Thermodynamic Properties Lab Kyungpook National University, Sangju CampusDepartment of Energy Chemical Engineering
Department of Energy Chemical Engineering
Kyungpook National University, Sangju Campus 2559 Gyeongsang-daero Sangju-si, 37224 Korea
Instructor: Prof. Moon Gab Kim E-mail: mg_kim@knu.ac.kr
Phone: 054-530-1332
Chemical Engineering
Thermodynamics
Chapter 1:
Introduction
Thermodynamics is an abstract and intangible,
yet perfect and logical, language, in which no
irregular verbs can be found.
Accordingly, it is critical to learn how to think in
thermodynamics. I wish that, together, we will
explore
the
challenging
subject
–
Thermodynamics – and I hope you will find that
the study of thermodynamics is objectively
interesting,
technically
rewarding,
and
intellectually stimulating.
Thermodynamics is a funny subject. The first time
you go through it, you don’t understand it at all.
The second time you go through it, you think you
understand it, except for one or two points. The
third time you go through it, you know you don’t
understand it, but by that time you are so used to
the subject, it doesn’t bother you anymore.
Arnold Sommerfeld (1868 - 1951)
1.1 THE SCOPE OF THERMODYNAMICS
The science of thermodynamics was developed in the
19th century
as a result of the need
• to describe the basic operating principles of the newly invented steam engine
• and to provide a basis for relating the work produced to the heat supplied.
Thus the name itself denotes power generated from heat.
From the study of steam engines, there emerged two of the
primary generalizations of science:
• the First and Second Laws of Thermodynamics.
All of classical thermodynamics is implicit in these laws.
Their statements are very simple, but their implications are
profound.
The First Law simply says that energy is conserved, meaning
that it is neither created nor destroyed.
It provides no definition of energy that is both general and precise.
No help comes from its common informal use where the word has imprecise meanings.
However, in scientific and engineering contexts, energy is recognized as appearing in various forms, useful because each form has mathematical definition as a function of some recognizable and measurable characteristics of the real world.
Thus kinetic energy is defined as a function of velocity, and gravitational potential energy as a function of elevation.
Conservation implies the transformation of one form of energy
into another.
Windmills have long operated to transform the kinetic energy of the wind into work that is used to raise water from land lying below sea level.
• The overall effect is to convert the kinetic energy of the wind into potential energy of water. Wind energy is now more widely converted to electrical energy.
Similarly, the potential energy of water has long been transformed into work used to grind grain or saw lumber. Hydroelectric plants are now a significant source of electrical power
The Second Law is more difficult to comprehend because it depends on entropy, a word and concept not in everyday use. Its consequences in daily life are significant with respect to environmental conservation and efficient use of energy. Formal treatment is postponed until we have laid a proper foundation.
The two laws of thermodynamics have no proof in a mathematical sense. However, they are universally observed to be obeyed. An enormous volume of experimental evidence demonstrates their validity. Thus, thermodynamics shares with mechanics and electromagnetism a basis in primitive laws.
These laws lead, through mathematical deduction, to a network of equations that find application in all branches of science and engineering.
Included are calculation of heat and work requirements for physical, chemical, and biological processes, and the determination of equilibrium conditions for chemical reactions and for the transfer of chemical species between phases.
Practical application of these equations almost always requires information on the properties of materials.
Thus, the study and application of thermodynamics is inextricably linked with the tabulation, correlation, and prediction of properties of substances.
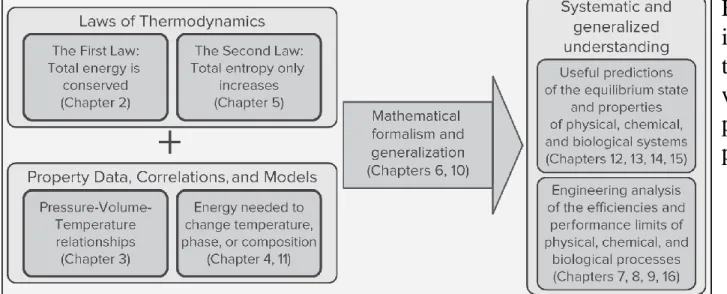
Fig. 1.1 illustrates schematically how the two laws of thermodynamics are combined with information on material properties to yield useful analyses of, and predictions about, physical, chemical, and biological systems.
Examples of questions that can be answered on the basis of the laws of thermodynamics
combined with property information include the following:
How much energy is released when a liter of ethanol is burned (or metabolized)? What maximum flame temperature can be reached when ethanol is burned in air?
What maximum fraction of the heat released in an ethanol flame can be converted to electrical energy or work?
How do the answers to the preceding two questions change if the ethanol is burned with pure oxygen, rather than air?
What is the maximum amount of electrical energy that can be produced when a liter of ethanol is reacted with O2 to produce CO2 and water in a fuel cell?
In the distillation of an ethanol/water mixture, how are the vapor and liquid compositions related?
When water and ethylene react at high pressure and temperature to produce ethanol, what are the compositions of the phases that result?
How much ethylene is contained in a high-pressure gas cylinder for given temperature, pressure, and volume?
When ethanol is added to a two-phase system containing toluene and water, how much ethanol goes into each phase?
If a water/ethanol mixture is partially frozen, what are the compositions of the liquid and solid phases?
What volume of solution results from mixing one liter of ethanol with one liter of water? (It is not exactly 2 liters!)
FIGURE 1.1 Schematic illustrating the combination of the laws of thermodynamics with data on material properties to produce useful predictions and analyses.
The application of thermodynamics to any real problem starts with the specification of a particular region of space or body of matter designated as the
system. Everything outside the system is called the surroundings.
The system and surroundings interact through transfer of material and energy across the system boundaries, but the system is the focus of attention.
Many different thermodynamic systems are of interest.
• A pure vapor such as steam is the working medium of a power plant. • A reacting mixture of fuel and air powers an internal-combustion engine. • A vaporizing liquid provides refrigeration.
• Expanding gases in a nozzle propel a rocket.
Once a system has been selected, we must describe its state.
There are two possible points of view, the macroscopic and the microscopic.
The macroscopic relates to quantities such as composition, density, temperature, and pressure.
• These macroscopic coordinates require no assumptions regarding the structure of matter. They are few in number, are suggested by our sense perceptions, and are measured with relative ease.
• A macroscopic description thus requires specification of a few fundamental measurable properties. • The macroscopic point of view, as adopted in classical thermodynamics, reveals nothing of the
microscopic (molecular) mechanisms of physical, chemical, or biological processes.
The microscopic description depends on the existence and behavior of molecules, is not directly related to our sense perceptions, and treats quantities that cannot routinely be directly measured.
• Nevertheless, it offers insight into material behavior and contributes to evaluation of thermodynamic properties.
• Bridging the length and time scales between the microscopic behavior of molecules and the macroscopic world is the subject of statistical mechanics or statistical thermodynamics, which applies the laws of quantum mechanics and classical mechanics to large ensembles of atoms, molecules, or other elementary objects to predict and interpret macroscopic behavior.
• Although we make occasional reference to the molecular basis for observed material properties, the subject of statistical thermodynamics is not treated in this book.1
1.2 INTERNATIONAL SYSTEM OF UNITS
Descriptions of thermodynamic
states
depend
on
the
fundamental dimensions of science..
A dimension is a property that can be measured, such as length, time, mass, temperature, and amount of substance etc, or calculated by multiplying or dividing other dimensions such as length/time(=velocity), length3(=volume), mass/length3 (= density).
These dimensions are primitives, recognized through our sensory perceptions, and are not definable in terms of anything simpler.
The arbitrary magnitudes(scales) assigned to the dimensions are
called units
Primary units have been set by international agreement, and are codified as the International System of Units (abbreviated SI, for Système International)
A measured or counted quantity has a numerical value (e.g. 2.47) and a unit (whatever there are 2.47 of). It is necessary in engineering calculations to report both the value and the unit.
Dimensions mass length time
Fundamental Units
Time, second, s
the duration of 9,192,631,770 cycles of radiation associated with a specified transition of the cesium-133 atom.
Length, meter, m
the length or distance of the path traveled by light in a vacuum during a time interval of 1/299,792,458 seconds”
Light
1 m
t = 0 s t = 1/299,792,458 s
Mass, kilogram, kg
the mass of a platinum/iridium cylinder kept at the International Bureau of Weights and Measures at Sèvres, France. (The gram, symbol g, is 0.001 kg.)
Temperature, Kelvin, K
1/273.16 of the (kelvin) temperature interval from absolute zero to the triple point of water.
Amount of a substance, mole, mol
the amount of a substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12”
This is equivalent to the “ gram mole” commonly used by chemists.
Electric current, ampere, A
unit of electric current in the International System of Units (SI), used by both scientists and technologists. In 2018 the General Conference on Weights and Measures (CGPM) agreed that on May 20, 2019, the ampere would henceforth be defined such that the elementary charge would be equal to 1.602176634 ×10−19 coulomb. The coulomb is defined as the quantity of electricity transported in one second by a current of one ampere.
Luminous intensity, candela, cd
the luminous intensity of a source that emits monochromatic radiation of frequency 540 × 1012 Hz and that has a radiant intensity of 1/683 watt per steradian.
Chemical engineers use SI units for measurements
All SI units are based on a set of seven measured base
units:
Summary; Base Unit in SI units
Measurement
Unit
Symbol
Time second s
Length meter m
Mass kilogram kg Temperature kelvin K
Amount of substance mole mol
Electrical current ampere A
SI unit of derived units
The derived units always reduce to combinations of primary units. Force, newton, N Force=(mass)(acceleration)=ma=(1 kg)(1m·s-2)=1 kg·m·s-2 (=1 N) Pressure, pascal, Pa Pressure=force/area=N/m2 =1 kg·m-1·s-2(=1 Pa) Energy, joule, J Energy=N·m=1 kg·m2·s-2(=1 J)
Measurement Formula SI Units
Area length x width m2
Volume length x width x height m3
Velocity distance/time m/s Acceleration velocity/time m/s2
Density mass/volume kg/m3
Difference between weight and mass
Mass is a fundamental quantity
Weight is the gravitational force applied to a body
A spring scale provides correct mass readings only when used in the gravitational field of its calibration.
Dimensional Homogeneity
Basic idea – you can’t add apples and oranges
Every term in an expression must have the same units Valuable tool for detecting errors
The U.S. Customary units are related to SI units by fixed conversion factors. To convert the units of a measured value, the value is multiplied by a
conversion factor to produce a result with the desired units.
unit new in number new unit original unit new units original in Number Conversion Factor Quantity to express in new units Quantity now expressed in new units
(
(
)
)
(
)
(
)
(
)
)
(
EXAMPLE 1.1: An astronaut weighs 730 N in Houston, Texas, where the
local acceleration of gravity is g = 9.792 m·s−2. What are the astronaut's mass and weight on the moon, where g = 1.67 m·s−2?
SOLUTION
By Newton's law, with acceleration equal to the acceleration of gravity, g,
2 1 2 2
730
74.55
(
)
74.55
9.792
F
N
m
N
kg m s
m s
kg
g
m s
This mass of the astronaut is independent of location, but weight depends on the local acceleration of gravity. Thus on the moon the astronaut's weight is:
F(moon)=m×g(moon)=74.55 kg×1.67 m⋅s−2
or
EXAMPLE 1.1-1: An object at sea level has a mass of 400 kg.
1) Find the weight of this object on earth.
2) Find the weight of this object on the moon where the local gravitational acceleration is one-sixth that of earth.
SOLUTION 2 2 2 2 1 (400 )(9.807 ) 1 1 (400 )(9.807 ) 1 3922.8 c mg F g m kg kgm s Ns m N kg kgm s s N
1)
or
2 2 9.807 1 (400 )( ) 1 6 653.8 c mg F g m N kg kgm s s N 2)
EXAMPLE 1.1-2: An object has a mass of 180 lbm. Find the weight of this object at a location where the local gravitational acceleration is 30 ft/s2.
SOLUTION 2 2
1
(180
)(30
)
32.2
167.7
c m m f fmg
F
g
ft
lb
lb ft
s
lb s
lb
1.3 MEASURES OF AMOUNT OR SIZE
Three measures of amount or size are in common use:
Mass, m Number of moles, n Total volume, Vt
or
m
n
m
Mn
M
Specific volume :
Molar volume :
Specific or molar density is defined as the reciprocal of specific or molar volume: ρ ≡ V−1.
These quantities (V and ρ) are independent of the size of a system, and are examples of intensive thermodynamic variables.
For a given state of matter (solid, liquid, or gas) they are functions of temperature, pressure, and composition, additional quantities independent of system size.
or
t tV
V
V
mV
M
or
t tV
V
V
nV
n
1.4 TEMPERATURE
The notion of temperature, based on sensory perception of heat and cold, needs no explanation. It is a matter of common experience. However, giving temperature a scientific role requires a scale that affixes numbers to the perception of hot and cold.
Heat energy transfers spontaneously from a hot object to a cold object.
A useful property for identifying pure elements and compounds - the
temperature at which a solid melts (melting point) and at which a liquid
boils (boiling point).
A physical property that determines the direction of heat flow in an object on contact with another object.
Heat is transferred from the body at higher
temperature to the one at lower
temperature until both bodies attain the same temperature (thermal equilibrium)
Celsius Scale, °C
Invented by the Swedish astronomer Anders Celsius (1701-1744).
0°C is defined as the freezing point of pure water and 100°C is defined as the boiling point of water.
Scale commonly used throughout the world and in the scientific community.
Fahrenheit Scale, °F
Invented by the German physicist Gabriel Fahrenheit (1686-1736).
0 °F is defined as the freezing point of a water solution saturated with
salt and 100 °F was intended to be normal human body temperature
(actually 98.6 °F).
Water freezes at 32 °F and boils at 212 °F.
Kelvin Scale, K
Invented by the Scottish natural philosopher William Thomson (1824-1907),
who was known as Lord Kelvin.
Uses the same unit size as Celsius but defines 0 K as absolute zero
(-273.15 °C).
Kelvin temperature is also called the absolute temperature scale. Adopted as the international standard (SI temperature) for science.
International Temperature Scale of 1990 (ITS−90)
Scientific and industrial practice depends on the International
Temperature Scale of 1990 (ITS−90)
Relationship between the Kelvin (SI), Celsius,
and Fahrenheit temperature scales.
( ) ( ) 273.15 ( ) ( ) 459.67 ( ) 1.8 ( ) ( ) 1.8 ( ) 32 T K T C T R T F T R T K T F T C
( ) 1 ( ) 32 1.8 T C T F ℃ (˚F ) (˚F ) ℃ ℃ ℉ ℃ ℉ ℉ - -= 32 + 459.67 = 212 + 459.67 = –459.67 + 459.67 = 100 + 273.15 = 0 + 273.15 2 1 2 1 2 1 2 1 ( ) = ( C + 273.15) - ( C + 273.15) = C - C = ( C) ( ) ( F + 459.67) - ( F + 459.67) F - F ( F) T K T T T T T T R T T T T T The magnitudes of each division of 1 K and 1ºC are identical, and so are the magnitudes of each division of 1 R and 1ºF. That is,
1.5 PRESSURE
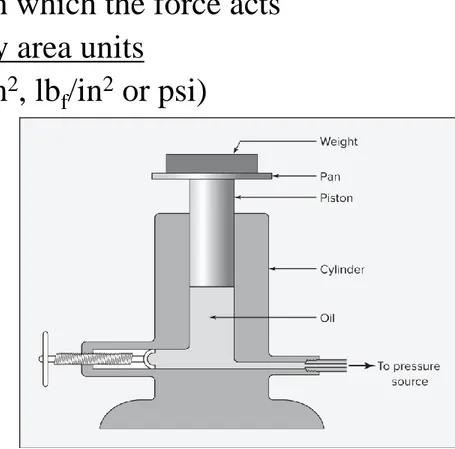
The primary standard for pressure measurement is the dead-weight
gauge shown in Fig. 1.2.
▪ Pressure ratio of a force to the area on which the force acts
▪ Pressure units are force units divided by area units
(e.g., N/m
2or pascal (Pa), dynes/cm
2, lb
f
/in
2or psi)
FIGURE 1.2 Dead-weight gauge.
(gauge pressure)
Force
F
mg
P
Area
A
A
Some values of 1 atm of pressure;
= 101.325 kPa
= 0.101325 MPa
= 14.696 psia
= 760 mmHg
absolute/gauge/vacuum pressures
gauge pressure = the difference between the pressure of interest and
the pressure of the surrounding atmosphere.
absolute pressures = gauge pressure + local barometric pressure
=> used in thermodynamic calculations
The pressure used in all calculations of state is the absolute pressure
measured relative to absolute zero pressure(a perfect vacuum).
However, pressures are often measured relative to atmospheric
pressure, called gauge or vacuum pressures.
relationship of absolute and vacuum pressures relationship of absolute and gauge pressure
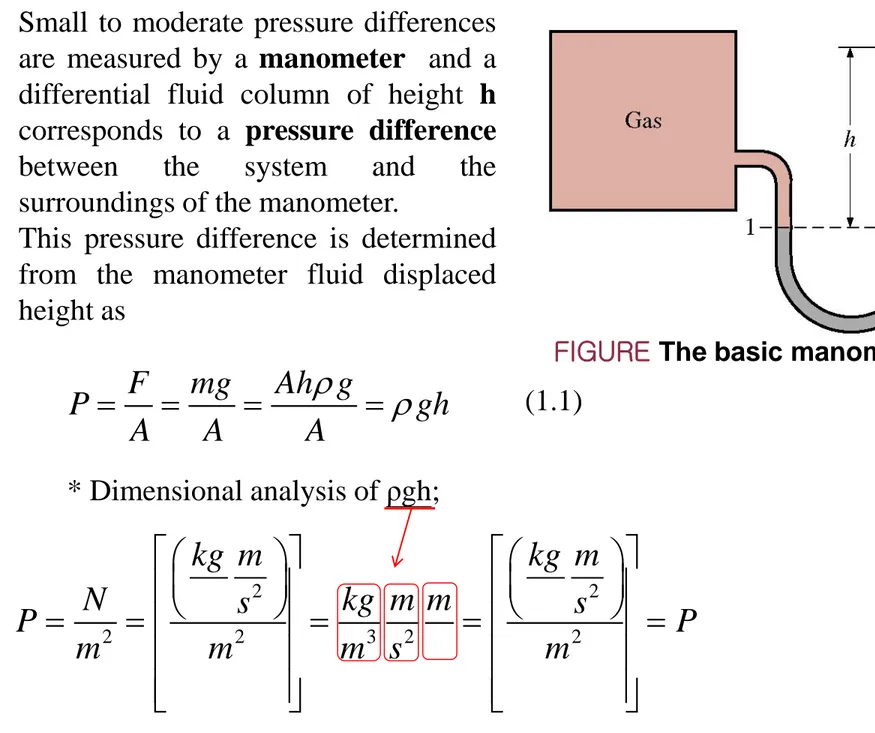
Small to moderate pressure differences are measured by a manometer and a differential fluid column of height h corresponds to a pressure difference
between the system and the
surroundings of the manometer.
This pressure difference is determined from the manometer fluid displaced height as
FIGURE The basic manometer.
F
mg
Ah g
P
gh
A
A
A
* Dimensional analysis of ρgh; 2 2 2 2 3 2 2kg m
kg m
N
s
kg m m
s
P
P
m
m
m s
m
(1.1)Pressure
,
atmospheric pressure
The earth’s atmosphere can be considered a column of fluid
with
zero pressure at the top.
The fluid pressure at the base of this column (e.g., at sea level)
is
the atmospheric pressure, P
atm.
* The homogeneous atmosphere
The density of air, under standard conditions, is about 800 times less
than the density of water — almost 1.225 kg per cubic meter.
So the height of a column of air needed to exert the standard
atmospheric pressure of 1013.25 hPa would be about 8 thousand
meters (8 km), if it were all the same density — i.e., homogeneous.
That height is “the height of the homogeneous atmosphere.”
101325/9.807/1.225 ≒ 8,430m
2 2 3 2101325
(
)
(1.225
)(9.807
)
8, 430
P
Pa kgms m
h
g
kgm
ms
m
P
gh
EXAMPLE 1.2: A dead-weight gauge with a piston diameter of 1 cm is
used for the accurate measurement of pressure. If a mass of 6.14
kg (including piston and pan) brings it into balance, and if g = 9.82
m·s
−2, what is the gauge pressure being measured? For a barometric
pressure of 0.997 bar, what is the absolute pressure?
SOLUTION
The force exerted by gravity on the piston, pan, and “weights” is: 2 5 2 2 5 5 5 2 (6.14 )(9.82 ) 60.295 60.295 7.677 10 ( ) (1 / 4)( )(0.01 ) 767.7
The absolute pressure is therefore:
7.677 10 0.997 10 8.674 10 ( ) 867.4 F mg kg m s N F N Gauge pressure Nm Pa A m kPa P Nm Pa kPa
EXAMPLE 1.3: At 27°
C the reading on a manometer filled with
mercury is 60.5 cm. The local acceleration of gravity is 9.784 m·s
−2. To
what pressure does this height of mercury correspond?
SOLUTION
P=ρgh, ρ of Hg at 27 ℃ is 13.53 gcm
-3.
Then, 3 2 2 2 2 2 2 2 2 2 13.53 9.784 60.5 8,009 8,009 1 1 (100 ) 8.009 80.09 0.8009 1000 1 (1 ) P gcm ms cm g m s cm g m s kg N cm N m kPa bar cm g kg m s m or
1.6 WORK
Work W is performed whenever a force acts through a distance.
2 1
(A is constant)
Integrating,
t t t t V t VdW
Fdl
V
PAd
A
PdV
W
PdV
F mg Ah g P A A A Work is regarded as
positive when the displacement is in the
same direction as the applied force
negative when they are in opposite
directions
(1.2)
(1.3) (1.4)
Equation (1.4) expresses the work done by
a finite compression or expansion process.
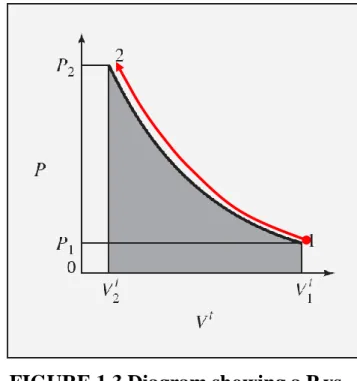
Figure 1.3 shows
a path for compression of a gas from point 1, initial volume Vt
1 at pressure
P1, to point 2, volume Vt
2 at pressure P2.
This path relates the pressure at any point of the process to the volume. The work required is given by Eq. (1.4) and is proportional to the area under the curve of Fig. 1.3. 2 1 t t V t V
W
PdV
(1.4)FIGURE 1.3 Diagram showing a P vs. Vt path.
1.7 ENERGY
The general principle of conservation of energy was established about
1850. The germ of this principle as it applies to mechanics was implicit
in the work of Galileo (1564–1642) and Isaac Newton (1642–1726).
Energy is the capacity to do work or to produce heat
However, the capacity to utilize energy to do work is limited (entropy)
Kinetic Energy (E
K)
:
energy due to the translational motion of the system as a wholeIntegration for a finite change in velocity from u
1to u
2gives:
dW Fdl ma dl du dl m dl m du mu du dt dt , ( velocity= ) du dl a u dt dt
or
2
1
2
K
E
mu
(1.6)
[kg][m/s]
2
kg ms m
2
Nm
J
Potential Energy (E
P)
: energy due to the position of the system in a
potential field (e.g., earth’s gravitational field (g=9.8 m/s
2))
F
ma
mg
2 1 2 1(
)
(
)
W
F z
z
mg z
z
mg z
mgz
E
p
(1.7) (1.8) 2 2[kg][m/s ][m]
kg ms m
Nm
J
Energy Conservation
2 2 2 2 1 2 12
0
mu
mu
or
0
2
2
K P K Pmu
W
E
W
E
mzg
E
E
mz g
mz g
The work done on a body in elevating it produces a change in its potential energy: The work done on an accelerating body produces a change in its kinetic energy:
An elevated body, allowed to fall freely (i.e., without friction or other resistance), gains in kinetic energy what it loses in potential energy. Mathematically,
The validity of this equation has been confirmed by countless experiments. Thus the development of the concept of energy led logically to the principle of its conservation for all purely mechanical processes, that is, processes without friction or heat transfer.
EXAMPLE 1.4 :An elevator with a mass of 2500 kg rests at a level 10 m above the base of an elevator shaft. It is raised to 100 m above the base of the shaft, where the cable holding it breaks. The elevator falls freely to the base of the shaft and strikes a strong spring. The spring is designed to bring the elevator to rest and, by means of a catch arrangement, to hold the elevator at the position of maximum spring compression. Assuming the entire process to be frictionless, and taking g = 9.8 m⋅s−2, calculate:
a. The potential energy of the elevator in its initial position relative to its base.
b. The work done in raising the elevator.
c. The potential energy of the elevator in its highest position.
d. The velocity and kinetic energy of the elevator just before it strikes the spring.
e. The potential energy of the compressed spring.
f. The energy of the system consisting of the elevator and spring (1) at the start of the process, (2) when the elevator reaches its maximum height, (3) just before the elevator strikes the spring, (4) after the elevator has come to rest.
SOLUTION
a. Potential energy is defined by Eq. (1.8):
EP1=mz1g=2500 kg×10 m×9.8 m⋅s−2=245,000 kg⋅m2⋅s−2=245,000 J
b. Work is computed by Eq. (1.7). Units are as in the preceding calculation: W=mg(z2−z1)=(2500)(9.8)(100−10)=2,205,000 J
c. Again by Eq. (1.8),
EP2=mz2g=(2500)(100)(9.8)=2,450,000 J Note that W = EP2 − EP1.
d. The sum of the kinetic- and potential-energy changes during the process
from state 2 to state 3 is zero; that is,
ΔEK2→3+ΔEP2→3=0 or EK3−EK2+EP3−EP2=0
However, EK2 and EP3 are zero; hence EK3 = EP2 = 2,450,000 J.
With 3 32 1 2 K E mu 2 2 2 3 2 2 3 1 3 2 2 2, 450, 000 2 2, 450, 000 1960 2500 2500 44.272 K E J kgm s u m s m kg kg u ms
e. The changes in the potential energy of the spring and the kinetic energy of the elevator must sum to zero:
ΔEP(spring)+ΔEK(elevator)=0
The initial potential energy of the spring and the final kinetic energy of the elevator are zero; therefore, the final potential energy of the spring equals the kinetic energy of the elevator just before it strikes the spring. Thus the final potential energy of the spring is 2,450,000 J.
f. With the elevator and spring as the system, the initial energy is the potential energy of the elevator, or 245,000 J.
• The only energy change of the system occurs when work is done in raising the elevator. This amounts to 2,205,000 J, and the energy of the system when the elevator is at maximum height is 245,000 + 2,205,000 = 2,450,000 J.
• Subsequent changes occur entirely within the system, without interaction with the surroundings, and the total energy of the system remains constant at 2,450,000 J. It merely changes from potential energy of position (elevation) of the elevator to kinetic energy of the elevator to potential energy of configuration of the spring. • This example illustrates the conservation of mechanical energy. However, the
entire process is assumed to occur without friction, and the results obtained are exact only for such an idealized process.
EXAMPLE 1.5: A team from Engineers Without Borders constructs a
system to supply water to a mountainside village located 1800 m above
sea level from a spring in the valley below at 1500 m above sea level.
(a) When the pipe from the spring to the village is full of water, but no
water is flowing, what is the pressure difference between the end of the
pipe at the spring and the end of the pipe in the village?
(b) What is the change in gravitational potential energy of a liter of water
when it is pumped from the spring to the village?
(c) What is the minimum amount of work required to pump a liter of
water from the spring to the village?
SOLUTION
(a) Take the density of water as 1000 kg⋅m−3 and the acceleration of gravity as 9.8 m⋅s−2. By Eq. (1.1):
P=hρg=(300 m) ×(1000 kg⋅m−3) ×(9.8 m⋅s−2)=2 9.4×105 kg⋅m−1⋅s−2
Whence P=29.4 bar or 2940 kPa
(b) The mass of a liter of water is approximately 1 kg, and its potential-energy change is:
ΔEP=Δ(mzg)=mgΔz=(1 kg) ×(9.8 m⋅s−2) ×(300 m) = 2940 N⋅m=2940 J
(c) The minimum amount of work required to lift each liter of water through an elevation change of 300 m equals the potential-energy change of the water. It is a minimum value because it takes no account of fluid friction that results from finite-velocity pipe flow.
1.8 HEAT
At the time when the principle of conservation of mechanical energy emerged, heat was considered an indestructible fluid called caloric.
Heat, like work, is recognized as energy in transit (Fig. 1). A simple example is the braking of an automobile (Fig. 2).
We know from experience that a hot object brought in contact with a cold object becomes cooler, whereas the cold object becomes warmer.
A reasonable view is that something is transferred from the hot object to the cold one, and we call that something heat Q
A kitchen refrigerator running on electrical energy must transfer this energy to the surroundings as heat.
More precisely, the rate of heat transfer from one body to another is proportional to the temperature difference between the two bodies; when there is no temperature difference, there is no net transfer of heat (Fig. 3).
Fig. 3 Fig. 1 Fig. 2
Units of Heat
Heat is energy in transit, and is measured in energy units. The SI unit is the joule (J), or Newton-metre (Nm).
Historically, heat was measured in terms of the ability to raise the temperature of water.
The calorie/kilocalorie (kcal) or Calorie(Cal), or “little calorie/big calorie”: amount of heat needed to raise the temperature of 1 g/kg of water by 1 ºC (from 14.5 ºC to 15.5 ºC), respectively.
In industry, the British thermal unit (Btu) is still used: amount of heat needed to raise the temperature of 1 lbm of water by 1 ºF (from 63ºF to 64ºF)
= 252.2 cal
1cal = 4.1840 J
1.9 SYNOPSIS
After studying this chapter, including the end-of-chapter problems, one
should be able to:
Describe qualitatively the scope and structure of thermodynamics
Solve problems involving the pressure exerted by a column of fluid
Solve problems involving conservation of mechanical energy
Use SI units and convert from U.S. Customary to SI units
Apply the concept of work as the transfer of energy accompanying the
action of a force through a distance, and by extension to the action of
pressure (force per area) acting through a volume (distance times
area)
Chapter 2:
The First Law and Other Basic Concepts
Introduction
In this chapter, we introduce and apply the first law of thermodynamics, one of the two fundamental laws upon which all of thermodynamics rests. Thus, in this chapter we:
Introduce the concept of internal energy; i.e., energy stored within a substance
Present the first law of thermodynamics, which reflects the observation that energy is neither created nor destroyed
Develop the concepts of thermodynamic equilibrium, state functions, and the
thermodynamic state of a system
Develop the concept of reversible processes connecting equilibrium states
Introduce enthalpy, another measure of energy stored within a substance, particularly useful in analyzing open systems
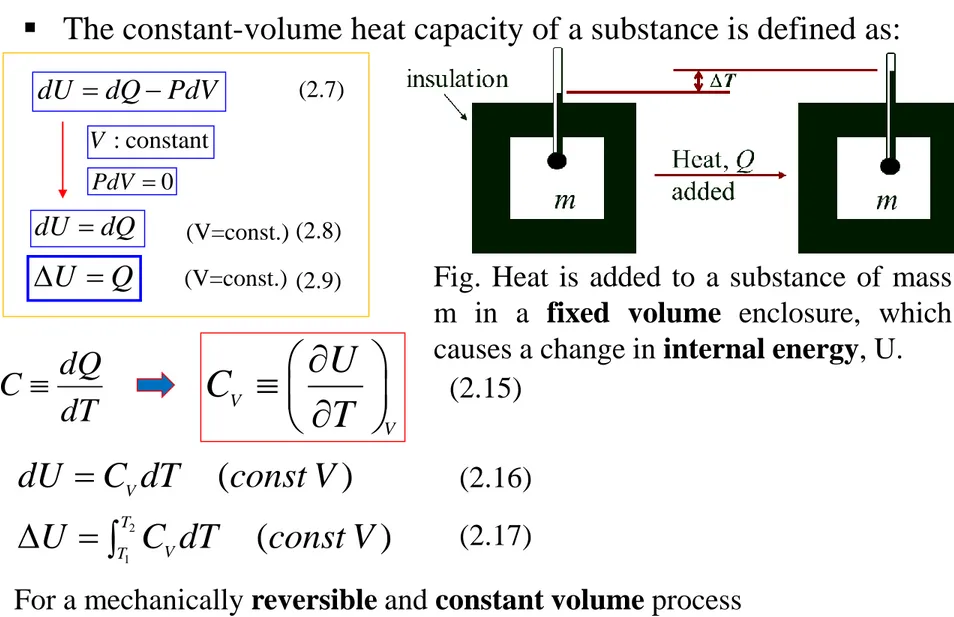
Use heat capacities to relate changes in the internal energy and enthalpy of a substance to changes in its temperature
2.1 JOULE’S EXPERIMENTS
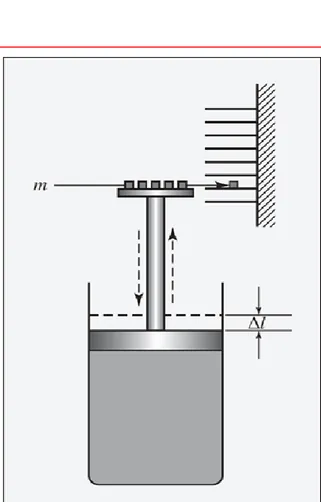
Modern concept of heat developed by James P. Joule (1818-1889) in the 1840s.
Illuminating the relationship between mechanical energy and heat (in the water tank)
• mechanical energy (falling weights and rotating shaft and paddles)
how the former is converted to the latter.
F M
x H2O
Joule’s Experiments
James P. Joule carried out his famous experiment , he placed known
amounts of water, oil, and mercury in an insulated container and
agitated the fluid with a rotating stirrer.
Simple experiments, but done carefully to ensure accuracy
Known amounts of water, oil, and mercury placed in an insulated container; fluid agitated with rotating stirrer
Work on the fluid by the stirrer and corresponding temperature changes in the fluid were carefully measured
Joule showed that for each fluid
a fixed amount of work per unit mass was required for each degree of temperature rise caused by the stirring, and that
the original temperature of the fluid was restored by the transfer of heat through simple contact with a cooler object.
Demonstrated the existence of a quantitative
relationship between work and heat, showed that heat is a form of energy.
2.2 INTERNAL ENERGY
Through Joule experiment what happen to energy between time it is added to water as work, and time it is extracted to heat?
e.g. energy added to a fluid as work is later transferred from the fluid as heat.
A rational answer to this question is that it is contained within the fluid in another form, which we call internal energy.
The internal energy of a substance
Does not include energy due to a substance’s macroscopic position/velocity Does include potential energy associated with intermolecular forces
Because of their ceaseless motion, all molecules possess kinetic energy of translation (motion through space); except for monatomic substances, they also possess kinetic energy of rotation and of internal vibration.
The addition of heat to a substance increases molecular motion, and thus causes an increase in the internal energy of the substance.
Work done on the substance can have the same effect, as was shown by Joule.
The internal energy of a substance also includes the potential energy associated with intermolecular forces.
Molecules attract or repel one another, and potential energy is stored through these interactions, just as potential energy of configuration is stored in a compressed or stretched spring. On a submolecular scale, energy is associated with the interactions of electrons and nuclei of atoms, which includes the energy of chemical bonds that hold atoms together as molecules.
This energy is named internal to distinguish it from the kinetic and potential energy associated with a substance because of its macroscopic position, configuration, or motion, which can be thought of as external forms of energy.
Internal energy has no concise thermodynamic definition. It is a thermodynamic primitive.
It cannot be directly measured; there are no internal-energy meters. As a result, absolute values are unknown.
However, this is not a disadvantage in thermodynamic analysis because only changes in internal energy are required. In the context of classical thermodynamics, the details of how internal energy is stored are immaterial. This is the province of statistical thermodynamics, which treats the relationship of macroscopic properties such as internal energy to molecular motions and interactions.
Internal Energy
Internal Energy
If we want to determine the total energy of a substance, then we must consider: its position (Ep) ;
its overall motion (Ek) ;
the internal motion of its protons, neutrons and electron (ek) ; the potential energy of these subatomic particles (ep).
E
T= E
k+ E
p+ e
k+ e
p= E
k+ E
p+ U
where U (Internal Energy) = ek + ep
Ek & Ep are interconvertable; however,
ET remains constant = Law of Conservation of Energy
Internal Energy (U) = sum of kinetic(ek) & potential (ep) energies of the
The total energy of a process system has three components 1. Kinetic Energy (Ek)
2. Potential Energy (Ep) 3. Internal Energy (U)
Internal Energy (U) – all energy possessed by system other than kinetic and potential energy, including the energy due to the:
rotational and vibrational motion of molecules within the system interactions between molecules within the system
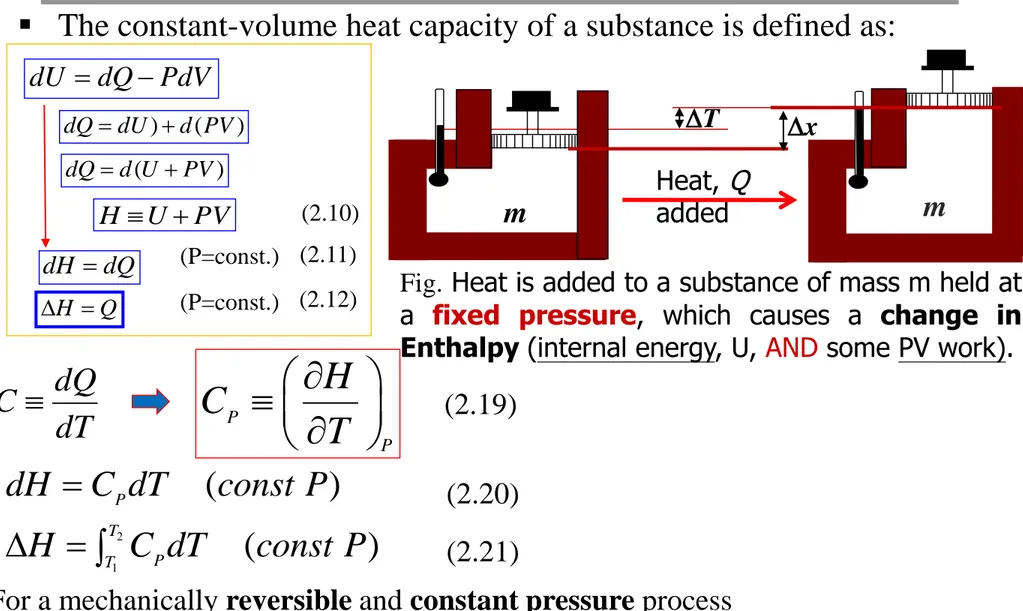
motion and interactions of electrons and nuclei within molecules Internal energy (U) is related to enthalpy (H)
U and H are a function of temperature, chemical composition, physical state (solid, liquid or gas) and only weakly a function of pressure
U and H are relative quantities
absolute values are unknown
values must be defined with respect to their reference state
PV U
H
2.3 THE FIRST LAW OF THERMODYNAMICS
Recognition of heat and internal energy as forms of energy makes
possible the generalization of the principle of conservation of
mechanical energy (Sec. 1.7) to include heat and internal energy in
addition to work and external potential and kinetic energy.
Overwhelming evidence of the validity of this generalization has
raised its stature to that of a law of nature, known as the first law of
thermodynamics. One formal statement is:
In application of this law to a given process, the sphere of influence of
the process is divided into two parts, the system and its surroundings.
Although energy assumes many forms, the total quantity of energy
is constant, and when energy disappears in one form it appears
simultaneously in other forms.
System:
• It is that part of the universe on which theoretical or experimental
investigations are carried out.
• A system is said to be a part of the universe to which we direct our attention for theoretical or experimental investigations and which is separated from the rest of the universe by a real or imaginary well-defined boundary.
Surroundings:
• The rest of the universe in the neighbourhood of the system is known as its surroundings.
• It lies outside the system and is also called the environment of the system. • The system can exchange matter or energy or both with its surroundings.
Boundary:
Anything that separates the system from the surroundings is known as a boundary.
However, the first law applies to the system and its surroundings; not
to the system alone. For any process, the first law requires:
the difference operator “Δ” signifies finite changes
In the context of thermodynamics, heat and work represent energy in
transit across the boundary, and are never stored or contained in the
system.
Potential, kinetic, and internal energy
reside with matter and are stored with matter.
Heat and work represent energy flows to or from a system,
while potential, kinetic, and internal energy represent quantities of
energy associated with a system.
0
)
(
)
(
2.4 ENERGY BALANCE FOR CLOSED SYSTEMS
All energy exchange between a closed system and its surroundings
is in the form of heat or work, and
the total energy change of the surroundings
equals the net energy transferred to or from it as heat and work.
• Open systems can exchange both matter
and energy with the environment.
• Closed systems exchange energy but not matter with the environment. Its mass is
necessarily constant.
• Isolated systems can exchange neither energy nor matter with the environment.
(Energy of surrounding) = ±Q ±W 0 ) ( ) (
• Heat Q and work W always refer to the system.
• Makes the numerical values of both quantities (heat and work) positive for transfer into the system from the surroundings.
(+) : work done to the system; energy is transferred into the system; (–) : work done by the system, energy is transferred out of the system.
• The corresponding quantities taken with reference to the surroundings, Qsurr and Wsurr, have the opposite sign, i.e., Qsurr = − Q and Wsurr = − W.
Sign convention
∴ (Energy of surrounding) = Qsurr+Wsurr=- Q - W
∴ (Energy of the system) = Q + W (2.2)
This equation states that the total energy change of a closed system equals the net energy transferred into it as heat and work
• Closed systems often undergo processes during which only the internal energy of the system changes.
Ut = Q + W
dUt = dQ + dW
(2.3)
(2.4)
Physical Properties (Intensive and Extensive Properties)
Extensive Properties: dependent on the quantity of material
Ex. total volume(Vt), total internal energy (Ut)
Intensive Properties: not dependent on the quantity of material present
Ex. temperature, pressure, density, ...., etc.)
Although Vt and Ut for a homogeneous system of arbitrary size are extensive
properties, specific and molar volume V and specific and molar internal energy U are intensive.
Note that the intensive coordinates T and P have no extensive counterparts.
• Dividing an extensive property by its quantity (mass or moles), yields an intensive property:
Specific Volume, V = Vt/m; Molar Volume, V = Vt/n
Specific Internal Energy, U = Ut/m; Molar Internal Energy, U = Ut/n
Axiom 2: (The First Law of Thermodynamics) The total energy of any system and its surroundings is conserved.
(
nU
)
n U
Q W
(
)
d nU
ndU
dQ
dW
For a closed system of n moles
(2.5) (2.6) If for n=1, Eqs. (2.5) and (2.6) become:
( )
U
U
Q W
dU
dU
dQ
dW
Axiom 1: There exists a form of energy, known as internal energy U, which is an intrinsic property of a system, functionally related to the measurable coordinates that characterize the system. For a closed system, not in motion, changes in this property are given by Eqs. (2.5) and (2.6).
• Equation (2.6) is the ultimate source of all property relations that connect the internal energy to measurable quantities.
• It does not represent a definition of internal energy; there is none. Nor does it lead to absolute values for the internal energy.
EXAMPLE 2.1:The Niagara river, separating the United States from Canada,
flows from Lake Erie to Lake Ontario. These lakes differ in elevation by about 100 m. Most of this drop occurs over Niagara Falls and in the rapids just above and below the falls, creating a natural opportunity for hydroelectric power generation. The Robert Moses hydroelectric power plant draws water from the river well above the falls and discharges it well below them. It has a peak capacity of 2,300,000 kW at a maximum water flow of 3,100,000 kg·s−1. In the following, take 1 kg of water as the system.
a. What is the potential energy of the water flowing out of Lake Erie, relative to the surface of Lake Ontario?
b. At peak capacity, what fraction of this potential energy is converted to
electrical energy in the Robert Moses power plant?
c. If the temperature of the water is unchanged in the overall process, how
much heat flows to or from it?
Solution
a. Gravitational potential energy is related to height by Eq. (1.8). With g equal to its standard value, this equation yields:
b. Recalling that 1 kW = 1000 J⋅s−1, we find the electrical energy generated per kg water is:
The fraction of the potential energy converted to electrical energy is 742/981 = 0.76. This conversion efficiency would be higher but for the dissipation of potential energy in the flow upstream and downstream of the power plant.
c. If the water leaves the process at the same temperature at which it enters, then its internal energy is unchanged. Neglecting also any change in kinetic energy, we write the first law, in the form of Eq. (2.2), as
Δ(Energy of the system) = ΔEp = Q + W
For each kilogram of water, W = −742 J and ΔEP = −981 J. Then
Q = ΔEP− W = −981 + 742 = −239 J This is heat lost from the system.
EXAMPLE 2.2: A typical industrial-scale wind turbine has a peak efficiency of
about 0.44 for a wind speed of 9 m·s−1. That is, it converts about 44% of the kinetic energy of the wind approaching it into usable electrical energy. The total air flow impinging on such a turbine with a rotor diameter of 43 m is about 15,000 kg·s−1 for the given wind speed.
a. How much electrical energy is produced when 1 kg of air passes through the turbine?
b. What is the power output of the turbine?
c. If there is no heat transferred to the air, and if its temperature remains unchanged, what is its change in speed upon passing through the turbine?
Solution
a. The kinetic energy of the wind on the basis of 1 kg of air is:
Thus, the electrical energy produced per kilogram of air is 0.44 ×40.5 = 17.8 J. b. The power output is:
c. If the temperature and pressure of the air are unchanged, then its internal energy is unchanged. Changes in gravitational potential energy can also be neglected. Thus, with no heat transfer, the first law becomes
2.5 EQUILIBRIUM & THE THERMODYNAMIC
STATE
Equilibrium is a word denoting a static condition, the absence of change. In thermodynamics, it means not only the absence of change but the absence
of any tendency toward change on a macroscopic scale.
Because any tendency toward change is caused by a driving force of one kind or another, the absence of such a tendency indicates also the absence of any driving force. Hence, for a system at equilibrium all forces are in exact balance.
A change of the system depends on resistance as well as on driving force.
like action and reaction
Different kinds of driving forces bring about different kinds of change. Examples:
Mechanical: pressures on both sides of a piston are equal, so there is no motion of the piston (forces in balance, no pressure driving force)
Thermal: temperatures of two objects in contact are equal, so there is no heat flow (no temperature driving force for heat flow)
Phase: chemical potentials of species in multiple phases are equal, so no net flow of material between phases (no chemical driving force)
The systems most commonly found in chemical technology are fluids,
for which the primary characteristics (properties) are temperature T,
pressure P, specific or molar volume V, and composition.
Such systems are known as PVT systems.
They exist at internal equilibrium when their properties are uniform
throughout the system, and conform to the following axiom:
This axiom prescribes an idealization, a model that excludes the
influence of fields (e.g., electric, magnetic, and gravitational) as well
as surface effects and other less common effects. It is entirely
satisfactory in a multitude of practical applications.
Axiom 3: The macroscopic properties of a homogeneous PVT system at internal equilibrium can be expressed as a function of its temperature, pressure, and composition.
Thermodynamic State and State Functions
Definitions:
• Thermodynamic Properties: physical attributes that are
perceived by the senses or are made perceptible by certain
experimental methods of investigation e.g. temperature,
pressure, molar/specific volume (or density)
• Thermodynamic State: macroscopic condition of the system,
as defined by T, P, and V (or ρ) at fixed composition
• State Function: property that depends only on the initial and
final states, not the path taken between the states (such as U)
• Path Function: property that does depend on the path taken
Ex. Thermodynamic State
For example, nitrogen gas at a temperature
of 300 K and a pressure of 105 Pa (1 bar) has a fixed specific volume or density and a fixed molar internal energy.
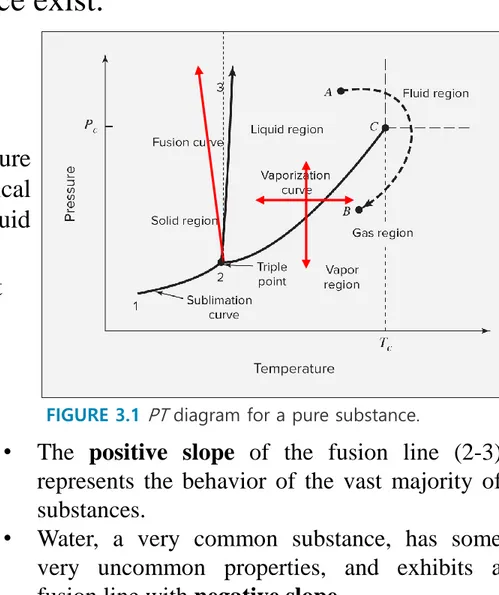
Gibbs’ phase rule
Degree of freedom=C-P+2
Ex. State Function
For a homogeneous pure substance, if two state functions are held at fixed values the thermodynamic state of the substance is fully determined.
If the gas is heated or cooled, compressed or expanded, and then returned to its initial temperature and pressure, its intensive properties are restored to
their initial values.
Such properties do not depend on the past history of the substance nor on the means by which it reaches a given state.
Such quantities are known as state functions.
Where from? Cooling or warming
A state function may therefore be expressed mathematically as a function of
other thermodynamic properties.
Its values may be identified with points on a graph.
2 2 1 2 1
and
1 2 1 P V PdP
P
P
P
VdV
V
V
V
and
dQ
Q
dW
W
A path function depends on the nature of the process causing the change, associated with areas rather than points on a graph
Differential state functions represent differential changes
Representation on a P-V Diagram
• Independent Thermodynamic
Properties =
P
,
V
t• Thermodynamic State = any
specific (
P
,
V
) pair/point
• State Function = any quantity
dependent only on states 1 and 2
• Thermodynamic Path = any curve
connecting state 1 to state 2
• Path Function = depends on the
process; may be associated with
area under the path rather than
individual points on the path
W and Q are not State Functions
- the work is negative for the “clockwise” cycle; if the cyclic process were carried out in the reverse order (counterclockwise), the net work done on the gas would be positive.
2 1
2 1
0
2 1 1 1 2 2
V
V
P
P
V
V
P
V
V
P
W
W
W
net AB CD
2 1)
,
(
2 1 V VP
T
V
dV
W
- we can bring the system from state 1 tostate 2 along infinite # of paths, and for eachpath P(T,V) will be different.
U is a state function, W - is not
thus, Q is not a state function either.
U = Q + W
Since the work done on a system depends not only on the initial and final states, but also on the
intermediate states, it is not a state function.
P V P2 P1 V1 V2 A B C D PV diagram P V T 1 2
2 1 2 1 2 1 2 1 2 1 1 2 1 2 1 20
net BA DCW
W
W
P V
V
P V
V
P V
V
P V
V
P
P
V
V
EXAMPLE 2.3: A gas is confined in a cylinder by a piston. The initial pressure
of the gas is 7 bar, and the volume is 0.10 m3. The piston is held in place by latches.
(a) The whole apparatus is placed in a total vacuum. What is the energy
change of the apparatus if the restraining latches are removed so that the gas suddenly expands to double its initial volume, the piston striking other latches at the end of the process?
(b) The process described in (a) is repeated, but in air at 101.3 kPa, rather than in a vacuum. What is the energy change of the apparatus? Assume the rate of heat exchange between the apparatus and the surrounding air is slow compared with the
rate at which the process
occurs.
Solution
EXAMPLE 2.4: When a system is taken from state a to state b in the
accompanying figure along path acb, 100 J of heat flows into the system and the system does 40 J of work.
(a) How much heat flows into the system along path aeb if the work done by
the system is 20 J?
(b) The system returns from b to a along path bda. If the work done on the
system is 30 J, does the system absorb or liberate heat? How much?
20 60 40 100 aeb aeb aeb t ab Q W Q J W Q U J Qaeb 80
30
60
bda bda bda t ab t baQ
W
Q
J
U
U
J Qbda 90 Qacb= 100 J Wacb= -40 J Qaeb=? J Waeb=-20 J=> Same initial and final points, ΔUacb= ΔUaeb
Qbda=? J Wbda=30 J Solution (b) (a) (a) (b)
2.6 THE REVERSIBLE PROCESS
Fig. 2.1: Expansion of a gas • Sudden removal of a finite mass
• Sudden removal of smaller mass
• dissipative process
=>Irreversible process
• Removal of an infinitesimal mass • Limiting case of removal of a
succession infinitesimal masses (powder)
=> Reversible process
A process is reversible when its direction can be reversed
at any point by an infinitesimal change in external
conditions.
Reversible Chemical Reaction
At equilibrium, this system exerts a
definite decomposition pressure of
CO
2for a given temperature.
When the pressure falls below this
value, then CaCO
3decomposes (→)
When the weight is added, CaCO
3produces(←)
When temp. of bath is raised, then
CaCO
3decomposes(→), the piston
is raised.(↑)
CaCO
3(S) ↔ CaO(s) + CO
2(g) ,
ΔH=+178kJ
Some chemical reactions can be carried out in an electrolytic cell, and
in this case they may be held in balance by an applied potential
difference.
The direction of reaction depends on potential
difference by electromotive force produced in
the cell
a slight decrease in opposing potential diff.
: forward direction (
)
A slight increase in the potential diff.
:
backward direction ( )Summary Remarks on Reversible Processes
A reversible process:
is frictionless
is never more than differentially removed from equilibrium traverses a succession of equilibrium states
is driven by forces whose imbalance is differential (infinitesimal) in magnitude can be reversed at any point by a differential change in external conditions
when reversed, retraces its forward path, and restores the initial state of the system and surroundings
A reversible process is ideal in that it produces the best possible
result. To approximate a real process, values from the reversible
process are combined with an appropriate efficiency
A reversible process is one that is carried out along a path in which all
intermediate states are equilibrium states
An equilibrium state is one in which an infinitesimal changes in opposite directions result in opposite changes in state
Thermal equilibrium between two systems at the same temperature Reversible changes are typically slow
Computing Work for Reversible Processes
Equation (1.3) gives the work of compression or expansion of a gas
caused by the displacement of a piston in a cylinder:
no more than infinitesimally displaced from a state of internal equilibrium, characterized by uniformity of temperature and pressure.
no more than infinitesimally displaced from mechanical equilibrium with its surroundings. (1.2) (1.3) (1.4) t t dW Fdl V PAd PdV A t
dW
PdV
2 1 t t V t VW
PdV
Integrating,
• This equation gives the work for the mechanically reversible expansion or compression of a fluid in a piston/cylinder arrangement.
Its evaluation clearly depends on the relation between P and Vt, i.e., on the
“path” of the process, which must be specified.
To find the work of an irreversible process for the same change in Vt, one