저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The regulation of stemness using the ligands of
human ES cell-specific factor
by
Jiin Kim
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
The regulation of stemness using the ligands of
human ES cell-specific factor
by
Jiin Kim
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for
the Degree of Master in Neuroscience
Supervised by
Myung Ae Lee, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
i
-ABSTRACT-
The regulation of stemness using the ligands of human ES cell-specific factor
Human embryonic stem cells (hESC) have been regarded as promising resources for therapeutic uses with self-renewal property. Recent studies have discovered various transcription factors underlying stemness of hESCs. However, no membrane receptors related to stemness are known so far. We identified a novel GPCR protein HECAT5 as a candidate molecule regulating stemness using digital differential display (DDD). When we overexpressed HECAT5 in hNSC and HEK293 cells, it dramatically increased cell proliferation as well as S phase population. While investigating signaling pathway underlying the effect of HECAT5 in cell proliferation, we found that treatment of Gq protein inhibitor, YM254890, did not affect cell proliferation induced by HECAT5. Furthermore, the level of ERK and AKT which are downstream molecules of β-arrestin pathway did not change in HECAT5-overexpressed cells.
Next, we aimed to identify ligands which may modulate HECAT5 activity. We found nonanoic acid which showed approximately 70% binding affinity to HECAT5 via ODORactor website. We observed that nonanoic acid increased cell proliferation, while there was no difference in ERK and AKT level, which means it is not HECAT5-specific ligand. Generally, GPCR goes through conformational change upon their activation and Toggle switch and Ionic lock are involved in this mechanism. Based on the fact that HECAT5 has both Toggle switch and ionic lock with highly conserved and candidate odors analyzed from reference dataset, we established the homology model of HECAT5. This results in approximately 140 candidate chemicals after screening various commercial chemical libraries. In this respect, we anticipate that HECAT5 activity modulators may play a role in increasing the efficiency of iPSC generation or in blocking certain type of cancer cells.
ii
TABLE OF CONTENTS
ABSTRACT ⅰ TABLE OF CONTENTS ⅱ LIST OF FIGURES .. ⅳ ABBREVIATION ⅴ Ⅰ. INTRODUCTION 1 Ⅱ. MATERIAL AND METHOD… 3 A. Digital differential display 3 B. RNA preparation/ Reverse transcription PCR 3 C. Molecular modeling 3 D. Cell culture 4 E. Cell proliferation assay 4 F. Western blot analysis after nonanoic acid treatment 4 G. Chemical preparation 5 Ⅲ. RESULTS 6 A. Identification of hESC-specific stemness factor, HECAT5 6 B. Characteristics of HECAT5 8 C. GPCR’s general signaling pathway 9 D. Gαq inhibitor did not affect the cell proliferation induced by HECAT5 10iii
E. HECAT5-mediated cell proliferation might not be caused by β-arrestin-dependent way 11 F. Conformational changes of GPCR family during activation 12 G. Toggle switch and Ionic lock are important motifs to trigger GPCR signaling 13 H. Structural dynamics in olfactory receptor 14 I. Discovery of HECAT5 ligand via ODORactor program 16 J. Treatment of nonanoic acid increased cell proliferation 17 K. Identification of candidate odors to modulate HECAT5 activity 18 Ⅳ. DISCUSSION 20 Ⅴ. CONCLUSION 22 REFERENCES 23 국문요약 26
iv
LIST OF FIGURES
Figure 1. Identification of hES cell-specific gene. 7 Figure 2. Characteristics of HECAT5. 8 Figure 3. Conventional GPCR downstream signaling pathway. 9 Figure 4. The treatment of Gq inhibitor to see whether cell proliferation is blocked. 10 Figure 5. ERK level without difference. 11 Figure 6. Two important motifs in GPCR activation. 13 Figure 7. Crucial motifs in OR activation and their conservation in HECAT5. 15 Figure 8. A list of possible ligands of HECAT5. 16 Figure 9. HECAT5 ligand nonanoic acid and its effect in cell proliferation 17 Figure 10. Construction of homology model of HECAT5. 19
v
ABBREVIATION
DDD : digital differential display hESC : human embryonic stem cell iPSC : induced pluripotent stem cell GPCR : G protein-coupled receptor TM : Transmembrane
1
Ⅰ. INTRODUCTION
Embryonic stem cells (ESCs) have the unlimited replicative potential and pluripotency, capable of differentiating into any somatic cell type (J. C. Mountford, 2008). Especially, the unrestricted proliferative trait has received tremendous attention due to its promising uses for therapeutic approaches (Charles E. Murry et al, 2008). Such immortality is observed by not only ESCs but also cancer cells. Cancer cells grow out of control starting from cancer stem cells (Christiana Hadjimichael et al, 2015). On the other hand, ESCs hold pluripotency and self-renewal properties under stringent regulation and lose these during differentiation after obtaining intrinsic and extrinsic cues. There must be various factors that grant both ESCs and cancer cells unlimited growth but make obvious difference between two. Oct4, Sox2, Nanog have been known for essential factors to maintain the pluripotency in ES cells (Laurie A. Boyer et al, 2005). In the last decade the introduction into somatic cells of transcription factors (Oct4, Sox2, Klf4, c-Myc) (Kazutoshi Takahashi and Shinya Yamanaka, 2006), microRNAs, and small molecules leads to the generation of induced pluripotent stem cells (iPSCs). Reprogramming of somatic cells into iPSCs by oncogenes like Myc and Klf4 means embryonic stem cells and cancer cells share common factors or mechanism for maintenance of unlimited proliferation.
From this aspect, we searched for sharing factors between ESCs and cancer cells in terms of cell proliferation and identified a set of genes which are co-expressed in both human ESCs and cancer cells, but not in normal tissues by using Digital Differential Display (DDD). We named this hESC-specific gene as HECAT5, which belongs to olfactory receptor family, the largest part of GPCR. G-protein-coupled-receptors (GPCRs) are 7-transmembrane protein responsible for transmission of signals from a variety of ligands, mediating multiple responses (Ji Young Park et al, 2016). During the embryonic development, hESCs keep communicating with external environment. This means the role of membrane protein as a
2
recipient of outside signals may be as important as that of transcriptional factor for maintenance of pluripotency in them.
In order to explore the function of HECAT5, we overexpressed HECAT5 in several cell lines. The overexpression of HECAT5 dramatically increased cell proliferation in F3, HEK293 cells as well as S phase of cell cycle. In addition, HECAT5-overexpressed F3, HEK293 cells showed an increase in number and size of neurosphere formation as well as of colony formation in soft agar assay. Still, NIH3T3 cell with HECAT5 induction did not exhibit any changes in cell proliferation. However, in xenograft tumor growth assay, only HEK293 cells overexpressed with HECAT5 generated tumor mass. Based on our data we hypothesized that HECAT5 may exhibit different action in the cell type-specific manner.
The mechanism of OR has been actively studied, however, there are multiple variables that affect the signaling pathways. This is because triggering the initial signal depends on different situation such as the binding affinity of ligand (Olivia Baud et al, 2013), the structural diversity of receptors binding pocket, and combinatorial ligand binding (Olivia Baud et al, 2015). Among these, the conformational change of GPCR has had attention for elucidating the mechanism of GPCR activation upon ligand binding and a myriad of signal transmission. Therefore, we aimed to identify how HECAT5 works in the cell and sends downstream signals to regulate stemness in cell type-specific manner based on general GPCR’s signaling pathways. Furthermore, based on molecular modeling and conformational change of GPCR, we planned to discover a set of ligand which may bind to HECAT5. This study may give a clue for deep understanding in the role and working mechanism of GPCR related with stemness, especially cell proliferation.
3
Ⅱ. MATERIALS AND METHODS
A. Digital differential display
DDD compares the Expressed Sequence Tags (ESTs) constituents of various tissue types, according to chosen libraries and then determines the relative representation of each sequence in the libraries being compared. The DDD output is in the web file that has link to Unigene clusters corresponding to the EST’s which are differentially expressed between two tissues.
B. RNA preparation and reverse transcription-polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer’s protocol. cDNA was synthesized using 2μg total RNA in the presence of Superscript Ⅱ (Invitrogen) and oligo(dT) (Invitrogen). The reaction mixture was incubated at 42°C for 50mins followed by incubation at 72°C for 15mins. 150ng of cDNA was then used as a template in 50ul PCR product containing 10X PCR buffer, 1.5mM MgCl2, 0.2mM deoxyribonucleoside-triphosphate (dNTP), 10pmol primer and 1U of Taq DNA polymerase (Invitrogen). The PCR condition of HECAT5 was as follow : 30 cycles as 94°C for 45 seconds, 56.7°C for 45 seconds, 72°C for 45 seconds and a final extension for 10minutes at 72°C. The primer seqences were as follow: 5’-ATG AAT CAT ATG TCT GCA TCT CTC-3’ and 5’-TAG ATC ACT CCC CAT TCC AAG C-3’. PCR products were separated using 1.2% agarose gel with TAE buffer.
C. Molecular modeling
This work was performed by DGMIF New drug development center. Our target protein sequence (HECAT5) was provided and one template sequence which showed the highest
4
sequence similarity (about 36%) with HECAT5 was selected. Target sequence and template sequence were aligned together and Homology modeling program by Schrodinger was used to generate HECAT5’ homology model. Next, virtual screening of chemicals were conducted based on molecular modeling and ligand binding active site prediction.
D. Cell culture
F3.HB1 (immortalized human neural stem cell, NCSs), HEK293 (human embryonic kidney cell) and NIH3T3 (mouse fibroblast cells) were maintained and passaged on uncoated culture dishes in Dulbecco’s modified medium (DMEM, Sigma Aldrich) with 10% Fetal bovine serum (FBS, Hyclone), 10ug/ml penicillin-streptomycin (Gibco). These cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
E. Cell proliferation assay
Cells were plated in 35mm3 dish at a density of 4x104 cells per dish. After 3days, cells were detached by trypsinization and dyed by 0.4% trypan blue solution and cell number was measured on a Countess™ Cell Counting slide (Invitrogen) by Countess™ automated cell counter (Invitrogen).
F. Western blot analysis after nonanoic acid treatment
After washed with PBS, cells were lysed with RIPA lysis buffer [1% sodium deoxycholoate, 1% Triton X-100, 0.1% SDS, 50mM Tris-HCl (pH7.5) and 50mM NaCl in distilled water] with both protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Roche). The concentration of protein was measured by the Bio-Rad DC Protein Assay. 20ug of the protein samples was resolved by 12% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (0.45um, Millipore). The PVDF membrane was blocked with TBST
5
(100mM NaCl, 10mM Tris, Tween 20) containing 5% skim milk (Becton Dickinson) and probed with polyclonal antibody specific for total ERK, p-ERK, total AKT, p-AKT (Cell Signaling) at a dilution of 1:2500. After washing, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies. Detection was performed with ECL (Bio-Rad).
G. Chemical preparation
Nonanoic acid was purchased from Sigma Aldrich and stocked into 1M with distilled water. In case of cell proliferation test, cells were incubated with different concentrations of nonanoic acid for 3days and then cell number was counted. For western blot, cells were treated with 10uM of nonanoic acid for 30 minutes and lysed.
YM-254890, Gαq inhibitor, was purchased from Wako and stocked with DMSO. To perform cell proliferation test with this chemical, cells were plated on 12 well plate. After 2 days, YM-254890 was diluted into cell culture media and treated with different concentrations for 5 minutes. After washing with pre-warmed PBS containing Ca2+, Mg2+, fresh media was added.
6
Ⅲ. RESULTS
A. Identification of hESC-specific stemness factor, HECAT5
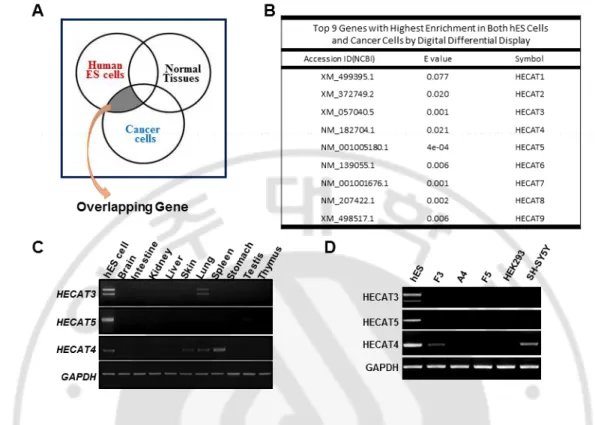
Human embryonic stem cells maintain their stemness by several core genes such as Oct4, Nanog, Sox2, and Klf4 known as Yamanaka factors. We identified genes which are expressed in both human ES cells and cancer cells. This shared gene may regulate the cell cycle system. This work was done by Digital differential display (DDD). We selected specific tissue libraries which were hES cell, cancer cell, and normal tissue. These pools were compared. DDD compares the Expressed Sequence Tags (ESTs) constituents of various tissue types, depending on selected libraries, resulting in the relative representation of each sequence in the libraries compared. We obtained a list of genes which are expressed both in hES cell and in cancer cell, but not in normal tissue and selected top 9 highly expressed genes (Figure 1A, 1B). According to our result, we confirmed that these 9 genes were expressed in hES cells. Finally, we narrowed down into three genes and choose HECAT5 as it showed expression level only in hES cells. This suggests that HECAT5 can be a candidate factor contributing to stemness. (Figure 1C, 1D)
7
Figure 1. Identification of hES cell-specific gene. (A)Venn diagram showing DDD concept.
(B) Top 9 genes with highest enrichment in both hES cells and cancer cells by DDD. (C) mRNA expression level of three candidate genes in different tissues and hES cell. (D) mRNA expression level of three candidate genes in different cell lines.
8
B. Characteristics of HECAT5
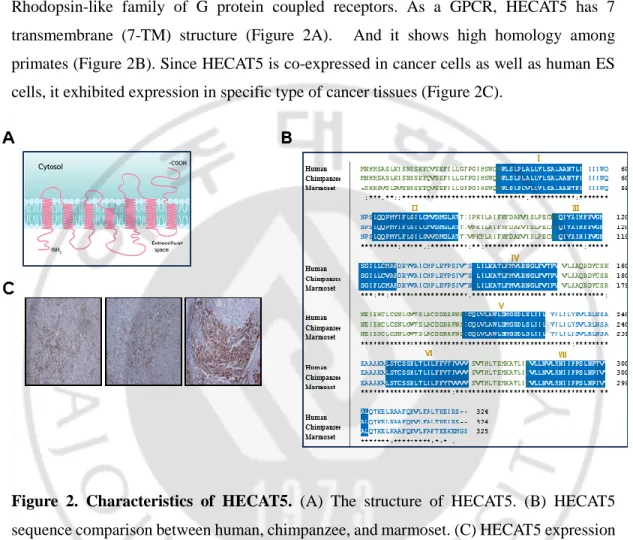
HECAT5 is one of the members of olfactory receptor, which is the biggest group in Rhodopsin-like family of G protein coupled receptors. As a GPCR, HECAT5 has 7 transmembrane (7-TM) structure (Figure 2A). And it shows high homology among primates (Figure 2B). Since HECAT5 is co-expressed in cancer cells as well as human ES cells, it exhibited expression in specific type of cancer tissues (Figure 2C).
Figure 2. Characteristics of HECAT5. (A) The structure of HECAT5. (B) HECAT5
sequence comparison between human, chimpanzee, and marmoset. (C) HECAT5 expression in specific cancer tissues.
9
C. GPCR’s general signaling pathway
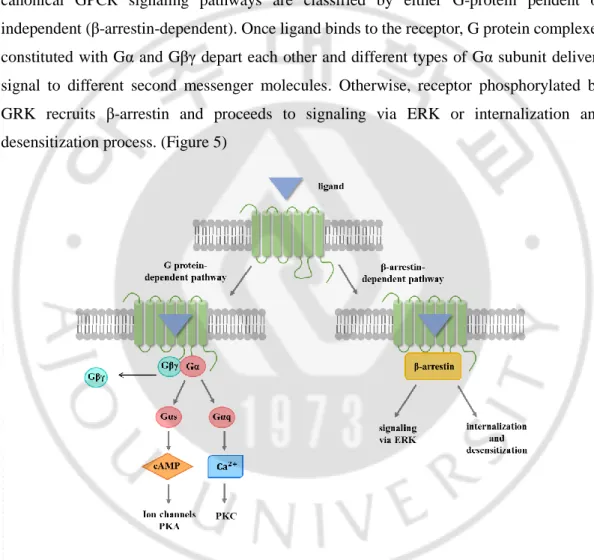
To further investigate the downstream signaling pathway underlying an increase in cell proliferation induced by HECAT5, we first studied GPCR’s general signaling pathway. Diverse options in GPCRs action may depend on different ligands binding, an interaction with other GPCRs, and different types of G protein that they associate with. However, canonical GPCR signaling pathways are classified by either G-protein pendent or independent (β-arrestin-dependent). Once ligand binds to the receptor, G protein complexes constituted with Gα and Gβγ depart each other and different types of Gα subunit delivers signal to different second messenger molecules. Otherwise, receptor phosphorylated by GRK recruits β-arrestin and proceeds to signaling via ERK or internalization and desensitization process. (Figure 5)
10
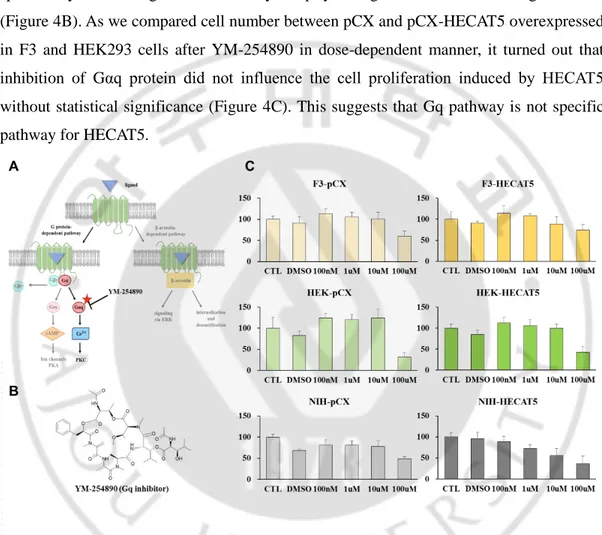
D. Gαq inhibitor did not affect the cell proliferation induced by HECAT5
First, we tried to dig out G protein-dependent pathway (Figure 4A). YM-254890 is a cyclic depsipeptide isolated from a soil bacterium Chromobacterium sp. QS3666 strain and specifically inhibits signals mediated by Gαq by acting on GDP/GTP exchange reaction (Figure 4B). As we compared cell number between pCX and pCX-HECAT5 overexpressed in F3 and HEK293 cells after YM-254890 in dose-dependent manner, it turned out that inhibition of Gαq protein did not influence the cell proliferation induced by HECAT5 without statistical significance (Figure 4C). This suggests that Gq pathway is not specific pathway for HECAT5.
Figure 4. The treatment of Gq inhibitor to see whether cell proliferation is blocked.
(A) The assumption if Gq pathway is activated by HECAT5. (B) The structure of Gq inhibitor, YM-254890. (C) Cell proliferation assay after Gq inhibitor with dose-dependent manner.
11
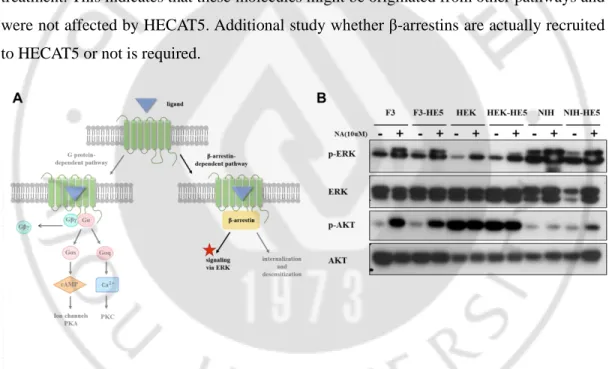
E. HECAT5-mediated cell proliferation might not be caused by β-arrestin-dependent way.
In addition, if HECAT5 trafficking leads to β-arrestin, we presumed that it might proceed to the creation of signal complexes involved in ERK pathway activation because GPCR internalization means signaling shutdown and GPCR recycling. For this reason, we checked the level of phospho-ERK and phospho-AKT by Western blot after nonanoic acid treatment (10uM, for 30min). Even though there was a change in phospho-ERK and AKT level with the presence of nonanoic acid or the absence of it, we could not find significant difference between pCX (empty vector) and pCX-HECAT5 with activation of HECAT5 by agonist treatment. This indicates that these molecules might be originated from other pathways and were not affected by HECAT5. Additional study whether β-arrestins are actually recruited to HECAT5 or not is required.
Figure 5. ERK level without difference. (A) The assumption if ERK pathway is activated
12
F. Conformational changes of GPCR family during Activation
Although we need to investigate each signaling pathways more deeply, now we assume that AC-cAMP-PKA pathway may account for HECAT5 signal trafficking pathway. On the other hand, the very first step of GPCR activation mechanism represents to be explored.
A myriad of variables have influenced GPCR signaling including the type of ligands, their combinational code, the activation status of GPCR, and the structure of GPCR binding pocket. Understanding the structural basis of GPCR’s dynamic signaling process is essential. (Naomi R et al, 2016) Upon ligand binds to an extracellular pocket of GPCR, this propagates conformational change, associates opening of a cleft between TM3 and TM6, and promotes binding and activation of downstream signaling effectors. It is likely that the diverse modes of agonist binding to extracellular structures and transmembrane domains result in different kind of signaling.
13
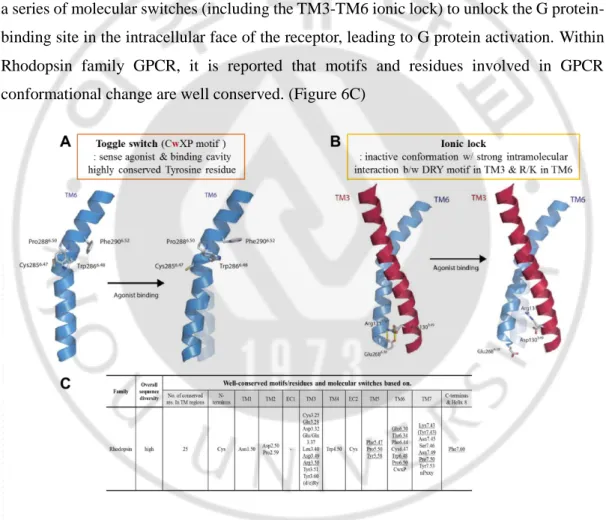
G. Toggle switch and Ionic lock are important motifs to trigger GPCR signaling
When GPCR conformational changes occur, TM3, TM5, TM6 are considered as playing a major role. There are two major motifs where conformational changes of TM happen. One is Toggle switch (Figure 6A): sense agonist and form binding cavity. In non-olfactory GPCR, CwXP motif in TM6 is highly conserved, especially Tyrosine residue. The other is ionic lock (Figure 6B): maintain inactive conformation with strong intramolecular interaction between DRY motif in TM3 and R/K residues in TM6 (X. Duepi et al, 2007). Ligand binding triggers a series of molecular switches (including the TM3-TM6 ionic lock) to unlock the G protein-binding site in the intracellular face of the receptor, leading to G protein activation. Within Rhodopsin family GPCR, it is reported that motifs and residues involved in GPCR conformational change are well conserved. (Figure 6C)
Figure 6. Two important motifs in GPCR activation. (A) Toggle switch. (B) Ionic lock.
(C) Table of well-conserved motifs/residues and molecular switches based on in Rhodopsin family.
14
H. Structural dynamics in olfactory receptor
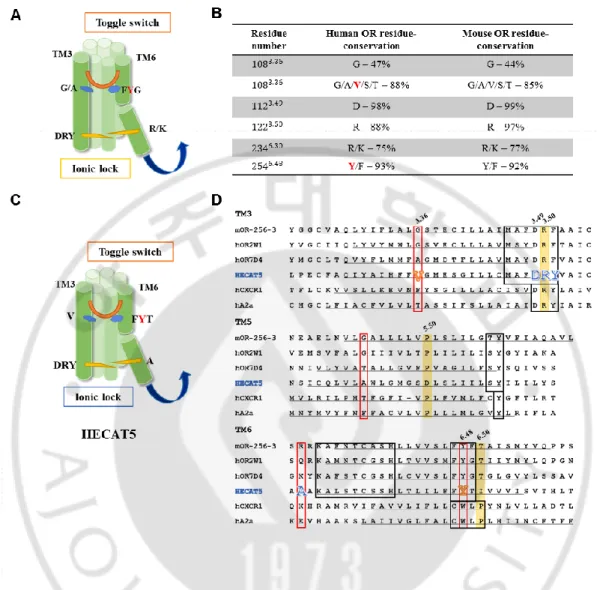
Even though OR have low sequence similarity with other class A GPCRs, they show the same highly conserved motifs in most TM domains. This means a conserved general mechanism work for their function. ORs contain some specific motifs such as MAYDRYVALCxPLxY in TM3 or KAFSTCxSH in TM6. However, ORs do not possess the CWxP motif which works as toggle switch for GPCR activation although the DRY motif remains highly conserved. According to the study about conformational change of OR, it is elucidated that FYG motif in TM6 is aligned with CwXP motif, suggesting the interaction between FYG motif in TM6 and G/A residue in TM3 play a role as toggle switch in OR. Furthermore, OR activation is also associated with a conformational change involving the ionic lock between TM3 and TM6 (Figure 7A) (CA de March et al, 2015)
In case of HECAT5, it has all known motifs with highly conserved. Based on the fact that residues attributed to conformational change in OR are considerably conserved between speicies, we compared sequence of known OR and HECAT5 and in turn we anticipated that HECAT5 also possesses those motifs and they may play a critical role in its function. (Figure 7B, C, D)
15
Figure 7. Crucial motifs in OR activation and their conservation in HECAT5. (A) OR
structure showing toggle switch motif and ionic lock between TM3 and TM6. (B) Table of how much residues are conserved in human OR and mouse OR. (C) HECAT5’s anticipated structure constituting TM3 and TM6. (D) Comparison of sequence in TM3, TM5, TM6 among several ORs and non-olfactory GPCRs.
16
I. Discovery of HECAT5 ligand via ODORactor program
According to structural characteristics, we wondered what would happen when ligand binds to HECAT5 and tried to discover ligands which are able to modulate the effect of HECAT5 in cell proliferation.
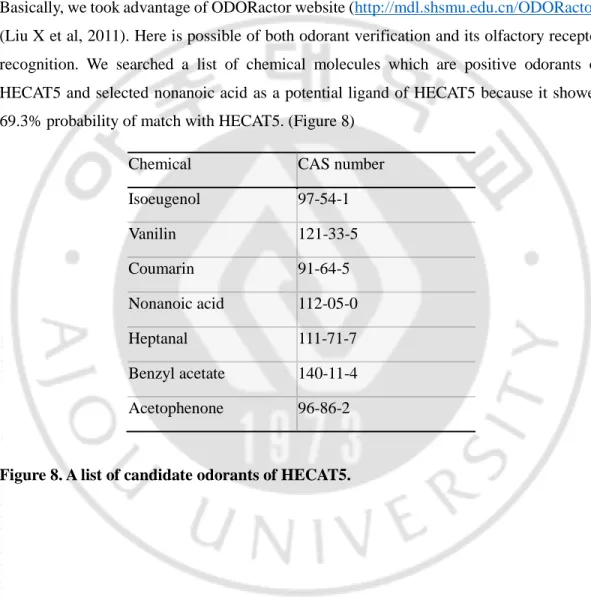
Basically, we took advantage of ODORactor website (http://mdl.shsmu.edu.cn/ODORactor) (Liu X et al, 2011). Here is possible of both odorant verification and its olfactory receptor recognition. We searched a list of chemical molecules which are positive odorants of HECAT5 and selected nonanoic acid as a potential ligand of HECAT5 because it showed 69.3% probability of match with HECAT5. (Figure 8)
Chemical CAS number Isoeugenol 97-54-1 Vanilin 121-33-5 Coumarin 91-64-5 Nonanoic acid 112-05-0 Heptanal 111-71-7 Benzyl acetate 140-11-4 Acetophenone 96-86-2
17
J. Treatment of nonanoic acid also increased cell proliferation
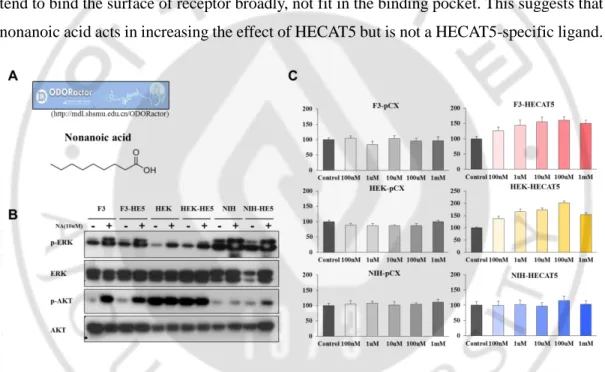
In order to identify the role of nonanoic acid as agonist or antagonist, we treated nonanoic acid to F3, HEK293, NIH3T3 cell lines with increasing concentration and counted cell number after 4 days. Only F3 and HEK293 cells overexpressed with HECAT5 exhibited dramatic increase in cell number, suggesting that nonanoic acid worked as an agonist to HECAT5 and boosted the effect of HECAT5 on cell expansion (Figure 9C). However, this chemical did not affect in phosphorylated ERK and AKT level in HECAT5-dependent manner. This may be because that chemicals which possess long chain like nonanoic acid tend to bind the surface of receptor broadly, not fit in the binding pocket. This suggests that nonanoic acid acts in increasing the effect of HECAT5 but is not a HECAT5-specific ligand.
Figure 9. HECAT5 ligand nonanoic acid and its effect in cell proliferation.
(A) Nonanoic acid structure. (B) Phospho-ERK and phospho-AKT level with nonanoic acid treatment. (C) Cell proliferation assay with treatment of nonanoic acid. Each cells were seeded with the number of 4x104 in 6 well plate. After 12hours, different concentrations of nonanoic acid were treated. All cells were counted at day4. Experiment was performed in triplicate.
18
K. Identification of candidate odors to modulate HECAT5 activity
We tried to identify more specific ligands to HECAT5 which are able to modulate the activity of HECAT5. During literature investigation, we came to know that J. D. Mainland et al conducted high-throughput screen of 73 odorants against a clone library of 511 human olfactory receptors (J. D. Mainland, 2015). We found out HECAT5 variant form with one amino acid difference in this screening dataset, and it responded to six candidate odorants. It has been well known that the sequences in crucial motifs for ligand-receptor binding such as toggle switch and ionic lock are conserved between members of OR family (de March et al, 2015). Comparison of sequence between HECAT5 and other ORs showed that those motifs are conserved in HECAT5.
This leads us to harness the interaction of HECAT5 variant with those odorants as a docking models (J. D. Mainland, 2015) to aim at identification of strong ligands to modulate HECAT5 activity. Based on comparison of amino acid sequence similarity between HECAT5 with other OR members which their 3D sequence already have been analyzed, we selected one OR with the highest similarity with HECAT5. By using homology modeling program from Schrodinger, HECAT5 sequence was co-aligned with the 3D structure of selected OR protein, which designated the homology model of HECAT5. Then, androstenone, a candidate odorant, was merged into the homology model to establish ligand docking molecular model of HECAT5 protein. Using this docking model, more than 140 chemicals were selected from ‘GPCR ligand focus library’ as well as various commercial chemical libraries. The candidate chemicals need to be analyzed their functions to act as agonist or antagonist.
19
Figure 10. Construction of homology model of HECAT5. Virtual structure of HECAT5
20
Ⅳ. DISCUSSION
HECAT5 has great potential to increase self-renewal property in vitro. It is a novel observation that olfactory receptor can regulate cell cycle even when only overexpressed. And it appears that HECAT5 possesses different action in different cell types. We are struggle for finding modulators of HECAT5 because controlling the effect of HECAT5 with external molecules can be applied for therapeutic uses. However, there are some limitations to be addressed.
Above all, we need to define the biological function of HECAT5 in human embryonic stem cells. It can be figured out whether HECAT5 works in hESCs as proliferation regulator by using siRNA of HECAT5. This will support our hypothesis that hESC-specific HECAT5 regulates cell proliferation and give a hint how olfactory receptor has functioned in embryonic stem cells as a gatekeeper receiving external signals.
Furthermore, protein level of HECAT5 has not been detected by Western blot and Immunocytochemistry. This is because sole isolation of membrane protein was very difficult. According to a research group, they used Rho tag to promote olfactory receptor protein expression on the cell surface as well as accessary factor constructs such as RTP1S, Ric8b (Hanyi Zhuang and Hiroaki Matsunami, 2008). Thus, tagging Rho to HECAT5 is under progress. We hope this would make it possible that we can check protein expression level of HECAT5 and its location within the cell.
GPCRs mediate many physiological processes by transmitting a variety of extracellular signals into intracellular responses (Xavier et al, 2007). In case of olfactory receptors, it is generally known that OR sends intracellular signaling through Gαs protein. And we were able to exclude other possible pathways such as Gαq pathway and ERK pathway as we witnessed that neither Gq inhibitor blocked the effect of HECAT5 nor phosphorylated ERK level was affected by HECAT5. Still, the assay to confirm whether HECAT5 follows cAMP-PKA downstream pathway starting from Gαs is required.
21
When it comes to therapeutic use of HECAT5, we are aware of the fact that GPCRs are targeted by approximately 25% of the drugs on the market today, which testifies the significant physiological roles GPCRs play in transducing signals from outside of cells to the inside (KA. Jacobson, 2015). However, knowledge about GPCR structure, conformation that bias receptor signaling, and each downstream signaling should be prior to finding the target of GPCRs. To be concrete, very first step which ligands bind may determine which signals they actually send to. In this respect, it is considered very significant to better understand the dynamic range and diverse mechanisms of GPCR activation (DB Staus, 2016). Based on the OR structure, active ligand binding site of HECAT5 from molecular modeling, and candidate ligands, we plan to explore each effect of chemical on cell proliferation. To list up of candidate chemicals is actually not enough because binding pocket of OR is spacious than that of other GPCR, resulting in both agonist and antagonist are able to bind. This makes it hard to distinguish which chemical acts as agonist or antagonist. Thus, we will figure out how outcome of HECAT5, increased cell proliferation, has been changed after treatment of each candidate chemicals and determine their mode of action as well as drugability.
Further, robust and efficient iPSCs generation without genomic insertion of transgenes has emerged as a challenge to be solved. Many research groups are developing iPSC induction methods including miRNA, small molecules, and nonintegrating virus in order with a minimum number of transgenes (Nasir Malik and Mahendra S. Rao, 2013). In this sense, we anticipate that while HECAT5 may act with other reprogramming factors, agonist of HECAT5 may play a role to increase the efficiency of iPSC generation (Dharmendra Kumar et al, 2017), on the other hand, antagonist of HECAT5 may act as a cancer cell blocker (Jane E. Visvader et al, 2012).
22
Ⅴ. CONCLUSION
Using digital differential display (DDD), we identified HECAT5 gene which is expressed both in hES cell and cancer cell, not in normal tissues. HECAT5 is a member of olfactory receptor and has 7-transmembrane structure. Overexpression of HECAT5 dramatically increased cell proliferation and this effect was enhanced by treatment of HECAT5’s candidate ligand, nonanoic acid. Of three major pathways in GPCR downstream signaling, we confirmed that neither Gαq pathway nor ERK pathway are specific to HECAT5. Next, going to upper stage where ligand and HECAT5 encounters, we expect to reveal the mechanism of HECAT5 activation according to conformational change in HECAT5 and the chemical screening.
23
REFERENCE
1. B. Trzaskowski, D. Latek, S. Yuan, U. Ghoshdastider, A. Debinski and S. Filipek : Action of Molecular Switches in GPCRs - Theoretical and Experimental Studies.
Current Medicinal Chemistry 19 :1090-1109, 2012
2. Charles E. Murry, Gordon Keller : Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell 132: 661–680, 2008
3. Christiana Hadjimichael, Konstantina Chanoumidou, Natalia Papadopoulou, Panagiota Arampatzi, Joseph Papamatheakis, Androniki Kretsovali : Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells 7 : 1150-1184, 2015
4. Claire A. de March, Yiqun Yu, Mengjue J. Ni, Kaylin A. Adipietro, Hiroaki Matsunami, Minghong Ma, and Jérôme Golebiowski : Conserved Residues Control Activation of Mammalian G Protein-Coupled Odorant Receptors. J. Am. Chem. Soc. 137 :8611−8616, 2015
5. Dharmendra Kumar, Taruna Anand, Wilfried A. Kues : Clinical potential of human-induced pluripotent stem cells :Perspectives of human-induced pluripotent stem cells. Cell
Biol Toxicol 33 :99-112, 2017
6. Dinorah Friedmann‐Morvinski, Inder M Verma : Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO reports 15: 244-253, 2015 7. Elmar Krieger, Sander B. Nabuurs, and Gert Vriend : Homology modeling.
Structural Bioinformatics 44 :507-521, 2013
8. Hanyi Zhuang and Hiroaki Matsunami : Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat
24 Protoc. 3: 1402–1413 2008
9. Jane E. Visvader and Geoffrey J. Lindeman : Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 10 :717-728, 2012
10. J. C. Mountford : Human embryonic stem cells: origins, characteristics and potential for regenerative therapy. Transfusion Medicine 18: 1–12, 2008
11. Jennifer Nichols and Austin Smith : Pluripotency in the Embryo and in Culture.
Cold Spring Harb Perspect Biol 4: a008128, 2012
12. J. Venkatakrishnan, Xavier Deupi, Guillaume Lebon, Christopher G. Tate, Gebhard F. Schertler & M. Madan Babu : Molecular signatures of G-protein-coupled receptors. Nature 494 :185-194, 2013
13. Ji Young Park, Su Youn Lee, Hee Ryung Kim, Min-Duk Seo, Ka Young Chung : Structural mechanism of GPCR-arrestin interaction: recent breakthroughs.
Archives of Pharmacal Research 39 :293-301, 2016
14. Kenneth A. Jacobson : New paradigms in GPCR drug discovery. Biochem
Pharmacol. 98: 541–555, 2015
15. Kazutoshi Takahashi and Shinya Yamanaka : Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676, 2006
16. Laurie A. Boyer, Tong Ihn Lee, Megan F Cole, Sarah E. Johnstone, Stuart S. Levine, Jacob P. Zucker, Matthew G. Guenther, Roshan M. Kumar, Heather L. Murray, Richard G. Jenner, David K : Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947-956, 2005
17. Liu X, Su X, Wang F, Huang Z, Wang Q, Li Z, Zhang R, Wu L, Pan Y, Chen Y, Zhuang H, Chen G, Shi T, Zhang J : ODORactor: a web server for deciphering olfactory coding. Bioinformatics 27 :2302-3, 2011
25
18. Naomi R. Latorraca, A. J. Venkatakrishnan, and Ron O. Dror : GPCR Dynamics: Structures in Motion. Chem. Rev 117 :139−155, 2017
19. Nasir Malik and Mahendra S. Rao : A Review of the Methods for Human iPSC Derivation. Methods Mol Biol 997: 23–33, 2013
20. Olivia Baud, Shuguang Yuan, Luc Veya, Slawomir Filipek2 Horst Vogel & Horst Pick : Exchanging ligand-binding specificity between a pair of mouse olfactory receptor paralogs reveals odorant recognition principles. Scientific Reports 5:14948, 2015
21. Takahashi K, Ymanaka S : Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 : 663-76, 2006 22. Staus DP, Strachan RT, Manglik A, Pani B, Kahsai AW, Kim TH, Wingler LM, Ahn S, Chatterjee A, Masoudi A, Kruse AC, Pardon E, Steyaert J, Weis WI, Prosser RS, Kobilka BK, Costa T, Lefkowitz RJ : Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature 535 :448-52, 2016
23. Xavier Deupi and Brian Kobilka : Activation of G protein-coupled receptors.
26 -국문요약-
인간 배아줄기세포-특이적 인자의 리간드를 이용한 세포 줄기성 조절
아주대학교 대학원 의생명과학과 김 지 인 ( 지도교수 : 이 명 애 ) 인간 배아줄기세포는 자가 증식능, 다분화능의 특징을 가지며 세포치료제로써 각광받고 있는 재료이다. 최근 인간 배아줄기세포의 줄기성을 관장하는 여러 가지 전사인자들이 밝혀지고 있다. 하지만, 줄기세포의 줄기성을 조절하는 세포 막 수용체는 알려진 바가 없다. 우리는 DDD를 통해 인간 배아줄기세포 특이적 인 유전자 HECAT5를 발굴하였다. HECAT5의 과발현은 세포 내에서 세포 증 식을 촉진시켰다. 이와 관련된 세포 내 신호전달 과정을 조사하는 중, Gq 단백 질 저해제인 YM254890을 처리하였으나 세포 증식에는 영향을 주지 않았고, β-arrestin 전달경로의 마지막 분자인 ERK, AKT 단백질 양 또한 변화가 없 었다.우리는 HECAT5의 활성을 조절할 수 있는 리간드를 찾고자 하였다. 먼저, ODORactor를 통해 HECAT5와 약 70%의 결합가능성을 가지는 nonanoic acid를 찾았다. Nonanoic acid의 처리는 세포 증식을 촉진시켰으나, ERK와 AKT의 양은 그대로였기에 HECAT5 특이적인 리간드는 아닌 것으로 밝혀졌 다. GPCR은 활성화가 되면 세포막 통과 도메인에 구조적인 변화가 일어나는데, 이 때 Toggle switch와 Ionic lock이 중요한 역할을 한다. 우리는 시퀀스 비교 를 통해 중요한 두 가지 모티프가 HECAT5에도 잘 보존되어 있음을 확인하였 다. 또한 레퍼런스 공개 자료를 분석하여, HECAT5와 반응을 보이는 천연 화
27 합물 6가지를 발굴하였다. 후각수용체의 구조적인 변화와 후보 물질들을 기반 으로 하여 HECAT5의 호몰로지 모델을 구축하였고, 여러 가지 화합물 라이브 러리를 스크리닝하여 약 140개의 화합물을 HECAT5 활성 조절인자 후보군으 로 선별하게 되었다. 추후 이들의 역할을 조사하여 역분화세포의 효율을 높이 거나 혹은 특정 암세포의 저해제를 개발하는 등의 응용을 기대하고 있다. 핵심어 : 배아줄기세포, 줄기성, 세포 증식, GPCR