Chronic Pain after traumatic,
Non-compressive Myelopathy: Characteristics and
Predictors for Neuropathic pain
by
Young InEom, M.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Chronic Pain after traumatic,
Non-compressive Myelopathy: Characteristics and
Predictors for Neuropathic pain
by
Young InEom, M.D.
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the
Requirements for the Degree of
Master of Science in
Medicine
Supervised by
In Soo Joo, M.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Young In Eom is approved.
SUPERVISORY COMMITTEE
ByungGon Kim
Jin Soo Lee
In Soo Joo
The Graduate School, Ajou University
i - Abstract –
Chronic Pain after Non-traumatic, Non-compressive Myelopathy:
Characteristics and Predictors for Neuropathic Pain
Introduction: Chronic pain is one of the most common and serious consequences of myelopathy. The aim of this study was to survey chronic pain experience in a neurology out-patient clinic and to determine potential predictors for neuropathic pain after non-traumatic, non-compressive(NTNC) myelopathy.
Methods: We analyzed54 patients with a history of NTNC myelopathy at the neurology out-patient clinic. All patients completed questionnaires on pain severity, descriptors and impact on quality of life (QOL) and underwent neurologic examination with bedside sensory testing. The Short Form McGill Pain Questionnaire (SF-MPQ) and the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) were used to assess pain. Neuropathic pain was diagnosed by LANSS score of 12 or more. Health-related QOL was evaluated by the Short Form 36-item (SF-36) health survey, while Hospital Anxiety and Depression Scale (HADS) and Patient Global Impression of Change (PGIC) were utilized to evaluate emotion and response to treatment for pain, respectively.
Results: Out of 54 patients, 48 reported pain; of these, 41 (85.4%) reported the initiation of pain during the first 3 months of myelopathy onset. The median (min-max) pain duration and SF-MPQ score was 41 (3.4-166) months and 10 (1-34), respectively. Thirty five (72.9%) patients reported continuous pain throughout the day. The most common pain descriptions were exhausting, gnawing and heavy. In total, 16 (33.3%) patients experienced neuropathic pain. Mean age was statistically significantly lower in patients with neuropathic pain than in patients with non-neuropathic pain (39.1 ± 12.5 vs. 49.8 ±
ii
9.3, P = 0.002). A binary logistic regression revealed that onset age under 40, non-idiopathic etiologysuch as neuromyelitis optica, multiple sclerosis were independent predictors of the occurrence of neuropathic pain. Both SF-MPQ and LANSS scores were significantly correlated with SF-36 scores, adjusted by age, sex, presence of diabetes mellitus, and current EDSS scores (r = –0.624, P< 0.0001 for SF-MPQ; r = -0.357, P = 0.017 for LANSS). Patients who showed clinical improvement (PGIC scores >2) with treatment were female, non-idiopathic etiology or lengthy lesion (> 3 vertebral segments). But the presence of diabetes was related to a poor treatment response.
Conclusion: Chronic pain is one of annoying complications in patients with NTNC myelopathy and also affects their quality of life. Onset age and etiology of
myelopathyare important factors in the development of neuropathic pain in NTNC myelopathy. Pain relief research is expected to improve health-related QOL in these patients..
Key words: Chronic pain, Non-traumatic non-compressive myelopathy, Neuropathic pain, Predictors
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FIGURES ··· ⅳ LIST OF TABLES ··· ⅴ . INTRODUCTION Ⅰ ··· 1. MATERIAL AND METHOD Ⅱ ··· 3
A. SUBJECTS ··· 3
B. MYELOPATHY CHARACTERISTICS ··· 3
C. PAIN ASSESSMENT ··· 3
D. ASSESSMENT OF FUNCTIONAL AND EMOTIONAL STATUS ··· 4
E. PAIN RESPONSE TO TREATMENT ··· 4
F. STATISTICAL ANALYSIS ··· 5
. RESULTS Ⅲ ··· 6
A. GENERAL CHARACTERISTICS OF SUBJECTS WITH NON-TRAUMATIC, NON-COMPRESSIVE MYELOPATHY ··· 6
B. PAIN PROFILES ··· 6
C. PAIR-WISE COMPARISONS BETWEEN NOCICEPTIVE AND NEUROPATHIC PAIN GROUPS ··· 7
D. PREDICTORS FOR NEUROPATHIC PAIN ··· 7
E. PAIN EFFECT ON QUALITY OF LIFE ··· 8
F. PAIN RESPONSE TO TREATMENT ··· 8
. DISCUSSION Ⅳ ··· 9 . CONCLUSION Ⅴ ··· 15 REFERENCES ··· 30 국문요약 ··· 36
iv
LIST OF TABLES
Table 1. Clinical characteristics of patients ... 16
Table 2.Pain characteristics in myelopathy ... 17
Table 3.Pain descriptors used in patients with myelopathy ... 18
Table 4. Predictive factors for occurrence of neuropathic pain ... 19
v
LIST OF FIGURES
Fig. 1. Korean version of Short Form McGill Pain Questionnaire... 21
Fig. 2. Korean version of Leeds Assessment of Neuropathic Symptoms and Signs pain scale ... 22
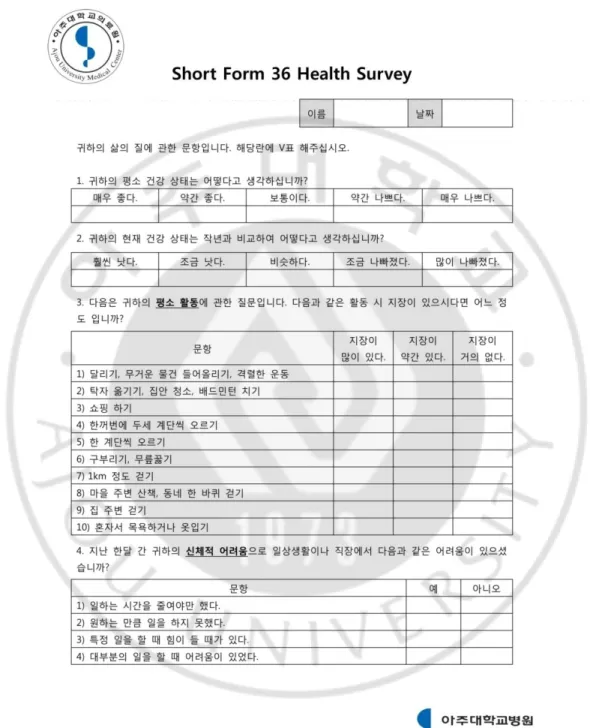
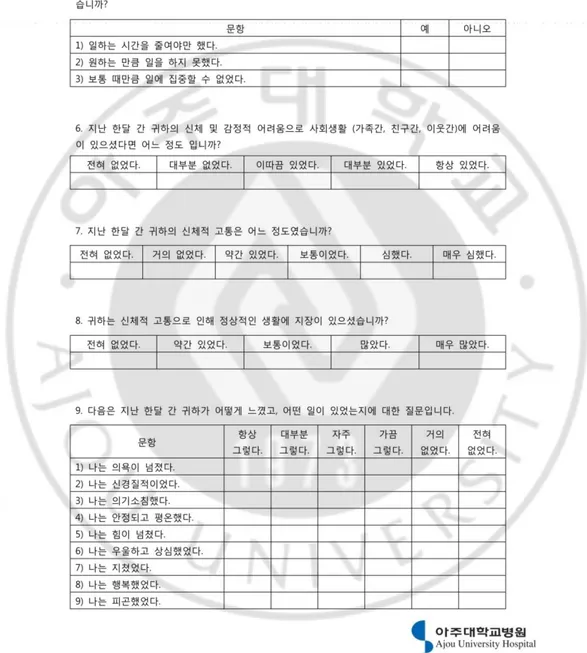
Fig. 3. Korean version of Short Form 36 Health Survey ... 23
Fig. 4. Korean version of Hospital Anxiety and Depression Scale ... 26
Fig. 5. Korean version of Patients’ Global Impression of Change scale ... 27
Fig. 6.Flowchart of patient recruitment and participation... 28
Fig. 7. Relationship between quality of life and total pain score and neuropathic pain score after adjusted by age, sex, DM, current EDSS score ... 29
1
Ⅰ. Introduction
Myelopathy refers to any neurologic deficit related to the spinal cord. Patients with myelopathy experience a loss in the ability to move, feel, and control bowel and bladder function. While loss of motor function is generally considered to be the most serious sequela of myelopathy, pain can have the greatest impact on the patient. Furthermore, pain can interfere with emotions and directly affect the patient’s ability to recover levels of activity.
Chronic pain is a frequent problem for patients with myelopathy. Approximately 60 to 80% ofsuch patients report pain and almost a third rate their pain as severe(Siddall et al., 1999; Siddall et al., 2003; Wollaars et al., 2007).
The new International Spinal Cord Injury Pain (ISCIP) classification divides spinal cord injury (SCI) pain into nociceptive, neuropathic, and other pain(Bryce et al., 2012). According to previous studies, nociceptive pain is the most common type associated with myelopathy (Siddall et al., 1999). However, approximately 30 to 40% of the pain manifests as neuropathic pain(Bonica, 1991; Siddall et al., 1997; Finnerup et al., 2001).
Neuropathic pain is caused by a lesion or disease of the somatosensory nervous system(Jensen et al., 2011) and is a result of nervous system dysfunction without peripheral nociceptive stimulation(Widerstrom-Noga and Turk, 2003). Neuropathic pain syndrome is associated with highlyheterogeneous clinical conditions(Yucel et al., 2004; Calmels et al., 2009). In myelopathy, neuropathic pain is related to the damage of nerve root and spinal cord; it tends to be chronic and responds poorly to treatment, resulting in various complications such as joint contractures, muscle atrophy, pressure sores, and cardiovascular problems(Wu et al., 2013).Therefore, it is important to diagnose neuropathic pain accurately and rapidly; however, little is known about the predictive factors of neuropathic pain in myelopathy.
Mostprevious studies ofmyelopathy pain have been limited to traumatic and compressive causes. Numerous patients with non-traumatic, non-compressive (NTNC)myelopathy, such as acute transverse myelitis (ATM), neuomyelitisoptica (NMO), multiple sclerosis (MS) or infective myelopathy experience painfor a long
2
period after disease onset. Whilst pain continues to be a significant problem in these patients, it has been relatively understudied. Other studies have described the prevalence, characteristics,and risk factors of pain in patients with NTNC myelopathy. One study found that neuropathic pain is relatively rare in acute transverse myelitis, although below-level neuropathic pain can be a chronic debilitating factor(Laffey et al., 1999).A few recent studies reported frequent pain and pain related quality of life impairment in patients with demyelinating myelopathy including NMO and MS (Solaro et al., 2004; Kanamori et al., 2011). However, these studies were limited to a specific disease and did notcomprehensively evaluate pain characteristics, severity, and treatment response.
The objective of this study was to surveychronic pain experience and to determine potential predictors for neuropathic after NTNC myelopathy. In addition, it aimed to investigate the influence of pain on daily life and emotion.
3
Ⅱ. Materials and Methods
A. Patients
All patients with NTNC myelopathy who visited the Department of Neurology, Ajou University Hospital, Suwon, Korea, between March 2014 and September 2014 were included. We interviewed a total 54 patient who met the inclusion criteria; 1) NTNC myelopathy at least 6 months of pain duration after onset of myelopathy, 2)aged over 18 years,3) an abnormal intra-cord signal demonstrated by spinal MRI. Patients with dementia and those who experienced pain from other diseases were excluded. Patients were examined and interviewed by an experienced neurologist at their regular visit to the out-patient clinic. All patientscompleted questionnaires for pain and quality of life. For analysis of predictors for neuropathic pain, patients were dived by the LANSS score 12; non-neuropathic pain group and neuropathic pain group. We also divided patients according to PGIC score 2; poor response to pain treatment group and good response to pain treatment group. The study was approved by the Institutional Review Board of the AjouUniverity Hospital and all patients provided informed consent prior to participation.
B. Myelopathy characteristics
The etiology of myelopathy was classified as idiopathic, demyelinating diseases (NMO, MS), infection, or other causes. The diagnosis of NMO was made based on the 2006 Wingerchuk criteria(Wingerchuk et al., 2006),while MS was diagnosed according to the 2010 McDonald criteria(Hawkes and Giovannoni, 2010). The level of myelopathy was categorized as cervical, thoracic, lumbar, or multi-level lesion. Results from cerebrospinal fluid and electrophysiological tests, in addition to a number of laboratory tests for differentiating cause of myelopathy were also reviewed.
4
The Short Form McGill Pain Questionnaire (SF-MPQ, Figure 1) was used to evaluate the type of pain and its severity. Neuropathic pain was diagnosed if patients scored 12 or more on the Leeds Assessment of Neuropathic Symptoms and Signs(LANSS, Figure 2). The LANSS scale, first invented by Bennett(Bennett, 2001), is a useful tool which helps distinguish neuropathic pain from nociceptive pain. The validation and reliability of the Korean versions of the SF-MPQ and LANSS have been confirmed previously(Yun et al., 2012).
Patients were interviewed about the location, patterns, and characteristics of their pain. The length of time between onset of disease to pain occurrence, and the total pain duration were also recorded. When neuropathic pain was present, it was classified as either above-level, at-level, or below-level neuropathic pain(Siddall et al., 1997).
D. Assessment of functional and emotional status
Patient functional status was assessed using the Short Form-36 (SF-36, Figure 3)(Han et al., 2004). This is a general health status measure, containing eight domains: physical functioning, physical role limitation, mental health, bodily pain, general health, vitality, social functioning, and emotional role limitation. The eight domains are aggregated into two summary scales, generating a physical component score and a mental component score. The validity and reliability of the Korean version of the SF-36 has been confirmed previously(Han et al., 2004). Depression and anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS, Figure 4), a14-items questionnaire, in which high scores indicate poor emotional wellbeing(Zigmond and Snaith, 1983). The validation and reliability for the Korean version has also been confirmed(Oh et al., 1999).
E. Pain response to treatment
5
response of pain to treatment(Farrar et al., 2001).It comprises a seven-point scale in which patients rate any change in their overall pain status since the initiation of treatment; clinical improvement was defined as a PGIC score more than 2. The Korean version of questionnaire(Moon et al., 2010) was completed by all patients.
F. Statistical analysis
The results for the overall patient group and sub-groups were presented as absolute numbers and percentages. The χ2-test and Fisher’s exact test were used to analyze the
association between categorical variables. For continuous variables with normal distribution and non-normal distribution, the t-test and Mann-Whitney U test were used, respectively. Binary logistic regression analyses were performed to adjust for various factors such as age, sex, and etiology laboratory test result. The relationship between variables was examined by Spearson’s correlation coefficient. All statistical analyses were performed by the Statistical Package for Social Sciences 11.0 software. P<0.05 was considered statistically significant.
6
Ⅲ. Results
Of the 58 patients interviewed, four patients were subsequently excluded due todementia (n = 2) and refusal to study consent (n = 2). The results of 48 patients who reported pain and 6 patients who did not have pain were analyzed (Figure 6).
A. General characteristics ofpatients with NTNC myelopathy
Demographics and clinical characteristics of patients with pain are summarized in Table 1. In pain(+) group, the mean (±standard deviation [SD])patient age was 46.2±11.5 years; 27 patients (56.3%) were men. Eighteen (37.5%) patients had recurrent myelopathy and six (12.5%) patients had diabetes mellitus (DM). Median (range) initial and current Expanded Disability Status Scale (EDSS) scores were 3.0(0-9) and 2.2 (0-7.5), respectively. The majority of cases had idiopathic etiology (n=36, 75%), and the most common level of lesion was in the thoracic cord (n=23, 47.9%). In six patients who did not have pain, the mean (±SD) age was 36.5±21.7 years; 4 patients were men. Median (range) initial and current EDSS score were 3.5 (2-8) and 3 (0-9). Current EDSS score of three patients were 0, while another 3 patients showed the score over 6. Unlike the pain (+) group, the most common etiology in the pain (-) group was NMO (n=3, 50%) without statistical significance due to low number of patients. Thoracic cord was also the most common level(n= 4, 66.7%) in pain (-) group.
B. Pain profiles
In total, 41 (85.4%) patients stated that pain initiated during the first 3 months of myelopathy onset. Median (range)pain duration was 52 (3.5-162) months and the median (range) SF-MPQ score was 12 (1-34). Thirty five (72.9%) patients reported continuous pain throughout the day. The most common words used by patients to describe their pain wereexhausting, gnawing and heavyin order.
7
Non-neuropathic pain (LANSS score < 12) was present in 32 (66%) patients and was more common than neuropathic pain (33%). In all cases of neuropathic pain, below-level neuropathic pain was the most frequently reported (in 93.7% of patients), followed by at-level neuropathic pain(37.5%); only one patient reported above-at-level neuropathic pain (data not shown).
C. Pair-wise comparisons between non-neuropathic and neuropathic pain groups
Mean (±SD) age was statistically significantly lower in patients with neuropathic pain than in patients with non-neuropathic pain (39.1 ± 12.5 vs. 49.8 ± 9.3, P = 0.002). However, overall median pain score (assessed by SF-MPQ) was statistically significantly higher in patients with neuropathic pain (8 vs 17.5, P = 0.004). There were no statistically significant differences in the other variables including gender, DM, etiology of myelopathy, CSF study, pain duration, total SF-36 score, and HADS score between the two pain groups (Table 2).
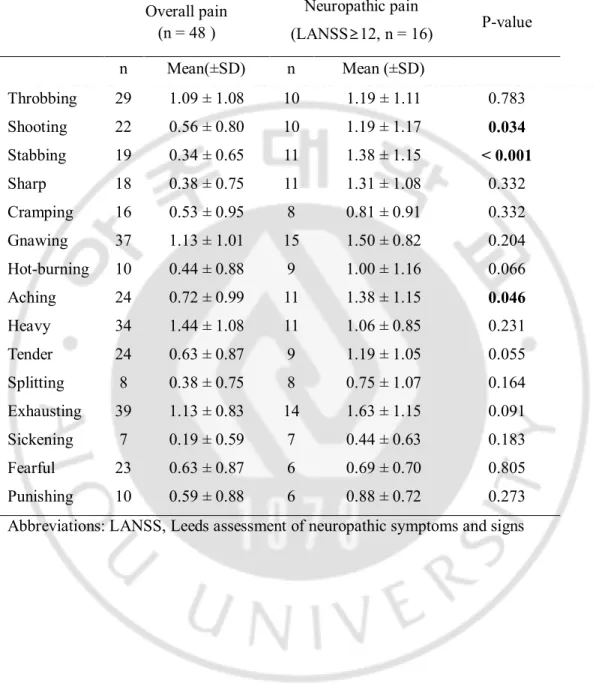
Similar to the all types of pain, the most common descriptions for neuropathic pain were gnawing, exhausting and heavy. However, in the presence of neuropathic pain, shooting, stabbing, and aching were used statistically significantly more than for overall pain (Table 3).
D. Predictors for neuropathic pain
A binary logistic regression was performed to evaluate the predictive factors for the development of neuropathic pain in NTNC myelopathy. The following factors were entered into the model: (1) age of myelopathy onset > 40 year old, (2) gender, (3) etiology, idiopathic or non-idiopathic, (4) initial EDSS score. The results showed that onset age under 40, non-idiopathic etiology were independent predictors of the occurrence of neuropathic pain (Table 4) The Nagelkerke R2 value was 0.383, suggesting
a strong association between neuropathic pain and the independent variables in the regression analysis.
8
E. Effect of pain on quality of life
Both the SF-MPQ and LANSS scores showed significant correlation with SF-36 scores adjusted by age, sex, presence of DM, and current EDSS scores (r = -0.624, P< 0.0001 for SF-MPQ; r = -0.357, P = 0.017 for LANSS). However, SF-MPQ scores, which indicated total pain, showed a higher correlation with SF-36 scores than the LANSS scores (Figure 7).
F. Pain response to treatment
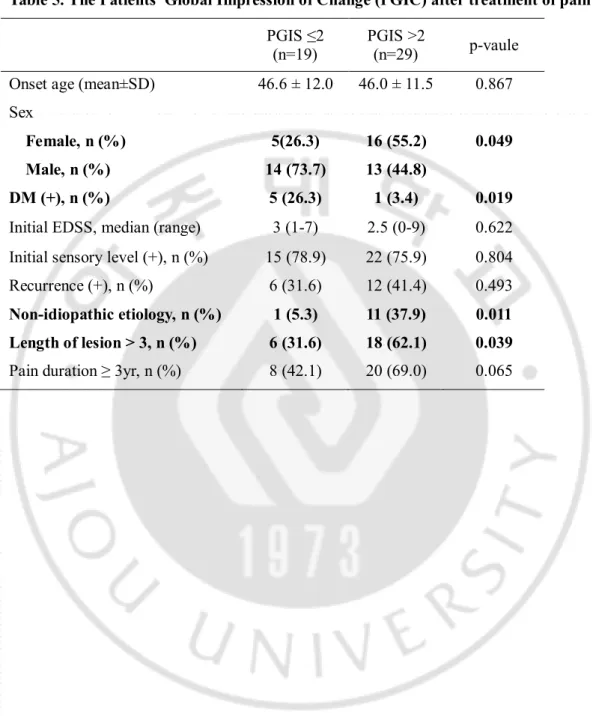
Patients were divided into two groups based on their PGIC score; those with no improvement or worsening pain (score ≤ 2) and those with improvement of pain after treatment (score > 2). Patients showing clinical improvementwith treatment were female (n=16, 55.2%), non-idiopathic etiology (n=11, 37.9%) or long lesion (n=18, 62.1%). But the presence of diabetes was related to poor pain treatment response (Table 5).
9
Ⅳ. Discussion
In this study, almost all patients with NTNC myelopathy reported chronic pain and one third of the pain had neuropathic features. Patients with neuropathic pain were younger and had higher SF-MPQ scores than those with non-neuropathic pain. Being aged under 40 and having non-idiopathic etiology were independent predictive factors for the development of neuropathic pain. Patients responded well to pain treatment tended to be ofthe female gender, have non-idiopathic etiology, and have a lesion length longer than 3 vertebral segments. In contrast,the presence of diabetes was related to poor response to pain treatment.
It is common for patients with myelopathy to develop serious and often life-threatening complications after the acute phase has passed. Chronic pain, a common secondary complication following SCI, has a wide reported range of prevalence, ranging from 11 to 94%(Defrin et al., 2001). Recent studies have shown that more than 80% of patients with SCIexperience pain (Siddall et al., 2003)and a third of them rated the pain as severe(Cruz-Almeida et al., 2005).Several studies of non-traumatic myelopathy have reported a 20 to 60% prevalence of chronic pain (New et al., 2002; Pidcock et al., 2007; Werhagen et al., 2007).Pain after idiopathic myelitis is generally considered rare (Laffey et al., 1999);however, one study found that 75% of patients with ATM had acute pain; chronic pain developed in 54% of patients(Pidcock et al., 2007).Furthermore, recent studies have reported frequent pain and pain related quality of life impairment in patients with demyelinating myelopathy including NMO and MS(Solaro et al., 2004; Kanamori et al., 2011; Qian et al., 2012).In the present study, 88.9% of NTNC myelopathy patients interviewed reported pain. Patients without pain were classified as either of two groups; EDSS score 0 or severely disabled status (EDSS ≥ 6.5) with no sensory function at all. There were no significant differences in epidemiological or clinical data between patients with pain and without pain.
Numerous studies have found that pain typically occurs within the first 6 months of myelopathy(Ravenscroft et al., 2000; Norrbrink Budh et al., 2003b; Henwood and Ellis, 2004), though in some studies it has been reported within the first year (Stormer et al.,
10
1997).The present results show that 80% of pain began within the first 3 months of disease onset; earlier than that reported in the previous myelopathy pain studies(Miguel and Kraychete, 2009).This difference is assumed to be due to the inclusion of non-traumatic, non-compressive causes of myelopathy inthe present study. At the onset of NTNC myelopathy, many patients complain of sensory symptoms, in contrast to traumatic myelopathy where motor symptoms are most frequently reported.According to previous studies, almost all patients with myelopathy pain report continuous pain, which is usually intensified in the evening(Demirel et al., 1998; Celik et al., 2012).Similarly, 35 (72.9%) patients in the present studyalso reported continuous pain. The various descriptions of pain have included pricking, tingling, and tiring(Stormer et al., 1997; Finnerup et al., 2001; Celik et al., 2012). In contrast, the most frequently used pain descriptions in our data included exhausting, gnawing and heavy. This difference may be due to variations in patient populations between studies, or cultural differences in pain perception.
Nociceptive pain is the most common type of myelopathy pain both in the acute and chronic phase(Siddall et al., 2003).It can be identified by location (above- or at-lesion level in patients with complete spinal cord damage) and by pain features (aching, dull, worse with activities)(Siddall et al., 1997).Shoulder pain due to acute phase trauma or overuse, muscle weakness, and spasticity, in the chronic phase is common(Akbar et al., 2011). Pain related to spasms, muscle contractures, and pain in the back and wrist are also examples of nociceptive pain(Finnerup, 2013).Nociceptive pain is less severe than the other types andusually presents early on following the onset of disease or trauma(Siddall et al., 2003).In NTNC myelopathy, unlike traumatic SCI, there is no injury or compression to the structures surrounding the spinal cord.Therefore, neuropathic pain could be considered more common than nociceptive pain in NTNC myelopathy. However, the present study revealed that 32 (67%) patients had non-neuropathic pain (LANSS score <12). We speculate that this result had several causes, Firstly, we only evaluated pain in the chronic phase. After the acute stage of myelopathy, nociceptive pain due to weakness, overuse, and spasticityincreases naturally. Secondly, patients with NTNC myelopathy are older than those with traumatic SCI(Burney et al.,
11
1993; New et al., 2002). Even if we excluded patients who experienced pain from other diseases, the elderly would have more pain-causing problems such as arthritis or muscle cramps. These factors could affect the majority of our patients having NTNC myelopathy with non-neuropathic pain.
There are two types of neuropathic pain in myelopathy: myelopathy-related neuropathic pain and other neuropathic pain. Myelopathy-related neuropathic pain is further classified as either at-level or below-level neuropathic pain. Other neuropathic pain describes pain which is not a direct consequence of myelopathy (e.g. post-thoracostomy pain, and pain due to carpal tunnel syndrome fromwheelchair use), which may occur above-, at-, or below- lesion level(Finnerup, 2013).
The mechanisms of myelopathy-related neuropathic pain are multiple and not yet fully understood. At-level neuropathic paincould result from a lesion in the nerve roots or spinal cord, whilebelow-level neuropathic pain is considered as central pain due to spinal cord lesions, suggesting a different underlying cause.At-level neuropathic paincan be initiated early following damage and it therefore appears to share some of the characteristics of nociceptive pain. However, at-level neuropathic pain is more likely to be described as severe, and persists despite treatment. Below-level neuropathic paingenerally occurs later than nociceptive or at-level neuropathic pain and is also described as severe and excruciating. Neuroplasticity is an important component of the spontaneous recovery from a myelopathy; however, it can generate negative consequences such as spasticity, autonomic dysreflexia, and neuropathic pain(Brown and Weaver, 2012).Increasing evidence has indicated that neuroplasticity may limit the effects of experimental myelopathy therapy due to undesired results such as neuropathic pain(Brown and Weaver, 2012).Central sensitization is considered to be the main cellular change responsible for central neuropathic pain and manifests as a decrease in pain thresholds, increased response to synaptic input, and expansion of receptor fields(Woolf, 2011). Additionally, an imbalance between descending inhibitory activity and facilitation, and the loss of dorsal interneuron containing inhibitory function may contribute to central sensitization(Gwak and Hulsebosch, 2011).
12
of neuropathic pain in NTNC myelopathy. While many studies have shown neuropathic pain to be a common and serious problem following myelopathy, the understanding of the factors associated with its development is limited.
In this study, several variables were significantly related to the development of neuropathic pain after the onset of myelopathy. First, the age of patients in the neuropathic pain group was significantly lower than that of the non-neuropathic group. Generally, old age is known as a predictor for persistent neuropathic pain(Jung et al., 2004; Kehlet et al., 2006; Werhagen et al., 2007; Margot-Duclot et al., 2009).Numerous studies have shown that increasing age is a predictive factor for chronic pain following surgery(White et al., 1997), illness(Dworkin, 1997), or injury(Baldwin et al., 1996). This may be due to age related changes in endogenous pain control mechanism at the central level(Washington et al., 2000; Gibson and Helme, 2001; Edwards et al., 2003; Karp et al., 2008).In contrast, age and neuropathic pain following SCI have not been found to be related(Ravenscroft et al., 2000; Norrbrink Budh et al., 2003a; Nakipoglu-Yuzer et al., 2013).Furthermore, one study found that myelopathy-related neuropathic pain was mainly observed in patients aged between 30 and 39 years, but there was no correlation with age and pain severity (Werhagen et al., 2004).
With regard to age, binary logistic regression analysis confirmed findings of the paired comparison analysis, showing that onset age below 40 is an independent predictive factor for the development of neuropathic pain. Additionally, known etiologywas anothersignificant predictor for neuropathic pain. While the mechanisms of neuropathic pain in myelopathy are not yet fully understood, a recent study found a marked difference in the effectiveness of diffuse noxious inhibitory control (DNIC) between a group of healthy participants aged between 40 to 55 years old and a group of patients aged between 20 and 35 years (Lariviere et al., 2007).DNIC, which is also referred to as bulbospinal endogenous pain control mechanism, is one of the most understood descending inhibitory pain mechanisms. It has been suggested that pain perception and endogenous pain modulation function decline by middle age and continue to deteriorate thereafter (Lariviere et al., 2007). The present findings support this because there was a significantly higher occurrence of neuropathic pain in patients under the age of 40.
13
One study reported that 55.4% of patients with myelopathy related neuropathic pain are tetraplegic(Widerstrom-Noga et al., 2001).Another study failed to find a relationship between the existence of pain and the level of lesion or completeness of injury, although below-level neuropathic pain was more common in patients with tetraplegia(Siddall et al., 2003).It has also been documented that there is no relationship between patient demographics and myelopathy features, pain score, localization, or severity(Ullrich et al., 2008).Furthermore, another study failed to find a relationship between completeness of injury and the type of pain(Yap et al., 2003). Patients with malignant etiology of myelopathy have been shown to experience neuropathic pain more frequently than other etiologic subgroups. In the present study, non-idiopathic etiology such as multiple sclerosis, neuromyelitis optica, and infection was associated with a higher frequency of neuropathic pain. However, these patients tended to show a better response to treatment than those with idiopathic myelopathy (as assessed by PGIC score), though the reason for these results is presently unknown. While there was no relationship found between recurrence of myelopathy and the PGIC score, targeted therapy according to the etiology would reduce pain intensity, even if further neuropathic pain occurred.
Myelopathy pain is intense and constant, aggravated by various stimuli encountered during daily activities, and interferes with quality of life beyond just the limiting of motor functions. In one study, 70% of patients with myelopathy reported that pain affected their life to a great extent(Werhagen et al., 2004) and in another report, more than 90% of such patients stated that their pain interfered with daily activities(Finnerup et al., 2001).Furthermore, numerous other studies have found that the health related quality of life of patients with neuropathic pain is worse than in those with non-neuropathic pain(Smith et al., 2007; Smith and Torrance, 2012).In the present study, both SF-MPQ and LANSS scores showed a significant correlation with SF-36 scores after adjustment for age, sex, presence of DM, and current EDSS score. In contrast to previous studies, SF-MPQ scores exhibited a higher correlation to SF-36 than LANSS scores. This may be because nociceptive pain is the most common type of pain during myelopathy, and secondly, a relatively low number of patients were included in this study.
14
The classification of pain type, its underlying mechanisms, and the consideration of the multidimensional aspects of pain is also required. In surveys of medical treatments, simple analgesics including non-steroidal anti-inflammatory drugs have been the most frequently used to treat overall myelopathy pain(Finnerup et al., 2001; Nakipoglu-Yuzer et al., 2013; Yamashita et al., 2014).While these drugs are effective at relieving nociceptive pain, they may be ineffective for treating neuropathic pain. Instead, anticonvulsants such as gabapentin andpregabalin, as well as antidepressants such as amitriptyline and duloxetine, could be effective treatments of myelopathy-related neuropathic pain. Gabapentin was the most frequently used medication by the patients in this study.
A major limitation of this study is that consecutive patients were not enrolled. Therefore, since the case series could be confounded by a selection bias, an accurate prevalence of pain could not be reported. Additionally, despite careful clinical examination and use of various questionnaires for pain assessment, it can be difficult to clearly differentiate between nociceptive and neuropathic pain. Furthermore, the LANSS pain scale might not to be a suitable tool for evaluating the neuropathic pain in myelopathy, despite its widespread use for classifying myelopathy pain.
15
Ⅴ. Conclusion
In this study, the characteristics of chronic pain in NTNC myelopathy were assessed and the predictive factors for development of neuropathic pain were investigated. The age at myelopathy onset and etiology of myelopathy were related to the occurrence of neuropathic pain. A significant relationship was also found between pain and quality of life. This research focused on chronic pain in NTNC myelopathy, which has been relatively poorly studied in comparison to traumatic or compressive SCI. These findings enhance the understanding of pain in NTNC myelopathy and may contribute to the advancement of research in this field and the development of effective treatment.
16
Table 1. Clinical characteristics of patients
Pain (+) (n = 48) Pain (-) (n=6) Age Mean ± SD 46.2 ± 11.5 36.5 ± 21.7 Range 19-71 17-77 Gender Male, n (%) 27 (56.3) 4 (66.7) Female, n (%) 21 (43.7) 2 (33.3) DM, n (%) 6 (12.5) 0 (0) Recurrent myelopathy, n (%) 18 (37.5) 3 (50) Median EDSS (range)
Initial 3.0(0-9) 3.5 (2-8) Current 2.2(0-7.5) 3 (0-9) Etiology of myelopathy Idiopathic Monophasic, n(%) 26 (54.2) 1 (16.7) Recurrent, n(%) 10 (20.8) 2 (33.3) NMO, n (%) 6 (12.5) 3 (50) MS, n (%) 2 (4.2) 0 (0) Infection, n (%) 3 (6.3) 0 (0) Others, n (%) 1 (2.1) 0 (0) Lesion level Cervical, n (%) 21 (43.8) 2 (33.3) Thoracic, n (%) 23 (47.9) 4 (66.7) Lumbosacral, n (%) 0 (0) 0 Multi-level, n (%) 4 (8.3) 0
Abbreviations: SD, standard deviation; DM, Diabetes mellitus; EDSS, Expanded Disability Status Scale; NMO, Neuromyelitis optica; MS, Multiple sclerosis
17
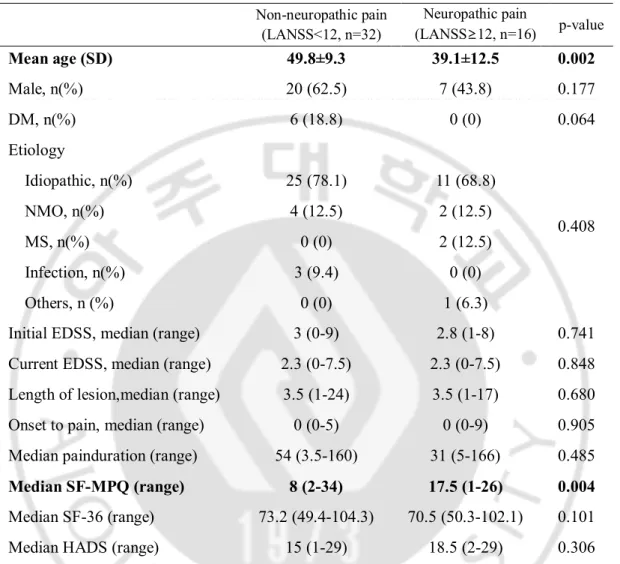
Table 2.Pain characteristics in myelopathy
Non-neuropathic pain (LANSS<12, n=32) Neuropathic pain (LANSS≥12, n=16) p-value Mean age (SD) 49.8±9.3 39.1±12.5 0.002 Male, n(%) 20 (62.5) 7 (43.8) 0.177 DM, n(%) 6 (18.8) 0 (0) 0.064 Etiology 0.408 Idiopathic, n(%) 25 (78.1) 11 (68.8) NMO, n(%) 4 (12.5) 2 (12.5) MS, n(%) 0 (0) 2 (12.5) Infection, n(%) 3 (9.4) 0 (0) Others, n (%) 0 (0) 1 (6.3)
Initial EDSS, median (range) 3 (0-9) 2.8 (1-8) 0.741 Current EDSS, median (range) 2.3 (0-7.5) 2.3 (0-7.5) 0.848 Length of lesion,median (range) 3.5 (1-24) 3.5 (1-17) 0.680 Onset to pain, median (range) 0 (0-5) 0 (0-9) 0.905 Median painduration (range) 54 (3.5-160) 31 (5-166) 0.485 Median SF-MPQ (range) 8 (2-34) 17.5 (1-26) 0.004 Median SF-36 (range) 73.2 (49.4-104.3) 70.5 (50.3-102.1) 0.101 Median HADS (range) 15 (1-29) 18.5 (2-29) 0.306 Abbreviations: LANSS, Leeds assessment of neuropathic symptoms and signs; SD, standard deviation; DM, Diabetes mellitus; NMO, Neuromyelitis optica; MS, Multiple sclerosis; CSF, Cerebrospinal fluid; MPQ, short form McGill pain questionnaire; SF-36, short form 36; HADS, Hospital anxiety depression scales
18
Table 3.Pain descriptors used in patients with myelopathy Overall pain (n = 48 ) Neuropathic pain (LANSS≥12, n = 16) P-value n Mean(±SD) n Mean (±SD) Throbbing 29 1.09 ± 1.08 10 1.19 ± 1.11 0.783 Shooting 22 0.56 ± 0.80 10 1.19 ± 1.17 0.034 Stabbing 19 0.34 ± 0.65 11 1.38 ± 1.15 < 0.001 Sharp 18 0.38 ± 0.75 11 1.31 ± 1.08 0.332 Cramping 16 0.53 ± 0.95 8 0.81 ± 0.91 0.332 Gnawing 37 1.13 ± 1.01 15 1.50 ± 0.82 0.204 Hot-burning 10 0.44 ± 0.88 9 1.00 ± 1.16 0.066 Aching 24 0.72 ± 0.99 11 1.38 ± 1.15 0.046 Heavy 34 1.44 ± 1.08 11 1.06 ± 0.85 0.231 Tender 24 0.63 ± 0.87 9 1.19 ± 1.05 0.055 Splitting 8 0.38 ± 0.75 8 0.75 ± 1.07 0.164 Exhausting 39 1.13 ± 0.83 14 1.63 ± 1.15 0.091 Sickening 7 0.19 ± 0.59 7 0.44 ± 0.63 0.183 Fearful 23 0.63 ± 0.87 6 0.69 ± 0.70 0.805 Punishing 10 0.59 ± 0.88 6 0.88 ± 0.72 0.273 Abbreviations: LANSS, Leeds assessment of neuropathic symptoms and signs
19
Table 4. Predictive factors for occurrence of neuropathic pain
Variables OR (95% CI) p-value
Sex 2.899 (0.549-15.315) 0.210
Age < 40 16.596 (3.044-90.477) 0.001
Non-idiopathic etiology 2.126 (1.373-12.116) 0.049
20
Table 5. The Patients’ Global Impression of Change (PGIC) after treatment of pain PGIS ≤2
(n=19)
PGIS >2
(n=29) p-vaule Onset age (mean±SD) 46.6 ± 12.0 46.0 ± 11.5 0.867 Sex
0.049 Female, n (%) 5(26.3) 16 (55.2)
Male, n (%) 14 (73.7) 13 (44.8)
DM (+), n (%) 5 (26.3) 1 (3.4) 0.019 Initial EDSS, median (range) 3 (1-7) 2.5 (0-9) 0.622 Initial sensory level (+), n (%) 15 (78.9) 22 (75.9) 0.804 Recurrence (+), n (%) 6 (31.6) 12 (41.4) 0.493 Non-idiopathic etiology, n (%) 1 (5.3) 11 (37.9) 0.011 Length of lesion > 3, n (%) 6 (31.6) 18 (62.1) 0.039 Pain duration ≥ 3yr, n (%) 8 (42.1) 20 (69.0) 0.065
21
22
Figure 2.Korean version of Leeds Assessment of Neuropathic Symptoms and Signs pain scale.
23
24
25
26
27
28
29
Figure 7.Relationship between quality of life and total pain score (A) and neuropathic pain score (B) after adjusted by age, sex, DM, current EDSS score (r=-0.624, p<0.0001 for SF-MPQ; r=-0.357, p=0.017 for LANSS).
30
REFERENCES
1. Akbar M, Brunner M, Balean G, Grieser T, Bruckner T, Loew M, Raiss P: A cross-sectional study of demographic and morphologic features of rotator cuff disease in paraplegic patients. J Shoulder Elbow Surg 20: 1108-1113, 2011 2. Baldwin M, Johnson W, Butler R: The error of using returns-to-work to measure
the outcomes of health care. Am J Ind Med 29: 632-641, 1996
3. Bennett M: The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 92: 147-157, 2001
4. Bonica JJ: Pain and Central Nervous System Disease: The Central Pain Syndrome. 13-29, 1991
5. Brown A, Weaver LC: The dark side of neuroplasticity. Exp Neurol 235: 133-141, 2012
6. Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, Norrbrink C, Richards JS, Siddall P, Stripling T, Treede RD, Waxman SG, Widerstrom-Noga E, Yezierski RP, Dijkers M: International spinal cord injury pain classification: part I. Background and description. March 6-7, 2009. Spinal Cord 50: 413-417, 2012
7. Burney RE, Maio RF, Maynard F, Karunas R: Incidence, characteristics, and outcome of spinal cord injury at trauma centers in North America. Arch Surg 128: 596-599, 1993
8. Calmels P, Mick G, Perrouin-Verbe B, Ventura M, Sofmer: Neuropathic pain in spinal cord injury: identification, classification, evaluation. Ann Phys Rehabil Med 52: 83-102, 2009
9. Celik E, Erhan B, Lakse E: The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord 50: 585-589, 2012
10. Cruz-Almeida Y, Martinez-Arizala A, Widerstrom-Noga E: Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J Rehabil Res Dev 42: 585-594, 2005
31
somatosensory function in spinal cord injury subjects. Pain 89: 253-263, 2001 12. Demirel G, Yllmaz H, Gencosmanoglu B, Kesiktas N: Pain following spinal cord
injury. Spinal Cord 36: 25-28, 1998
13. Dworkin RH: Which indiviuals with acute pain are most likely to developed a chronic pain syndrome? Pain Forum 6: 127-136, 1997
14. Edwards R, Fillingim J, Ness T: Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 101: 155-165, 2003
15. Farrar J, Young J, LaMoreaux L, Werth J, Poole R: Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94: 149-158, 2001
16. Finnerup N: Pain in patients with spinal cord injury. Pain 154 Suppl 1: S71-76, 2013
17. Finnerup N, Johannesen I, Sindrup S, Bach F, Jensen T: Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord 39: 256-262, 2001 18. Gibson SJ, Helme RD: Age-related differences in pain perception and report.
Clin Geriatr Med 17: 433-456, v-vi, 2001
19. Gwak YS, Hulsebosch CE: GABA and central neuropathic pain following spinal cord injury. Neuropharmacology 60: 799-808, 2011
20. Han C, Lee E, Iwaya T, Kataoka H, Kohzuki M: Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med 203: 189-194, 2004
21. Hawkes C, Giovannoni G: The McDonald Criteria for Multiple Sclerosis: time for clarification. Mult Scler 16: 566-575, 2010
22. Henwood P, Ellis JA: Chronic neuropathic pain in spinal cord injury: the patient's perspective. Pain Res Manag 9: 39-45, 2004
23. Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD: A new definition of neuropathic pain. Pain 152: 2204-2205, 2011
32
neuralgia in patients with herpes zoster. Neurology 62: 1545-1551, 2004
25. Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T, Kanaoka-Suzuki C, Misu T, Fujihara K, Itoyama Y: Pain in neuromyelitis optica and its effect on quality of life: a cross-sectional study. Neurology 77: 652-658, 2011
26. Karp JF, Shega JW, Morone NE, Weiner DK: Advances in understanding the mechanisms and management of persistent pain in older adults. Br J Anaesth 101: 111-120, 2008
27. Kehlet H, Jensen TS, Woolf CJ: Persistent postsurgical pain: risk factors and prevention. Lancet 367: 1618-1625, 2006
28. Laffey JG, Murphy D, Regan J, O'Keeffe D: Efficacy of spinal cord stimulation for neuropathic pain following idiopathic acute transverse myelitis: a case report. Clin Neurol Neurosurg 101: 125-127, 1999
29. Lariviere M, Goffaux P, Marchand S, Julien N: Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 23: 506-510, 2007
30. Margot-Duclot A, Tournebise H, Ventura M, Fattal C: What are the risk factors of occurence and chronicity of neuropathic pain in spinal cord injury patients? Ann Phys Rehabil Med 52: 111-123, 2009
31. Miguel M, Kraychete D: Pain in patients with spinal cord injury: a review. Rev Bras Anestesiol 59: 350-357, 2009
32. Moon D, Lee L, Lee S, Song S, Yoon D, Yoon M, Kim H: Efficacy and Tolerability of Pregabalin Using a Flexible, Optimized Dose Schedule in Korean Patients With Peripheral Neuropathic Pain: A 10-Week, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Clinical Therapeutics 32: 2370-2385, 2010
33. Nakipoglu-Yuzer GF, Atci N, Ozgirgin N: Neuropathic pain in spinal cord injury. Pain Physician 16: 259-264, 2013
34. New PW, Rawicki HB, Bailey MJ: Nontraumatic spinal cord injury: demographic characteristics and complications. Arch Phys Med Rehabil 83:
996-33 1001, 2002
35. Norrbrink Budh C, Lund I, Ertzgaard P, Holtz A, Hultling C, Levi R, Werhagen L, Lundeberg T: Pain in a Swedish spinal cord injury population. Clin Rehabil 17: 685-690, 2003a
36. Norrbrink Budh C, Lund I, Hultling C, Levi R, Werhagen L, Ertzgaard P, Lundeberg T: Gender related differences in pain in spinal cord injured individuals. Spinal Cord 41: 122-128, 2003b
37. Oh S, Min K, Park D: A Study on the Standardization of the Hospital Anxiety and Depression Scale for Koreans: A Comparison of Normal, Depressed and Anxious Groups. J Korean Neuropsychiatr Assoc. 38: 289-296, 1999
38. Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA: Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology 68: 1474-1480, 2007
39. Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, Naismith RT: Association of neuromyelitis optica with severe and intractable pain. Arch Neurol 69: 1482-1487, 2012
40. Ravenscroft A, Ahmed YS, Burnside IG: Chronic pain after SCI. A patient survey. Spinal Cord 38: 611-614, 2000
41. Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ: A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103: 249-257, 2003
42. Siddall PJ, Taylor DA, Cousins MJ: Classification of pain following spinal cord injury. Spinal Cord 35: 69-75, 1997
43. Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ: Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 81: 187-197, 1999
44. Smith BH, Torrance N: Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 16: 191-198, 2012
45. Smith BH, Torrance N, Bennett MI, Lee AJ: Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J
34
Pain 23: 143-149, 2007
46. Solaro C, Brichetto G, Amato MP, Cocco E, Colombo B, D'Aleo G, Gasperini C, Ghezzi A, Martinelli V, Milanese C, Patti F, Trojano M, Verdun E, Mancardi GL, Pa IMSSG: The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology 63: 919-921, 2004
47. Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, Aldinger W, Walker N, Zimmermann M, Paeslack V: Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord 35: 446-455, 1997
48. Ullrich PM, Jensen MP, Loeser JD, Cardenas DD: Pain intensity, pain interference and characteristics of spinal cord injury. Spinal Cord 46: 451-455, 2008
49. Washington LL, Gibson SJ, Helme RD: Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain 89: 89-96, 2000
50. Werhagen L, Budh CN, Hultling C, Molander C: Neuropathic pain after traumatic spinal cord injury--relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord 42: 665-673, 2004
51. Werhagen L, Hultling C, Molander C: The prevalence of neuropathic pain after non-traumatic spinal cord lesion. Spinal Cord 45: 609-615, 2007
52. White CL, LeFort SM, Amsel R, Jeans ME: Predictors of the development of chronic pain. Res Nurs Health 20: 309-318, 1997
53. Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP: Relationships among clinical characteristics of chronic pain after spinal cord injury. Arch Phys Med Rehabil 82: 1191-1197, 2001
54. Widerstrom-Noga EG, Turk DC: Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord 41: 600-609, 2003
55. Wingerchuk D, Lennon V, Pittock S, Lucchinetti C, Weinshenker B: Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485-1489, 2006
35
56. Wollaars MM, Post MW, van Asbeck FW, Brand N: Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain 23: 383-391, 2007
57. Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain 152: S2-15, 2011
58. Wu WT, Huang YH, Chen DC, Huang YH, Chou LW: Effective management of intractable neuropathic pain using an intrathecal morphine pump in a patient with acute transverse myelitis. Neuropsychiatr Dis Treat 9: 1023-1028, 2013
59. Yamashita T, Takahashi K, Yonenobu K, Kikuchi S: Prevalence of neuropathic pain in cases with chronic pain related to spinal disorders. J Orthop Sci 19: 15-21, 2014
60. Yap EC, Tow A, Menon EB, Chan KF, Kong KH: Pain during in-patient rehabilitation after traumatic spinal cord injury. Int J Rehabil Res 26: 137-140, 2003
61. Yucel A, Senocak M, Kocasoy Orhan E, Cimen A, Ertas M: Results of the Leeds assessment of neuropathic symptoms and signs pain scale in Turkey: a validation study. J Pain 5: 427-432, 2004
62. Yun D, Oh J, Kim B, Lim J, Bae J, Jeong D, Joo I, Park M, Kim B: Development of Korean Neuropathic Pain Questionnaire for Neuropathic Pain Screening and Grading: A Pilot Study. J Korean Neurol Assoc 30, 2012
63. Zigmond A, Snaith R: The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370, 1983
36 - 국문요약 –
비외상성비압박성척수병에서만성통증의양상및
신경병성통증발생의예측인자
아주대학교대학원의학과
엄영인
(지도교수: 주인수)
서론:만성통증은척수병이후에발생하는흔하고심각한후유증으로알려져있다. 본 연구에서는신경과외래에서추적관찰중인척수병환자를대상으로만성통증을조사 하였고, 신경병성통증발생의예측인자에대해알아보고자하였다. 연구대상및방법: 2014년 3월부터 9월까지아주대병원신경과외래를방문한환자중 비외상성비압박성척수병이있는환자를대상으로하였다. 모든환자들은척수병발생 후 6개월이상지난상태였으며, 인지기능장애가있거나다른원인에의한통증이있는 자는제외하였다. 모든환자들에대하여신경과의사가설문지를통하여통증및삶의질 에대한평가를시행하였다. 통증에대한설문지로는 Short Form McGill Pain Questionnaire (SF-MPQ) 와 Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) 를사용하였고, 삶의질평가를위해서는 Form 36-item (SF-36) health survey 가이용되었다.결과:총 54명의환자중 48명이통증을호소하였다. 통증이있는환자들을대상으로 분석하였을때통증의발생시기가질병발생후 3개월이내인경우가41명(85.4%)였다. 평균통증지속기간은41 (3.4-166)달이였고, 평균 SF-MPQ 점수는10 (1-34)점이였 다. 72.9% 의환자들이하루종일통증이지속된다고하였으며, 가장흔한통증의표현
37 은무기력한, 성가신, 묵직한순이었다.LANSS 12점을기준으로나눴을때신경병성 통증을가지고있는환자는16명으로33.3%였다. 이러한환자들은통각통증을가진환 자군에비하여발병연령이어리고(39.1±12.5 vs 49.8±9.3, p=0.002),더높은 SF-MPQ 점수를보였다(8 vs 17.5, p=0.004). 로지스틱회귀분석상 40세이하의나이와비특발 성원인이신경병성통증의발생에독립적인예후인자로밝혀졌다. 또한 SF-MPQ, LANSS 점수모두삶의질을평가하는지표인 SF-36 점수와밀접한관련성을보였다. 치료에의해통증이호전되는경우는주로여성, 원인이밝혀진경우, 혹은긴병변(>척 수 3분절)이었고당뇨가있으면반응이좋지않았다. 결론:만성통증은외상성척수손상뿐아니라비외상성, 비압박성척수병환자에서도 흔하게발생하는심각한문제이며삶의질에큰영향을끼친다. 그중신경병성통증의발 생에는발병나이와발생원인이관련되어있는것으로확인되었다.향후이러한환자에 서통증의양상및기전, 더나아가치료에대한추가연구가필요할것으로생각된다.