed a major protein band of about 50 kDa in SDS-PAGE (Fig. 4A). The
purified rNf-actin fusion protein showed strong immunoreactivity to the anti-Nf-actin polyclonal antibody, but not to normal mouse sera (Fig. 4B).
- 29 -
Fig. 4. SDS-PAGE and Western blot of the ITPG-induced recombinant Nf-actin protein. A; UN, uninduced E. coli extracts; IN, 1mM IPTG-induced E. coli extracts. P,
recombinant Nf-actin protein (fused with His-tag) purified using Ni-NTA column. B; Western blot of the rNf-actin reacted with normal mouse sera and mouse immunized with rNf-actin sera (IM). Normal mouse sera (N) did not show the reaction band. M indicated protein marker.
- 30 -
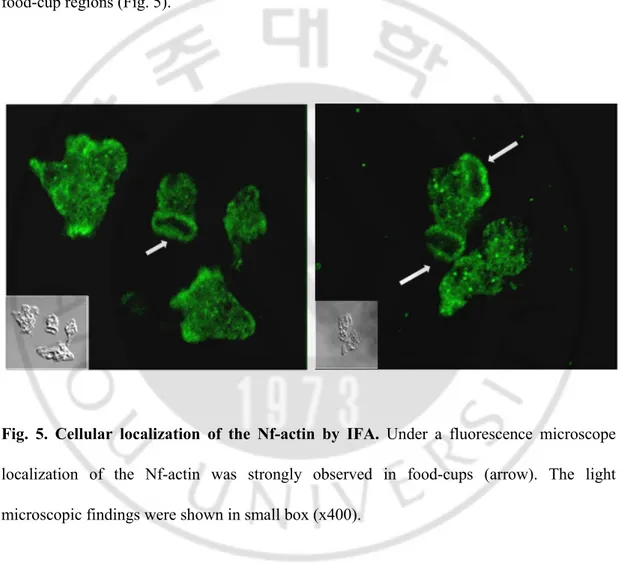
D.
Cellular localization of the Nf-actin by immunofluorescence assay (IFA)
Immunofluorescence assay using polyclonal anti-actin antibody showed that the Nf-actin protein was immunolocalized in cytosol, and compact signal was particularly shown in food-cup regions (Fig. 5).
Fig. 5. Cellular localization of the Nf-actin by IFA. Under a fluorescence microscope
localization of the Nf-actin was strongly observed in food-cups (arrow). The light microscopic findings were shown in small box (x400).
- 31 -
E. Localization of the Nf-actin in N. fowleri trophozoites co-cultured with target cells
To observe the role of the Nf-actin protein in N. fowleri co-cultured with target cells during cell contact, target cells were stained with CM-SNARF (red color) (Fig. 6). After N.
fowleri was co-cultured with CHO cells at 3 h, strong fluorescent signals were shown in
pseudopodia of N. fowleri trophozoties and in food-cups structures (Fig. 7). In addition, the Nf-actin protein was often observed around area contacted with CHO cells (Fig. 7).
- 32 -
Fig. 6. CHO cells stained with CM-SNARF. a; Red color from CHO cells observed by a
- 33 -
Fig. 7. Localization of the Nf-actin protein in N. fowleri trophozoites co-cultured with CHO cells. Strong fluorescent signals were shown in pseudopodia and food-cups of N.
fowleri (arrow). a; CM-SNARF stained CHO cells (red), b; fluorescein isothiocyanate-labeled Nf-actin (green), c; DIC-merged images, d; merged images. Arrows indicate food-cup structures in N. fowleri trophozoites (x200).
- 34 -
F. Function of the Nf-actin by treatment of actin inhibitor
1) Localization of the Nf-actin in N. fowleri treated with cytochalasin D
To observe the role of actin on the food-cup formation of N. fowleri, N. fowleri was treated with cytochalasin D, actin polymerization inhibitor. N. fowleri treated with Cytochalasin D showed shrinkage in food-cup structure. Morphologically, the N. fowleri trophozoites changed into cyst form at high concentration of cytochalasin D (Fig. 8). And then, On the results of inhibition of the Nf-actin in N. fowleri co-cultured with CHO cells,
CHO cells stained with CM-SNARF showed red color (Fig. 9). When N. fowleri without
cytochalasin D treatment was co-cultured with CHO cells at 3 h, N. fowleri trophozoites showed strong and compact Nf-actin signals around area contacted with CHO cells (Fig 9A). In contrast, N. fowleri treated with cytochalasin D and co-cultured with CHO cells showed decreasing the numbers of food-cups and showed food-cup formation (Fig. 9B).
- 35 -
Fig. 8. Localization of the Nf-actin in N. fowleri treated with cytochalasin D. N. fowleri
were treated with 20 μM or 100 μM of Cytochalasin D for 1 h. N. fowleri treated with cytochalasin D showed reducing the numbers of food-cups structures and weak food-cup formation. a; fluorescein isothiocyanate-labeled Nf-actin (green), b; light microscopic finding (x200).
- 36 -
Fig. 9. Inhibition of the Nf-actin with cytochalasin D in N. fowleri co-cultured with CHO cells. A; wild-type N. fowleri was co-cultured with CHO cells. B; N. fowleri with 20 uM cytochalasin D was co-cultured with CHO cells. a; CM-SNARF stained CHO cells (red), b; fluorescein isothiocyanate-labeled Nf-actin (green), c; DIC images, d; merged images. Arrows indicate food-cup structures in N. fowleri trophozoites (x200).
- 37 -
2) In vitro cytotoxicity against target cells in N. fowleri inhibited Nf-actin
To evaluate the role of Nf-actin on the N. fowleri cytotoxicity against CHO cells, LDH release assay was carried out. When CHO cells were co-cultured with N. fowleri trophozoites pretreated with cytochalasin D, the cytotoxicity of N. fowleri decreased 30 % at 6 h in comparison with untreated control (P < 0.001) (Fig. 10).
- 38 -
Fig. 10. In vitro cytotoxicity of the Nf-actin-inhibited N. fowleri. Cytotoxicity was
calculated at 3 and 6 h post-treated cytochalasin D and showed decreasing value in a
time-dependent manner (P < 0.001). N. fowleri; untreated trophozoites, DMSO; dimethyl
sulfoxide-treated trophozoites, Cytochalsin D; 20 μM cytochalasin D-treated trophozoites.
- 39 - G. Construction of eukaryotic expression vectors
Constuction of Ubi-pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 vectors using modified pEGFP-C2 vector containing expression of the green fluorescence protein in mammalian cells. Instead of CMV promoter, ubiquitin promoter was replaced. In the Ubi-pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 vectors, an nf-actin gene and nfa1 gene were inserted in downstream of the gene encoding EGFP which produced 7.7 kbp and 6.9 kbp, and nf-actin primer and nfa1 primer were used to amplify the 1.2 kb and 360 bp, respectively (Fig. 11). These results show that Ubi-pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 vectors were correctly constructed.
- 40 -
Fig. 11. Construction of eukaryotic expression vectors. In the Ubi-pEGFP-C2/nf-actin (A)
or Ubi-pEGFP-C2/nfa1 (B) vectors, ubiquitin promoter was replaced with CMV, and
nf-actin or nfa1 gene was inserted. Amplified PCR products of nf-nf-actin (A) or nfa1 (B) on
Ubi-pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 were shown. DNA size marker (M), Lane 1: amplified PCR product of nf-actin gene of Ubi-pEGFP-C2/nf-actin, Lane 2: amplified PCR product of nfa1 gene of Ubi-pEGFP-C2/nfa1.
- 41 -
H. Transfection of nf-actin and nfa1 gene and observation of Nf-actin and Nfa1 expresssion
On following 48 h post transfection and selection with 1 mg/ml of G418 for 7 days the expression of GFP in N. fowleri tranfected with C2/nf-actin or Ubi-pEGFP-C2/nf-actin vectors was examined. At 48 h post transfection, GFP expression was observed in the cytoplasm of N. fowleri transfected with C2/nf-actin or pEGFP-C2/nfa1 vector (Fig 12A). In N. fowleri transfected with pEGFP-C2/nf-actin or Ubi-pEGFP-C2/nfa1, GFP fusion proteins could be identified by Western blotting after 48 h of transfection (Fig. 12B).
- 42 -
Fig. 12. Fluorescence microscope findings and Western blot analysis in nf-actin or nfa1 overexpressed N. fowleri. A; The fluorescence of GFP was observed at 48 h after
transfection (x200). B; The lysates of wild-type N. fowleri (lane 1), N. fowleri transfected with Ubi-pEGFP-C2 (lane 2), Ubi-pEGFP-C2/nfa1 (lane 3) and Ubi-pEGFP-C2/nf-actin (lane 4) were reacted with GFP antibody.
- 43 -
I. Knock-down of nf-actin or nfa1 gene using antisense oligomer
To observe the inhibited role of nf-actin or nfa1 gene in the food-cup formation of N.
fowleri, nf-actin or nfa1 gene were knock-downed using transfection system with antisense
oligomer. The nf-actin mRNA levels were knock-downed about 42 %, and the Nf-actin protein expression was inhibited about 58.2 % (Fig. 13A). Under a fluorescent microscope, an nf-actin knock-downed N. fowleri showed decreasing numbers of food-cup and weak food-cup formation (Fig. 13B).
- 44 -
Fig. 13. Knock-down of the Nf-actin expression in N. fowleri treated with antisense oligomer. A; RT-PCR and Western blotting analysis in untransfected or nf-actin
knock-downed N. fowleri trophozoites. Total RNA profiles indicated that all loading sample
quantity was equal. B; Immunofluorescence assay with polyclonal anti-Nf-actin antibody in untransfected or nf-actin knock-downed N. fowleri trophozoites (x200). N; wild-type N.
- 45 -
J. Expression of the Nf-actin and Nfa1 in transgenic N. fowleri
To confirm the effect of overexpression or knock-down of nf-actin or nfa1 on N.
fowleri trophozoites, RT-PCR and Western blot ananlysis were performed. In nf-actin
overexpressed N. fowleri, the elevated mRNA level of nf-actin was induced compared to control groups (Fig. 14A). In nf-actin knock-downed N. fowleri, mRNA level and protein expression were reduced in comparison with control groups (Fig. 14A). In addition, nfa1 overexpressed N. fowleri showed increasing mRNA levels and protein expression in comparison with control groups (Fig. 14B). In nfa1 knock-downed N. fowleri, the reduced mRNA level and protein expression were showed in comparison with control groups (Fig. 14B).
- 46 -
Fig. 14. Expression of the Nf-actin and Nfa1 in nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri. The expressions of the Nf-actin (A) and Nfa1 (B) in
overexpressed or knock-downed N. fowleri were analyzed by RT-PCR and Western blotting. Total RNA profiles indicated that all loading sample quantity was equal. Lane 1; wild type N.
fowleri, lane 2; N. fowleri transfected with empty vector, lane 3; nf-actin or nfa1
- 47 -
K. Cytotoxicity against target cells in transgenic N. fowleri
On the observation by a fluorescent microscopic, CHO cells co-cultured with N. fowleri trophozoites for 24 h showed severe destruction (data not shown). On the contrary, CHO cells co-cultured with nf-actin or nfa1 knock-downed N. fowleri showed less destruction than wild type or overexpressed N. fowleri (data not shown). In nf-actin overexpressed N.
fowleri, the level of cytotoxicity was the highest about 74 % among the all experimental
groups. However, in case of N. fowleri transfected with empty vector, the level of cytotoxicity was 31 % (Fig. 15). In case of nfa1 gene, the nfa1 overexpressed N. fowleri showed increasing cytotoxicity levels from 45 % to 72 % in a time-dependent manner (Fig. 16). In addition, the nfa1 knock-downed N. fowleri showed weekly increasing cytotoxicity levels from 6 % to 22 %, respectively (Fig. 16).
- 48 -
Fig. 15. Cytotoxicity against CHO cells in nf-actin overexpressed or knock-downed N. fowleri by LDH release assay. CHO cells co-cultured with nf-actin overexpressed N.
fowleri showed a high cytotoxicity in a time dependent manner, but the cytotoxicity was
decreased in nf-actin knock-downed N. fowleri. Experiments were performed in triple and the data was shown with mean ± SD (P < 0.05).
- 49 -
Fig. 16. Cytotoxicity against CHO cells in nfa1 overexpressed or knock-downed N. fowleri by LDH assay. CHO cells co-cultured with nfa1 overexpressed N. fowleri showed a
high cytotoxicity in a time dependent manner, but the cytotoxicity was decreased in nfa1 knock-downed N. fowleri. Experiments were performed in triple and the data was shown with mean ± SD (P < 0.05).
- 50 - L. Ability of adherence in transgenic N. fowleri
To examine the ability of adherence in nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri, adhesion assay to extracellular matrix (ECM) was performed. The
nf-actin overexpressed N. fowleri had increased ability to attach ECM components in
comparison with bovine serum albumin (BSA; negative control). Particularly, nf-actin overexpressed N. fowleri have a significantly higher adhesion in fibronectin and fibrinogen (Fig. 17). Similarly, in case of nfa1 overexpressed N. fowleri, the ability of attachment to ECM components such as fibronectin was increased, and nfa1 knock-downed N. fowleri showed less adherence than control groups (Fig. 18).
- 51 -
Fig. 17. Adhesion activity to the ECM components in nf-actin overexpressed or knock-downed N. fowleri. The nf-actin overexpressed N. fowleri showed a significantly higher
adhesion to fibronectin, collagen I, collagen IV, laminin l and fibrinogen, and nf-actin knock-downed N. fowleri showed less adherence than control groups. Experiments were performed in triple and the data was shown with mean ± SD (P < 0.05).
- 52 -
Fig. 18. Adhesion activity to the ECM components in nfa1 overexpressed or knock-downed N. fowleri. The nfa1 overexpressed N. fowleri increased the ability of attachment to
fibronectin, collagen I, collagen IV and laminin I. Experiments were performed in triple and the data was shown with mean ± SD (P < 0.05).
- 53 - M. Phagocytic activity in transgenic N. fowleri
To examine the phagocytic activity of nf-actin (or nfa1) overexpressed (or knock-downed) N. fowleri, phagocytosis assay using zymosan particles was performed. As shown in Fig. 19 and 20, nf-actin or nfa1 overexpressed N. fowleri led to increase the phagocytic activity in comparison with control groups (wild type N. fowleri and N. fowleri transfected with empty vector). On the other hand, nf-actin or nfa1 knock-downed N. fowleri was decreasing phagocytic activity in comparison with control groups (Fig. 19, 20).
- 54 -
Fig. 19. Phagocytosis assay in nf-actin overexpressed or knock-downed N. fowleri. To
examine the phagocytic activity of N. fowleri, phagocytosis assay was performed in triple, and the data was shown with mean ± SD (P < 0.05). Lane N; Negative control, Lane 1; wild type N. fowleri, Lane 2; N. fowleri transfected with empty vector, Lane 3; nf-actin overexpressed N. fowleri, Lane 4; nf-actin knock- downed N. fowleri.
- 55 -
Fig. 20. Phagocytosis assay in nfa1 overexpressed or knock-downed N. fowleri. To
examine the phagocytic activity of N. fowleri, phagocytosis assay was performed in triple, and the data was shown with mean ± SD (P < 0.05). Lane N; Negative control, Lane 1; wild type N. fowleri, Lane 2; N. fowleri transfected with empty vector, Lane 3; nfa1 overexpressed N. fowleri, Lane 4; nfa1 knock-downed N. fowleri.
- 56 -
IV. DISCUSSION
N. fowleri causes an acute lethal CNS disease called PAM. N. fowleri trophozoites enter
the nasal cavity, then attach and invade the nasal mucosa and the olfactory nerve (Carter, 1968; Carter, 1972; Ma et al., 1990). PAM is typically leads to death with 1 to 2 weeks from the onset of symptoms (Apley et al. 1970; Shin and Im 2004).
Kim et al. (2008) reported that the contact-independent killing mechanisms of N.
fowleri induced by extracellular secreted proteins were related with amoebic pathgenicity,
secreted protein and could play a major role in immune response. Moreover, pathogenic mechanism of N. fowleri is destruction of host cells through a ‘food-cups’ structure on the amoeba surface (Cline et al., 1986 and Marciano-Cabral et al., 1983). Concerned with host-invasion, the adhesion to target cells is most important step in an contact-dependent mechanism of pathogenicity. N. fowleri trophozoties are able to enter the nervous system through the olfactory nerve and digest neuronal tissue by cytolysis and phagocytosis (Carter 1968; Anderson and Jamieson 1972). In addition, subsequent to adhesion the parasite produces a potent cytopathic effect leading to target host cell death in Acanthamoeba (De Jonckheere 1980). Although the food-cups or amoebastomes in N. fowleri were mentioned and described in previous studies, there are no studies related to the attachment and trogocytosis of trophozoites (Marciano-Cabral and John 1983).
In the previous study, an nfa1 gene was characterized, and this gene was located in pseudopodia and food-cup structure (Kang et al. 2005). So, the food-cup formation of N.
- 57 -
interest. To resolve this question, first, I screened with cytoskeleton proteins by using immunofluorescence assay with commercial usable antibodies. As a result, among the three common cytoskeletal proteins, myosin and tubulin were shown in dispersed cellular location of N. fowelri (data not shown), but actin was shown in cytosolic, pseudopodia, and food-cup structure with concentrated and compacted signals. There are many studies that reported actin cytoskeleton protein involved in diverse functions of cell such as adhesion (Gumbiner 1996), motility (Bretscher 1991), and phagocytosis (Swanson and Baer 1995). Therefore, I cloned and characterized an nf-actin gene to evaluate the role of nf-actin gene in pathogenic
N. fowleri. The nf-actin gene had the coding sequence of 1.2 kbp, producing a 50 kDa
recombinant fusion protein (Nf-actin). The sequence identity was 82 % with nonpathogenic
N. gruberi, but has no sequence identity with other mammals and human actin gene (Table
1). In immunofluorescence assay, the Nf-actin was located on the cytoplasm, pseudopodia, and especially, food-cup structure in N. fowleri trophozoites. When N. fowleri co-cultured with CHO target cells, the Nf-actin was strongly expressed on food-cup structure concerning trogocytosis. Although the presence of food-cups does not correlate with pathogenicity (Marciano-Cabral 1988), N. fowleri treated with cytochalasin D, actin polymerization inhibitor, reduced ability of food-cup formation (Fig. 9, 10) and in vitro cytotoxicity in this study. It suggests that food-cup formation was reduced by actin inhibition, and then, cytotoxicity of N. fowleri was reduced because of decreased attachment ability. These results suggest that Nf-actin play an important role in phagocytic activity and pathgoenicity of N.
- 58 -
In addition, eukaryotic transfection vectors, C2/nf-actin and Ubi-pEGFP-C2/nfa1 vector were constructed, and then, the effect of cell adhesion, cytotoxicity and phagocytosis in N. fowleri were observed. After transfection, GFP fluorescence using a microscope was observed in N. fowleri transfected with pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 vectors. Ubiquitin promoter was used in this study, because ubiquitin is a small regulatory protein in almost all eukaryotic cells (Jentsch S, 1992). It is involved in several basic cellular functions; making protein for rapid degradation, mediation of gene transcription, DNA repair, cell cycle progression, stress response and the modulation of the immune response. The ubiquitin gene was shown to be regulated during Acanthamoeba development from the actively growing stage toward the dormant cyst stage (Ahn and Henny 1994; Wulff et al., 1990). In Ubi-pEGFP-C2/nf-actin and Ubi-pEGFP-C2/nfa1 vectors, a CMV promoter was replaced with an ubiquitin promoter in order to transcribe the nf-actin or
nfa1 gene in transgenic N. fowleri, because the CMV promoter could poorly transcribe the nf-actin or nfa1 gene. Western blotting and RT-PCR was performed to indentify an
expressed Nf-actin or Nfa1 from nf-actin or nfa1 overexpressed N. fowleri. Also N. fowleri transfected with antisense oligonucleotides of nf-actin or nfa1 gene was examined by knock-down system. Antisense nf-actin or nfa1 oligomers were designed to anneal on the nf-actin or nfa1 ATG start codon. In this study, nf-actin or nfa1 knock-downed N. fowleri showed decreasing, number of food-cup and reducing of nf-actin or nfa1 mRNA and protein levels. These results show that the Nf-actin and Nfa1 proteins are the key molecules to contact and kill the target cells.