이
이
이학
학
학 박
박
박사
사학
사
학
학위
위
위 논
논
논문
문
문
C
C
Ce
e
el
l
ll
ld
l
d
de
e
ea
a
at
t
th
h
ha
a
an
n
nd
d
dP
Pr
P
r
ro
o
o-
-
-i
i
in
nf
n
f
fl
l
la
a
am
m
mm
m
ma
a
at
t
to
o
or
ry
r
y
yC
C
Cy
y
yt
t
to
o
ok
k
ki
i
in
n
ne
e
e
R
R
Re
e
el
l
le
e
ea
a
as
s
se
eo
e
o
of
f
fR
R
Ra
a
at
t
tM
M
Mi
i
ic
c
cr
r
ro
o
og
g
gl
l
li
i
ia
a
al
l
lC
C
Ce
e
el
l
ll
l
ls
s
sb
b
by
y
y
N
N
Na
a
ae
e
eg
gl
g
l
le
e
er
r
ri
i
ia
a
af
f
f
o
o
ow
w
wl
l
le
e
er
r
ri
i
i
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
의
의
의 학
학
학 과
과
과
박
박
박 창
창
창 은
은
은
C
C
Ce
e
el
l
ll
ld
l
d
de
e
ea
a
at
t
th
h
ha
a
an
n
nd
d
dP
Pr
P
r
ro
o
o-
-
-i
i
in
nf
n
f
fl
l
la
a
am
m
mm
m
ma
a
at
t
to
o
or
r
ry
y
yC
C
Cy
y
yt
t
to
ok
o
k
ki
i
in
n
ne
e
e
R
R
Re
e
el
l
le
e
ea
a
as
s
se
eo
e
o
of
f
fR
R
Ra
a
at
t
tM
M
Mi
i
ic
c
cr
r
ro
o
og
g
gl
l
li
i
ia
a
al
lC
l
C
Ce
e
el
l
ll
l
ls
s
sb
b
by
y
y
N
N
Na
a
ae
e
eg
g
gl
l
le
e
er
r
ri
i
ia
a
af
f
f
o
o
ow
w
wl
l
le
e
er
r
ri
i
i
by
Chang-Eun Park
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
DOCTOR
DOCTOR
DOCTOR
DOCTOR OF
OF
OF
OF PHILOSOPHY(SCIENCE)
PHILOSOPHY(SCIENCE)
PHILOSOPHY(SCIENCE)
PHILOSOPHY(SCIENCE)
Supervised by
Ho-Joon Shin, Ph.D.
Department
Department
Department
Department of
of
of
of Medical
Medical
Medical Sciences
Medical
Sciences
Sciences
Sciences
The
The
The
The Graduate
Graduate
Graduate
Graduate School,
School,
School,
School, Ajou
Ajou
Ajou
Ajou University
University
University
University
August,
August,
August,
박
박
박창
창
창은
은
은의
의 이
의
이
이학
학
학 박
박
박사
사
사학
학
학위
위
위 논
논
논문
문
문을
을
을 인
인
인준
준
준함
함
함.
.
.
심
심
심사
사
사위
위
위원
원
원장
장
장
박
박
박
선
선
선
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
안 명
안
안
명
명 희
희
희
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
신
신
신 호
호
호 준
준
준
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
김
김
김 경
경
경 민
민
민
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
임
임
임 경
경
경 일
일
일
인
인
인
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
2
2
20
0
00
0
08
8
8년
년
년 6
6
6월
월
월 2
2
23
3
3일
일
일
A
A
AC
C
CK
K
KN
N
NO
O
OW
W
WL
L
LE
ED
E
D
DG
G
GE
E
EM
M
ME
E
EN
N
NT
T
T
대학원의 수학 과정 동안 많은 것을 깨닫고 느꼈습니다.우선 사람은 혼자 살아가는 것이 아니라는 점을 간절히 느꼈습니다.앞으로는 이점을 깊이 간직하고 베풀어가면서 살아야겠 다는 마음을 다시 잡아봅니다.학위동안에 세세하게 여러 모로 힘이 되어주시고 챙겨주신 신호준 교수님,논문지도에 열성을 보여주신,박선 교수님,김경민 교수님,안명희 교수님, 임경일 교수님께 모두 머리 숙여 깊은 감사의 인사드립니다. 한편 인생의 많은 이정표가 되어주시는 홍성노 교수님,정하승 교수님,김대식 교수님,조 윤경 교수님 모두 감사의 인사를 드립니다.또한 자주 찾아뵙지는 못하지만 인생의 전환기 에 많은 도움을 주시고 계신 최돈찬 교수님,김세화 교수님,이웅빈 교수님,김판기 교수님, 최성부 교수님 감사의 인사를 드립니다. 서라벌대 임상병리과를 위해 많이 힘써주시는 정옥교 학장님,동경이 연구를 함께하는 최 석규 교수님,성기창 교수님,이은우 교수님,박순태 교수님께도 감사드립니다. 그리고 항상 저의 주변에서 아껴주시는 지영진 교수님,박진건 교수님,백형기 교수님,김 대중 실장님,박동엽 실장님,변영길 부장님,김주원 교수님,정종현 교수님,권수자 교수님, 김동현 교수님,민만기 교수님 모두 감사드립니다. 고마움을 주시고 도움을 주시는 많은 분들을 다 언급할 수 없지만 아무쪼록 감사의 인사를 드립니다. 이 자리에 오기까지는 무엇보다도 가족들의 이해와 협조가 없었으면 힘들었을 것입니다. 많은 뒷받침에 고마운 마음을 전합니다.언제나 저의 편이 되어주시는 어머니 윤영희 여사 님께도 진심으로 머리 숙여 감사 인사드립니다.묵묵히 뒤에서 지켜봐주고 힘이 되어주는 내 인생의 영원한 반려자,수빈이 엄마인 정숙이에게도 고마운 마음을 전합니다.함께하는 시간이 적어서 늘 미안한 마음뿐인 우리 딸,수빈이에게도 고마움을 전합니다. 마지막으로 학위논문을 받을 수 있도록 도와주신 모든 분들께 감사 인사드리며,내가 이 세상에서 살아 갈수 있도록 해주신 하늘나라에 계시는 아버님,어머님께 감사드립니다.-
ABSTRACT -
Cell Death and Pro-inflammatory Cytokine Release of Rat
Microglial Cells by Naegleria fowleri
Naegleria fowleri, a free-living amoeba, causes fatal primary amoebic meningoencephalitis in humans and experimental animals. In previous study, N. fowleri trophozoites showed the highly cytopathic activity and cytotoxicity against rat microglial cells by the morphological observation and 51Cr release assay.
In the present study, to determine whether a pathogenic N. fowleri lysate shows the cytopathic effects against primary cultured rat microglial cells, the morphological changes of microglial cells was observed by a light, scanning and transmission electron microscopes. And then, the cytotoxicity of N. fowleri lysate against rat microglial cells was also observed by 51Cr release assay. In addition, the pro-inflammatory cytokine release from microglial cells in co-culture system was estimated.
As results with a light and electron microscopes, most of microglial cells were severely destroyed by N. fowleri lysate, showing the necrotic (above 85%) and apoptotic cell death (below 15%) in a time- and dose dependent manner. As the results of 51Cr release assay, the cytotoxicity of N. fowleri lysate against microglial cells were 14.6, 21.9, 38.5 and 71.5% at 3, 6, 12 and 24 h post incubation, respectively.
And then, the amount of cytokines released from microglial cells in co-culture system at 3, 6 and 12 hr were 121.6, 90.4 and 81.0 pg/ml of TNF-α, 88.5, 92.7 and 190.1 pg/ml of IL-1β, and 298.8, 414.9 and 251.1 pg/ml of IL-6, respectively.
Keywords: Naegleria fowleri, amoebic meningoencephalitis, microglial cells,
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FIGURES ··· ⅴ LIST OF TABLES ··· ⅵ ABBREVIATION ··· ⅶ Ⅰ. INTRODUCTION ··· 1 A. NAEGLERIA FOWLERI ··· 1 1. Life cycle ··· 1 2. Incidence ··· 2 3. Symptoms ··· 24. Association with Water ··· 3
5. Pathogenesis ··· 4
B. Background ··· 5
C. Purpose ··· 8
Ⅱ. MATERIALS AND METHODS··· 9
A.
Amoeba and lysate
··· 9B.
Preparation of microglial cells
··· 9C.
Light microscopy
··· 10D.
Scanning electron microscopy
··· 10F. DNA fragmentation
··· 11G. Flow cytometry analysis ··· 12
H.
In vitro cytotoxicity by chromium release assay
··· 13I. ELISA for m
easurement of pro-inflammatory cytokines
··· 14Ⅲ. RESULTS ··· 15
A. Cytopathic changes of microglial cells by a light microscope
··· 15B. SEM and TEM findings for cytopathic changes of microglial cells
··· 19C. Apoptotic process of microglial cells by N. fowleri lysate
··· 22D. Cytotoxicity of N. fowleri lysate on microglial cells
··· 25E. Cytokines released from microglial cells treated with N. fowleri lysate
· 27 Ⅳ. DISCUSSION ··· 29Ⅴ. CONCLUSION ··· 35
REFERENCES ··· 36
LIST OF FIGURES
Fig. 1. Primary cultured rat microglial cells ··· 16
Fig. 2. Light microscopic findings of microglial cells treated with the N. fowleri lysate in a dose-dependent manner ··· 17
Fig. 3. Light microscopic findings of microglial cells treated with the N. fowleri lysate in a time-dependent manner ··· 18
Fig. 4. SEM and TEM findings of microglial cells ··· 20
Fig. 5. TEM and SEM findings of microglial cells treated with N. fowleri lysate ··· 21
Fig. 6. DNA fragmentation of microglial cells treated with N. fowleri lysate ··· 23
Fig. 7. FACS analysis of microglial cells stained with PI and Anexin-V in treated with N. fowleri lysate ··· 24
Fig. 8. Amounts of TNF-α , IL-1β and IL-6 secreted from microglial cells treated with N. fowleri lysate for 3, 6 and 12 h were determined by ELISA ··· 28
LIST OF TABLES
Table 1. The cytotoxicity of N. fowleri lysate against microlglial cells by 51Cr
ABBREVIATION
◆ anti-Nfa1 antibody: anti-Naegleri Fowleri 1 antibody ◆ CNS: Central Nervous System
◆ CPE : CytoPathic Effect
◆ DMEM: Dulbecco's Modified Eagle's Medium
◆ ED 1 antibody: FITC-conjugated mouse anti-rat CD68 ◆ ELISA: Enzyme-Linked Immunosorbent Assay
◆ FACS: Fluorescence Activated Cell Sorter ◆ FBS: Fetal Bovine Serum
◆ IL-1: Interleukin-l ◆ IL-1β: Interleukin-1 beta ◆ IL-6: Interleukin-6 ◆ LPS: Lipopolysaccharide
◆ PAME: Primary Amoeba MeningoEncephalitis ◆ PBS : Phosphate Buffered Saline
◆ PI : Propidium Iodide
◆ SEM: Scanning Electron Microscopes ◆ TEM: Transmission Electron Microscopes ◆ TNF-α: Tumor Necrosis Factor alpha
I. INTRODUCTION
A. NAEGLERIA FOWLERI
1. Life Cycle
Naegleria fowleri are three stages to the life cycle: the trophozoite, the flagellate, and the
cyst (Visvesvara, 1993). Trophozoites are active and usually elongated with broadly rounded processes called lobopodia. Their cytoplasm is granular and contains vacuoles, and they feed on bacteria such as Escherichia coli (Martinez and Visvesvara, 1991; Martinez, 1993; Visvesvara, 1993). The flagellate stage is pear shaped and motile and eventually reverts to the trophic stage (Martinez and Visvesvara, 1991; Bottone, 1993; Martinez, 1993; Visvesvara, 1993).
Cysts are usually spherical, smooth, double walled, and refractile, measuring about 10 mm in diameter (Martinez and Visvesvara, 1991; Visvesvara, 1993). Adverse environmental conditions cause the organisms to encyst (Martinez, 1993). The portals of entry for human infection are the olfactory neuroepithelium and nasal passages, which are usually exposed to the flagellate stage during periods of swimming or bathing in hot baths or hot springs (Martinez, 1993; Visvesvara, 1993; Kilvington and Beeching, 1995).
Infection can also occur by breathing infectious cysts present in dust or soil particles (Martinez and Visvesvara, 1991; Bottone, 1993; Martinez, 1993; Visvesvara, 1993). Once the organism has been inhaled, excystation occurs and the trophozoite penetrates the nasopharyngeal mucosa, migrates to the olfactory nerves, and invades the brain through the
cribriform plate (Bottone, 1993).
2. Incidence
While there are six species in the genus, N. fowleri is the primary human pathogen, producing primary amebic meningoencephalitis (PAME), a rapidly progressing meningoencephalitis which is almost always fatal. N. australiensis may be pathogenic to a lesser extent than N. fowleri (Martinez, 1993; Visvesvara, 1993; Kilvington and Beeching, 1995).
In 1997 years, there have been more than 192 reported cases of disease worldwide and more than 64 cases in the United States (Marshall et al., 1997). While the numbers of cases may appear low, exposure to the organisms may be relatively common since antibodies to
Naegleria spp. are widespread in human sera (Bottone, 1993).
3. Symptoms
No predisposing factors are necessary for human infections to occur (Martinez, 1993; Visvesvara, 1993). After a 2- to 7-day incubation period, the symptoms of PAME are evident. Onset is abrupt, with rapidly progressive headaches, fever, nausea, vomiting, pharyngitis, and nasal obstruction or discharge (Martinez and Visvesvara, 1991; Martinez, 1993). As the symptoms persist, lethargy, confusion, and stiff neck develop. Convulsions may also occur, with progressive deterioration to coma and death within 1 to 14 days. The mean time interval from onset to death is 6.4 days (Ma et al., 1990; Visvesvara and Stehr-Green, 1990; Martinez, 1993; Visvesvara, 1993; Kilvington and Beeching, 1995).
Other symptoms include abnormalities of taste and smell; seizures; cerebellar ataxia; nuchal rigidity; photophobia; palsies of the third, fourth, and sixth cranial nerves; and increased intracranial pressure. Cardiac abnormalities may also develop (Ma et al., 1990). Subclinical infections are possible in healthy people when these protozoa colonize the nose and throat (Ma et al., 1990; Martinez and Visvesvara, 1991).
4. Association with water
The preferred environment for N. fowleri is the soil; however, heavy rains and runoffs introduce this organism into lakes, ponds, and surface waters (Marciano-Cabral, 1988; Elder et al., 1994). Naegleria spp. Are distributed worldwide in thermally polluted streams, and they tolerate temperatures of 40 to 458℃ (Bottone, 1993; Martinez, 1993; Sparagano, 1993; Visvesvara, 1993). They can also be found in coastal water, freshwater, sewage, heating and ventilation units, poorly chlorinated swimming pools, artificial lakes, and warm water near discharge outlets of power plants (Martinez, 1993).
In Australia, one fatal case of PAME led to the detection of Naegleria spp. in the household water supply. This case emphasizes that PAME may be associated with washing and bathing as well as with swimming (Marciano-Cabral, 1988). Vertical distribution in freshwater has been correlated with physical, chemical, and biological parameters. Significant numbers of Naegleria spp. were found in water layers containing filamentous cyanobacteria and eubacteria, which serve as food sources.
In addition, large numbers of organisms have been isolated from water with increased iron and manganese concentrations. As expected, Naegleria spp. was found in increased
numbers in waters contaminated with coliforms. In addition, some species of Naegleria interact with Legionella spp. and are thought to play a possible role in the dissemination of
Legionella in water (Ma et al., 1990).
5. Pathogenesis
Naegleria species typically cause PAME in children and healthy adults who have been
swimming in polluted pond water or inadequately chlorinated swimming pools, as well as man-made or natural freshwater lakes. Organisms enter through the olfactory neuroepithelium at the level of the cribriform plate and invade the amyelinic submucosal nervous plexus (Culbertson, 1971, Carter, 1972). Symptoms begin after a 3-7 day incubation period, and infections caused by N. fowleri tend to be fulminant, with rapid progression to death in most cases (John, 1993). Pathological changes include acute hemorrhagic necrotizing meningoencephalitis with purulent exudates in the brain, brainstem and cerebellum (John, 1993). Patients who develop N. fowleri meningoencephalitis may have an immunoglobulin IgA deficiency, which would imply weaker defenses at the mucus membrane level (Reilly, 1983).
Acanthamoeba and Hartmanella are similar organisms that cause a more subacute form
of illness in immunocompromised, debilitated or malnourished individuals, including those undergoing suppressive therapy for organ transplant and HIV/AIDS patients (Gonzalez, 1986). These organisms enter the body through the respiratory tract or skin ulcerations, reaching the central nervous system (CNS) by hematogenous spread (Martinez and Visvesvara, 1997), causing a patchy, chronic granulomatous encephalitis with trophozoites
and cysts in the lesions. The incubation period is unknown but is thought to be more than 10 days (Gonzalez, 1986).
B. Background
Pathogenic N. fowleri, a free-living amoeba found in widespread environment, causes fatal PAME in experimental animal and humans. PAME occurs most commonly in healthy, young adults and non-immunocompromised children. It has been associated with swimming or bathing in contaminated warm waters (John, 1982; Im and Shin, 2004; Schuster and Visvesvara, 2004). The infection results from the introduction of water which contains amoebae into the nasal cavity and the subsequent passage of these organisms to the CNS via the olfactory apparatus (Ma et al., 1990; Carter, 2001).
CNS inflammation occurs in both disease and trauma, and is mediated in part by microglial cell, the resident immune cells of the CNS. Microglial cells originate from bone marrow and migrate into the CNS during early stages of development (Shuman et al., 1997). Microglial cell display graded levels of activation in the CNS, from resting, highly ramified microglial cell, to phagocytic macrophages (Streit et al., 1988).
microglial cell react quickly in response to CNS injury or disease (Kreutzberg, 1996), migrating into an injury site (Carbonell et al., 2005) and secreting a wide array of molecules that can be toxic to oligodendrocyte progenitor cells (OPCs) and oligodendrocytes, including tumor necrosis factor-α (TNF-α) (Selmaj and Raine, 1988; Dasgupta et al., 2003; Jana et al., 2003), glutamate (Nakamura et al., 2003), and free radicals (Benveniste, 1997). Furthermore,
molecules that induce oligodendrocyte death can also lead to microglial cell activation, such as glutamate (Christensen, 2006) and proinflammatory cytokines (Thery and Mallat, 1993).
In vitro, microglial cell is capable of inducing OPC death even without the two cell populations being in direct contact (Li et al., 2005). However, in vivo microglial cell has been observed in close proximity to dying oligodendrocytes after spinal cord injury (Shuman et al., 1997). This proximity after injury suggests a mechanism by which microglial cell may influence oligodendrocyte and OPC survival, as it has been shown in vitro that microglial cell in contact with oligodendrocytes can induce oligodendrocyte death via membrane-bound TNF-α which is more potent than soluble TNF-α (Zajicek et al., 1992). Additionally, any soluble factors secreted by microglial cell could have a higher effective concentration if secreted into a small space between cells.
Additionally, cytokines produced by microglial cell may aid in repair after injury, as mice lacking TNF-α undergo delayed remyelination (Arnett et al., 2004). Even the observations of Shuman and colleagues (Shuman et al., 1997) that activated microglial cells are found in contact with apoptotic oligodendrocytes after spinal cord injury, raises the question of whether microglial cell destroy oligodendrocytes that would otherwise survive after injury, or are simply phagocytosing oligodendrocytes already destroyed by other toxins in the damaged CNS. Some data suggest that microglial cell play a dual role in CNS injury, exacerbating damage in some instances or at some times, and promoting repair or regeneration at others (Popovich et al., 2002). Shuman and colleagues (Shuman et al., 1997) also reported that microglial cell undergo apoptosis after spinal cord injury.
important step in the mechanism of pathogenicity of N. fowleri, and a specific pseudopodia projection, called an amoebastome, is formed. Invasive amoeba capable of entering the nervous system usually digests neuronal tissue and other mammalian cells by effective cytolysis and phagocytosis, as observed in culture or in infected sections of brain tissue (Martinez, 1985; Marciano-Cabral, 1988).
Rat microglial cells exist in three morphological forms following cell differentiation, i.e., an amoeboid form during embryogenesis, a ramified shape in the mature brain, and a rod-shaped morphology around inflammatory lesions in the CNS (Giulian and Baker, 1986; Suzumura et al., 1991). Moreover, they function as phagocytotic cells and produce cytokines such as interleukin-l (IL-1), IL-6, and TNF-α (Chao et al., 1994; Oh et al., 2005). Thus, it has been suggested that microglial cells play important roles as inflammatory cells or as immunoregulatory cells in the protective immune system of the CNS (Suzumura et al., 1993).
In the previous study, it was seen that N. fowleri trophozoites in contact with microglial cells produced vigorous pseudopodia and a food-cup structure. Microglial cells were destroyed by N. fowleri trophozoites as seen from necrotic and apoptotic cell death in a time-dependent manner (Oh et al., 2005). As the results of 51Cr release assay, N. fowleri showed increasing cytotoxicity against microglial cells in a culture-time dependent manner.
And then, microglial cells co-cultured with N. fowleri trophozoites secreted the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 (Oh et al., 2005). In addition, When an anti-Nfa1 antibody which was made with a recombinant anti-Nfa1 protein expressed nfa1 gene cloned from a cDNA library of pathogenic N. fowleri was treated in a co-culture system, N. fowleri showed decreasing cytotoxicity against microglial cells, and the secretion of TNF-α from
microglial cells was inhibited (Cho et al., 2003; Jeong et al., 2004; Oh et al., 2005). A few attempts to study the cytopathic effects of pathogenic N. fowleri lysate against actual target cells of PAME, rat microglial cells, have been poorly reported.
C. Purpose
To determine whether pathogenic N. fowleri lysate showed cytopathic effects against primary cultured rat microglial cells, the morphological changes of microglial cells cultured with N. fowleri lysate were observed with light, scanning and transmission electron microscopes (SEM and TEM). The in vitro cytotoxicity of N. fowleri lysate against rat microglial cells was also subsequently observed. Additionally, the pro-inflammatory cytokines that are released from microglial cells in a culture system were estimated.
II. MATERIALS AND METHODS
A. Amoeba and lysate
The trophozoites of N. fowleri (Cater NF69 strain, ATCC NO. 30215) were axenically cultured at 37°C in Nelson's medium (Shin et al., 2001). Amoebic lysate was prepared according to the previous paper (Kang et al., 2005). After freezing and thawing of trophozoites, the soluble proteins (whole lysate) was filtered with 0.22-㎛-pore-size disk filters, and the protein concentration was determined by the method of Bradford assay (Kang et al., 2005).
B. Preparation of microglial cells
Rat microglial cells were prepared using a modified method of Giulian and Baker (Giulian and Baker, 1986). Mainly, the cortexes of the brain were obtained from newborn rats (Sprague-Dawley, purchased from KIST in Daejeon, Korea) and homogenized by pumping with 21-gauge syringe. The mixture was centrifuged at 300 × g for 5 min and suspended in Dulbecco's modified Eagle's medium (DMEM; Sigma) with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. The suspension was put into 75 cm3 tissue culture flasks. The flasks were then incubated for 14 days at 37°C, 5% CO2 humidified atmosphere. Upon 14 days of culture, microglial cells were harvested by vigorous shaking of each culture flask. They were then filtered with nylon wool to remove
any remaining astrocytes and were centrifuged at 300 × g for 5 min. The pellets were suspended in DMEM with 10% FBS, and the mixtures were incubated at 37°C for 2 h. After the supernatant was removed, the attached microglial cells were harvested and counted at a concentration of 2 × 105 per well in a 24-well culture plate with subjected to subsequent experiments. The purity of the microglial cells was determined by indirect immunofluorescent staining with a FITC-conjugated mouse anti-rat CD68 (ED 1 antibody) (Serotec, Bicester, United Kingdom) to be nearly 95%, as shown (Fig. 1).
C. Light microscopy
Microglial cells (2 × 105) were cultured with N. fowleri lysate (0.1, 0.5 or 1 mg/ml) in 24-well culture plates for 3, 6 or 12 h. After co-incubation, samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and post-fixed with 1% osmium tetroxide-1.5% potassium for 1 h. The cells were then examined with a light microscope.
D. Scanning electron microscopy
Microglial cells (2 × 105) were seeded onto the Lab-tek II chamber slide system (Nunc A/S, Roskilde, Belgium) and the lysate of N. fowleri were added to the monolayer. After incubation for 3, 6 and 12 h, samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and were dehydrated with increasing concentrations of ethanol. Samples were vacuum-dried and coated with ultra-thin layers (300 Å) of gold/pt in an ion
sputter (E-1010, Hitachi, Tokyo, Japan). An image analyzer program (Escan 4000, Bumi-Mi Universe Co., Ltd., Ansan, Korea) was used to capture the images of cells and modified surfaces. Samples were characterized using an SEM (S-800, Hitachi, Tokyo, Japan).
E. Transmission electron microscopy
After microglial cells were incubated with amoebic lysate in 24-well culture plates for 3, 6, or 12 h. They were then fixed in modified Karnovsky's fixative solution in cacodylate buffer (pH 7.4) and post-fixed in 1% osmium tetroxide-1.5% potassium ferrocyanide. The cells were stained en bloc in 0.5% uranyl acetate, dehydrated through a graded ethanol series, and embedded in resin (Polyscience, Warrington, Pa.). Then, the blocks were sectioned with Ultrostain 1H and 2 (Leica, Vienna, Austria). Specimens were observed and photographed with a Zeiss EM 902A TEM (Leo, Oberkohen, Germany).
F. DNA fragmentation
DNA extractions and agarose-gel electrophoresis were performed to observe DNA fragmentation of microglial cells treated with N. fowler lysate. Microglial cells were harvested by sterile cell scrapers after cultivation with an amoeba lysate (1 mg/ml) for 3, 6, 12 and 24 h. Then, microglial cells were washed with phosphate-buffered saline (PBS) (pH 7.4) and suspended in 0.5 ml of TBE buffer (45 mM Tris-borate buffer, 1 mM EDTA [pH 8.0]) containing 0.25% Nonidet P-40 and 1 mg of RNase A per ml. After the mixture was incubated at 37°C for 30 min, 1 mg of proteinase K per ml was added. The mixture was
incubated at 37°C for 30 min and resuspended in 0.1 ml of loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF, 30% glycerol). The suspended volume of 25 ml was put on 1.5% agarose gel containing 10 ml of ethidium bromide per ml. Electrophoresis was carried out at 2 V/cm for 6 h. A 123-bp DNA ladder and PCR marker containing fragments of 1,000, 750, 500, 300, 150, and 50 bp (Promega Corporation, Madison, Wis.) were used as molecular size standards.
G. Flow cytometry analysis
The apoptotic cell death from microglial cells treated with N. fowleri lysate were measured with the PI and Annexin V end labeling assay (Promega Corporation). Briefly, microglial cells cultured with 1 mg/ml of each amoeba lysate for 6 or 18 h were harvested and washed twice with PBS (pH 7.4). The cells were resuspended in 0.5 ml of PBS. The cells were fixed by adding 5 ml of 1% methanol-free formaldehyde for 20 min on ice, centrifuged, and resuspended in 0.5 ml of PBS. The cell suspension was mixed with 5 ml of 70% ice-cold ethanol and kept at - 20°C for 4 h. The mixture was centrifuged and resuspended in 1 ml of PBS. The suspended cells were adjusted to a concentration of 2 × 106 and transferred into a 1.5-ml microcentrifuge tube. After centrifugation, cells were resuspended in 80 ml of equilibration buffer (200 mM potassium cacodylate, 24 mM Tris-HCl, 0.2 mM dithiothreitol, 0.25 mg of bovine serum albumin/ml, 2.5 mM cobalt chloride [pH 6.6]). After centrifugation, the cells were resuspended in 50 ml of Annexin V incubation buffer. The suspended cells were incubated in a water bath for 60 min at 37°C. The reaction was terminated by adding 1 ml of 20 mM EDTA. Following centrifugation after the reaction,
the pelleted cells were resuspended in 0.5 ml of PBS containing Triton X-100 and 5 mg of bovine serum albumin/ml. The cells were washed twice with PBS, centrifuged, and resuspended in 0.5 ml of propidium iodide (PI) solution (freshly diluted to 5 mg/ml in PBS) containing 250 mg of DNase-free RNase A. After the cells were incubated for 30 min in the dark, the green fluorescence of fluorescein-Annexin V at 520 nm and the red fluorescence of PI at 620 nm were measured by FACScan flow cytometry (Becton Dickinson, Paramus, N.J.).
H. In vitro cytotoxicity by chromium release assay
Using the methods of a previous study (Oh et al., 2005), a 51Cr (chromium) release assay was performed to determine the cytopathic effects of N. fowleri lysate. Target microglial cells were labeled with 100 µCi of [Na]2 51CrO4 per 105 cells for 60 min at 37°C. The cells were washed to remove unbound radioisotope. Labeled microglial cells (5 × 104) were added to each well of a 96-well culture plate. They were then cultured with N. fowleri lysate (0.1, 0.5 or 1 mg/ml) in 5% CO2 for 3, 6 or 12 h. Spontaneous release from labeled microglial cells was determined by acquiring the counts per min (cpm) in the supernatant fluid without amoebic lysate. All assays were performed in triplicate. At the end of the experimental incubation period, plates were centrifuged at 300 × g for 3 min and the supernatant from each well was harvested. For maximal release, each of these was lysed with 5% (vol/vol) Triton X-100 and harvested. Following 3, 6, or 12 h, the supernatant fluid and lysed cells were counted in a gamma counter. The percentage of the radioisotope released from target microglial cells was determined to be the index of lysis using the following formula:
experimental release – spontaneous release
Cytotoxicity (%) = ×××× 100 maximum release – spontaneous release
I. ELISA for measurement of pro-inflammatory cytokines
To determine whether microglial cells released the pro-inflammatory cytokines as a result of a cytopathic effect (CPE) induced by pathogenic N. fowleri lysate, the amounts of tumor necrosis factor (TNF)-α, Interleukin (IL)-1β, and IL-6 released from microglial cells in the co-culture system were measured using enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, California, USA). The amounts of cytokines produced by the microglial cells were estimated by generating a standard curve according to the instructions of the manufacturer.
III. RESULTS
A. Cytopathic changes of microglial cells by a light microscope
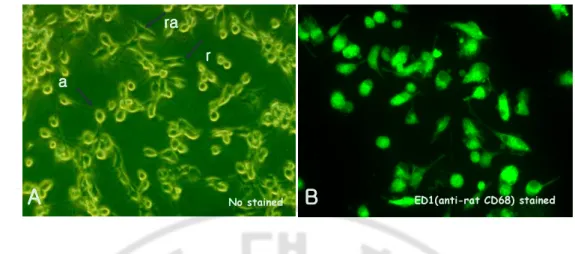
Light microscopic findings of primary cultured rat microglial cells showing three types; amoeboid form, rod form and rammified form (Fig. 1A). For the identification of microglial cells, fluorescent microscopic findings of microglial cells immunostained with a FITC conjugated mouse anti-rat CD68 was carried out (Fig. 1B).
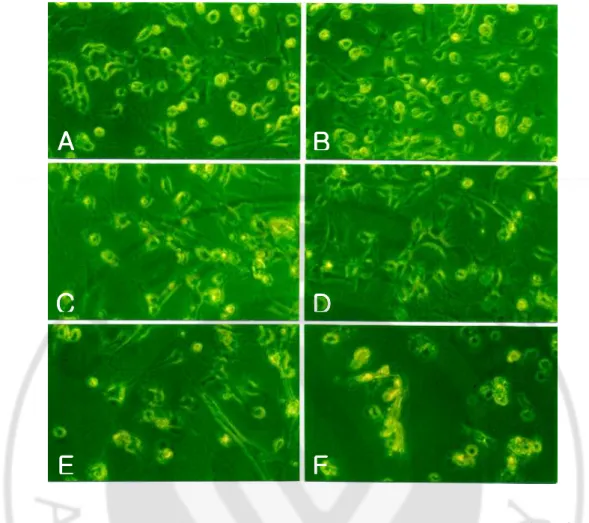
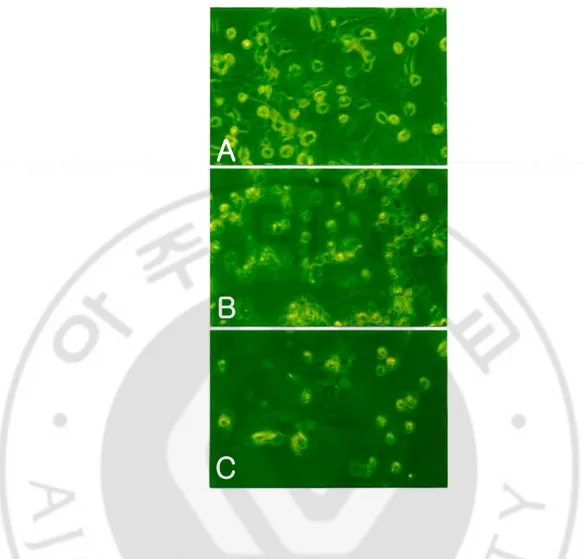
The microglial cells treated with N. fowleri lysate showed morphological changes such as the cell membrane destruction and reduction in number (Fig. 2). They were proceed in a dose- and time-dependent manner (Fig. 2, 3)
Fig. 1. Primary cultured rat microglial cells. A. Light microscopic findings of primary
cultured rat microglial cells showing three types; amoeboid form (a), rod form (r) and rammified form (ra). (x200). B. Fluorescent microscopic findings of microglial cells immunostained with a FITC conjugated mouse anti-rat CD68. (x400).
B
B
B
B
ED1(anti-rat CD68) stained a a a a ra ra ra ra r r r rA
A
A
A
No stainedB
B
B
B
B
B
B
B
ED1(anti-rat CD68) stainedED1(anti-rat CD68) staineda a a a ra ra ra ra r r r r
A
A
A
A
No stained a a a a ra ra ra ra r r r rA
A
A
A
No stainedFig. 2. Light microscopic findings of microglial cells treated with the Naegleria fowleri
lysate in a dose-dependent manner. Microglial cells were cultured for 12 h in DMEM
media only (A), treated with PBS (B) or LPS (C), and the lysate of N. fowleri (0.1, 0.5 and 1 mg/ml) (D, E and F), respectively. Microglial cells showed severe destruction in a dose-dependent manner. (×200).
A
A
A
A
C
C
C
C
B
B
B
B
D
D
D
D
E
E
E
E
F
F
F
F
Fig. 3. Light microscopic findings of microglial cells treated with the Naegleria fowleri
lysate in a time-dependent manner. Microglial cells were cultured in DMEM media only
(A), and treated with a lysate of N. fowleri (1 mg/ml) for 6 h(B) and 12 h (C). Microglial cells showed severe destruction in a time-dependent manner. (× 200).
A
A
A
A
B
B
B
B
C
C
C
C
A
A
A
A
B
B
B
B
C
C
C
C
B. SEM and TEM findings for cytopathic changes of microglial cells
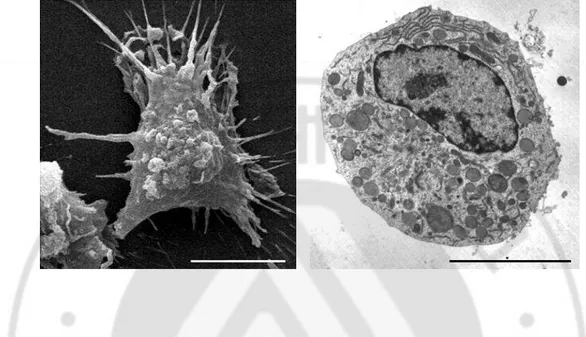
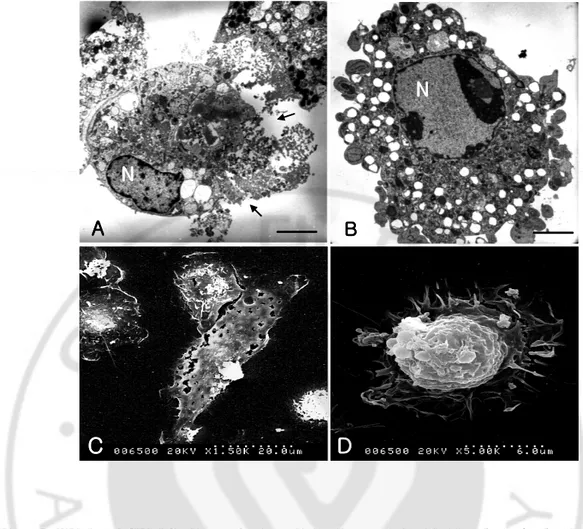
By SEM and TEM findings, a primary culture microglial cells show numerous cytoplasmic projections, and scattered chromatin materials in large nucleus were showed (Fig. 4).
In a co-culture system at 6 h, most of microglial cells were destroyed by N. fowleri lysate, which showed the necrotic process characterized by the swelling of the membrane and the bursting of the nuclear membrane and nucleus (Fig. 5A, C). And some of them showed an apoptotic feature such as blebing and chromatin condensation in nucleus (Fig. 5B, D).
Fig. 4. SEM and TEM findings of microglial cells. A. Microglial cell shows numerous
cytoplasmic projections by SEM. B. Scattered chromatin materials in large nucleus were showed by TEM. Bars, 5um.
Fig. 5. TEM and SEM findings of microglial cells treated with Naegleria fowleri lysate.
Microglial cells were cultured with N. fowleri lysate (1 mg/ml) for 12 hr. Microglial cells showed a necrotic process (A, C) and produced apoptotic bodies (B, D). Bars, 5 µm.
C
C
C
C
A
A
A
A
N
N
N
N
B
B
B
B
N
N
N
N
D
D
D
D
C
C
C
C
C
C
C
C
A
A
A
A
N
N
N
N
B
B
B
B
N
N
N
N
C
C
C
C
A
A
A
A
N
N
N
N
A
A
A
A
N
N
N
N
A
A
A
A
N
N
N
N
B
B
B
B
N
N
N
N
B
B
B
B
N
N
N
N
D
D
D
D
C
C
C
C
D
D
D
D
C
C
C
C
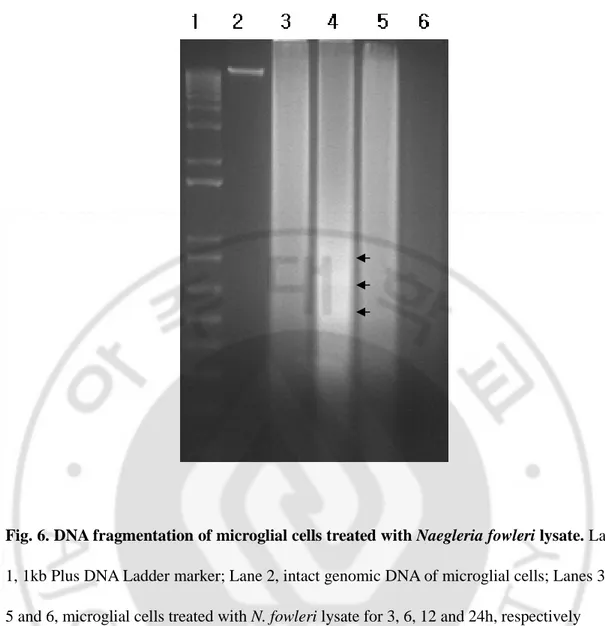
C. Apoptotic process of microglial cells by N. fowleri lysate
In the experiments to identify the apoptotic process of microglial cells by N. fowleri lysate, DNA fragmentation of microglial cells in a co-culture system was observed (Fig. 6). DNA fragmentation was not observed with total genomic DNA of microglial cells treated with PBS, but the development of DNA ladders was shown in microglial cells treated with N.
fowleri lysate (1 mg/ml) for 3, 6 and 12h, respectively.
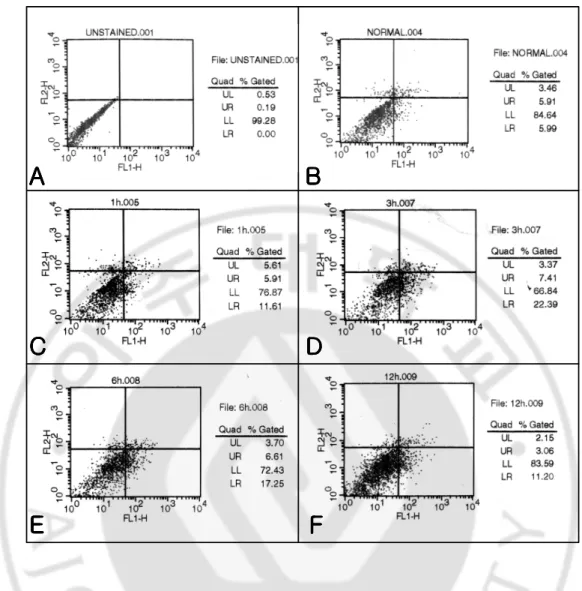
PI staining demonstrated that some microglial cells cultured with the N. fowleri lysate (1 mg/ml) for 6 h underwent apoptosis. Additionally, apoptotic cells stained with Annexin-V were observed about 22% at 3 h and 17% at 6 h post-incubation, as which showed increased intensity of Annexin-V stained apoptotic cells (Fig. 7D, E). By contrast, microglial cells cultured with N. fowleri lysate for 12 h did not show apparent changes in the intensity of fluorescence of the Annexin-V stained apoptotic cells (Fig. 7F).
Fig. 6. DNA fragmentation of microglial cells treated with Naegleria fowleri lysate. Lane
1, 1kb Plus DNA Ladder marker; Lane 2, intact genomic DNA of microglial cells; Lanes 3, 4, 5 and 6, microglial cells treated with N. fowleri lysate for 3, 6, 12 and 24h, respectively
Fig. 7. FACS analysis of microglial cells stained with PI and Annexin-V in treated with
Naegleria fowleri lysate. A and B, unstained and microglial cells only, respectively; C, D, E
and F, stained microglial cells cultured with N. fowleri lysate for 1, 3, 6 and 12 h, respectively. Maximum number of stained microglial cells showing the apoptotic process was observed at 3~6 hr post incubation.
A
A
A
A
B
B
B
B
C
C
C
C
E
E
E
E
D
D
D
D
F
F
F
F
A
A
A
A
B
B
B
B
C
C
C
C
E
E
E
E
D
D
D
D
F
F
F
F
D. Cytotoxicity of N. fowleri lysate on microglial cells
To determine whether N. fowleri lysate showed the cytotoxicity to microglial cells, 51
Cr release assay was carried out. When microglial cells were cultured with N. fowleri lysate (0.5 mg/ml), the cytotoxicity of amoeba on microglial cells was increased in a time-dependent manner, 7.9, 14.4, 23.8 and 51.5 % at 3, 6, 12, and 24 h, respectively (Table 1). When microglial cells were cultured with N. fowleri lysate (1 mg/ml), the cytotoxicity of amoeba on microglial cells was increased in a time-dependent manner, 14.6, 21.9, 38.5 and 71.5 % at 3, 6, 12, and 24 h, respectively (Table 1).
Table 1. The cytotoxicity of Naegleria fowleri lysate against microlglial cells by 51Cr release assay Times of co-cultivation Groups 3 h 6 h 12 h 24 h Microglia + lysate (0.5 mg/ml) 7.9%* 14.4% 23.8% 51.5% Microglia + lysate (1.0 mg/ml) 14.6% 21.9% 38.5% 71.5% *
% cytotoxicity calculated by the 51Cr amount released from radiolabelled microglial cells (mean ± standard variation)
E. Cytokines released from microglial cells treated with N. fowleri lysate
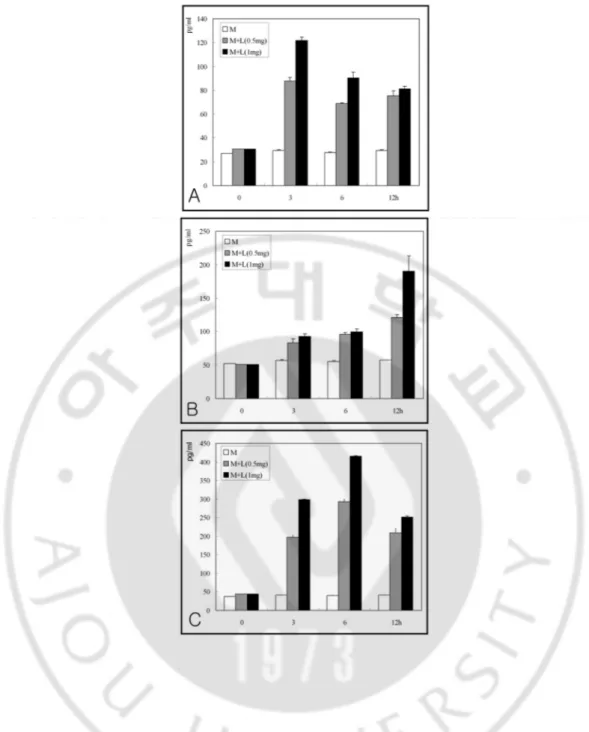
To determine whether microglial cells showed the release of cytokines induced by pathogenic N. fowleri lysate, pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, were measured with enzyme-linked immunosorbent assay kits. In a co-culture system with N.
fowleri lysate (1 mg/ml), the amount of TNF-α released from microglial cells was 121.6, 90.4 and 81.0 pg/ml at 3, 6 and 12 h, respectively, which was peaked at 3 h and decreased during the period of co-incubation (Fig. 8A). The amount of IL-1β was 88.5, 92.7 and 190.1 pg/ml at 3, 6 and 12 h, respectively, which was increased during the period of co-incubation (Fig. 8B). The amount of IL-6 was 298.8, 414.9 and 251.1 pg/ml at 3, 6 and 12 h, respectively, which was peaked at 6 h post co-incubation (Fig. 8C).
Fig. 8. Amounts of TNF-α (A), IL-1ββββ (B) and IL-6 (C) secreted from microglial cells treated with Naegleria fowleri lysate for 3, 6 and 12 h were determined by ELISA.
IV. DISCUSSION
N. fowleri is the principal protozoa, commonly referred to as pathogenic free-living
amoebae that cause PAM (Matinez, 1997; Parija and Jayakeerthee, 1999). PAME occurs very rarely but is usually fatal. Since the disease was first identified in 1965 (Fowler and Carter, 1965), fewer than 200 cases have been identified worldwide (Ockert, 1993; Matinez, 1997; Parija and Jayakeerthee, 1999).
N. fowleri are ubiquitous in the environment, in soil, water, and air (Rodriguez-Zaragoza,
1994). Infections in humans are acquired through water entering the nasal passages (usually during swimming) and by inhalation.
Most human victims of PAME are exposed to free-living amoebae while swimming in warm surface water. This may include ponds, lakes, streams, rivers, and improperly maintained swimming pools. The risk of acquiring PAME increases as water temperatures rise (Rodriguez-Zaragoza, 1994; Matinez, 1997). Transmission to humans occurs when the organism gains access to brain tissues through the nasal passages.
Invasion of the CNS and development of PAME are prominent features in fatal human and animal cases of N. fowleri infection. As the major route of invasion for N. fowleri infection, amoebas enter the nasal cavity, attach, and invade the nasal mucosa and olfactory nerve (Culbertson, 1971; Chang, 1979).
The organism can enter the nasal passages when water containing the organism is forced up the nose through activities such as diving, jumping into water, and underwater swimming. However, PAM is not transmitted from person to person (Ockert, 1993; Matinez, 1997;
Parija and Jayakeerthee, 1999).
N. fowleri, the causative agent of primary amoebic meningoencephalitis in humans, is
cytopathogenic for a variety of cultured mammalian cells (Chang, 1974; Visvesvara and Callaway, 1974; Brown, 1978; Cursons and Brown, 1978; Brown, 1979; Marciano-Cabral et al., 1982). The proposed mechanism(s) of the cytopathic action of this organism for mammalian cells include active phagocytosis of cells by pseudopod formation (Visvesvara and Callaway, 1974), trogocytosis or repeated nibbling by trophozoites (Brown, 1978; Brown, 1979), and secretion of cytolytic substances by N. fowleri (Chang, 1974). In the previous transmission electron microscopic study established that amoebae ingest portions of mammalian cells without cell lysis at 12 h of cocultivation with N. fowleri (Marciano-Cabral et al., 1982).
Several investigators have suggested that N. fowleri releases cytolytic substances, phospholipolytic enzymes and acid phosphatase. These substances account for the invasiveness and tissue damage in vivo and the cytopathogenicity in vitro (Feldman, 1977; Ferrante and Bates, 1988). It has been proposed that cytotoxic toxins and cytolytic proteases are influential in the destruction of target cells.
In another free-living amoeba, Acanthamoeba spp., its cytopathogenic effects on host cells require the adhesion of amoeba to the host cell (Moore et al., 1991; Yang et al., 1997; Shin et al., 2001), phagocytosis, and amoebic proteolytic enzymes, including serine proteases (Mitro et al., 1994), contact-dependent metalloproteases (Hadas and Mazur, 1993), elastases (Ferrante and Bates, 1988), cysteine proteases (Khan et al., 2000), and cytotoxic proteinases induced by mannose-mediated adhesion (Leher et al., 1988).
The lysate of N. fowleri has been observed to cause tissue destruction in vitro (Fulford et al., 1985; Marciano-Cabral and Fulford, 1986). Proteinases in N. fowleri could also be involved in tissue destruction and in its pathogenesis since this enzymes present in the cell lysate (Mat, 2004). The involvements of proteinases in the pathogenecity of some parasitic protozoa have been proven by Keene et al., (1990) and Robertson and Coombs (1992).
Proteinases in N. folweri observed in this study appear to be high molecular weight enzymes whereas proteinases reported from other protozoa generally have their apparent molecular weight in the range of 20 kDa to 96 kDa (Keene et al., 1990; North et al., 1990; Robertson and Coombs, 1992). Despite high molecular weight, the proteinases of this amoeba might be the cysteine proteinase. It is interesting to speculate why there are not many forms of proteinases present in pathogenic N. fowleri compared with other parasitic protozoa such as Trichomonas spp. (Lockwood et al., 1987) and Leishmania mexicana
mexicana (Robertson and Coombs, 1992). Details on the functional aspects of proteinases in N. fowleri however, need to be carried out to further verify if these enzymes are related to
the amoeba’s pathogenesis as have been observed in Entamoeba histolytica.
In this study, to determine whether N. foweri lysate has cytopathic effects, microglial cells cultured from the brains of newborn rats were used as target cells. As determined by morphological findings by a light microscope, an SEM and a TEM, microglial cells in co-culture systems with N. fowleri lysate were destroyed by showing necrotic and apoptotic process.
In previous reports, pathogenic A. culbertsoni lysate induced cytopathic effects in primary-culture rat microglial cells, with the effects characterized by necrosis and apoptosis
of microglial cells (Shin et al., 2000; Shin et al., 2001; Shin et al., 2001(a)). A. culbertsoni lysate also showed stronger destruction than Acanthamoeba castellanii (Marciano-Cabral et al., 2004).
Activated macrophages are efficient cytotoxic cells as well as the producers of over 100 secretory molecules (Nathan, 1987). Effector functions of activated macrophages are tightly regulated by a complex network of cytokines. Mouse peritoneal macrophages may be activated in vivo by infection with microorganisms or in vitro by exposure to lymphokines.
Macrophages activated for cytolysis of one target cell are not necessarily cytolytic for other target cells (Wing, 1977; Nacy et al., 1984; LeBlanc et al., 1990). Current studies have focused on identifying the effector molecules that mediate the direct, nonspecific cytotoxicity of activated macrophages. Reactive oxygen intermediates produced during the respiratory burst have been implicated as one of the major mechanisms of macrophage microbicidal effector functions (Ding et al., 1988; Schwamberger et al., 1991).
More recently, the cytotoxic capacity of nitric oxide produced by nitrogen oxidation of L-arginine has been demonstrated for parasites (Adams et al., 1990; James and Hibbs, 1990; Liew et al., 1990; Green et al., 1991; Vincendeau and Daulouede, 1991; Lin et al., 1992).
Also, pro-inflammatory cytokines are produced by activated macrophages, including tumor necrosis factor alpha (TNF-α), interleukin-1 alone or in combination with TNF-α, and cytolytic protease (Adams, 1980; Onozaki et al., 1985; Lachman et al., 1986; Ichinose, 1988; Last-Barney et al., 1988). TNF-α effects tumoricidal activity but has no direct cytolytic activity for a number of parasitic protozoa (DeTitto et al., 1986; Clark et al., 1990).
parasitic organisms by serving as an inducer of the L-arginine dependent cytolytic pathway (Liew et al., 1990). TNF-α has also been shown to synergize with gamma interferon in the presence of lipopolysaccharide (LPS) and induce the production of nitric oxide in the target cells themselves (Amber et al., 1991).
On the other hands, necrosis characterized by the disruption of cell membrane integrity and apoptosis by the membrane blebing and apoptotic bodies formation were determined to be two fundamental mechanisms in related to the cytolysis of target microglial cells by N.
fowleri trophozoites (Oh et al., 2005).
In this study, N. fowleri lysate induced cytopathic effects in primary-culture rat microglial cells, with the effects characterized by necrosis and apoptosis of microglial cells. More detailed studies are necessary in the future to elucidate what the cell death signaling pathways due to N. fowleri lysate are induced, and what the pathogenic elements produced from N. fowleri lysate are present.
It is well known that microglial cells produce pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-3, IL-6, IL-8, IL-12 and IL-15, as well as anti-inflammatory cytokines, such as IL-10 and TGF-β, for defense against parasites and brain injury (Benedetto et al., 2001; Marciano-Cabral et al., 2004).
In a previous study, to determine whether microglial cells release pro-inflammatory cytokines as a protective mechanism induced by N. fowleri trophozoites, the ELISA assay for cytokines, such as TNF-α, IL-1β and IL-6, was performed with culture supernatants (Oh et al., 2005). TNF-α and IL-6 peaked at 6 h post co-incubation and then decreased, whereas IL-1β peaked at 12 h and continued to accumulate in the medium through 24 h. In addition,
when microglial cells were co-cultured with N. fowleri trophozoites and an anti-Nfal antibody for 3 and 6 h, the amount of TNF-α secreted from microglial cells was inhibited about 20.3% and 14.1%, respectively. But the amount of IL-1β and IL-6 was not decreased (Oh et al., 2005).
In present study, to determine whether N. fowleri lysate induce the releasing of pro-inflammatory cytokines from microglial cells, amounts of TNF-α, IL-1β and IL-6 were checked. Secretion of TNF-α and IL-6 peaked at early periods of co-incubation and subsequently decreased, whereas IL-1β was increased during the periods of cultivation and continued to accumulate in the medium during the following 24 h (data not shown). More extensive studies on the cytokine responses of microglial cells due to N. fowleri are necessary in the future study.
V. CONCLUSION
In the present study, to determine whether a pathogenic N. fowleri lysate shows the cytopathic effects against primary culture rat microglial cells, the morphological changes of microglial cells was observed by a light, scanning and transmission electron microscope. And then, the cytotoxicity of N. fowleri lysate against microglial cells was also observed by 51
Cr release assay.
In addition, the pro-inflammatory cytokine release from microglial cells in the co-culture system was estimated. As results with a light and electron microscopes, most of microglial cells were severely destroyed by N. fowleri lysate, showing the necrotic (above 85%) and apoptotic cell death (below 15%) in a time- and dose dependent manner.
As the results of 51Cr release assay, the cytotoxicity of N. fowleri lysate against microglial cells were 14.6, 21.9, 38.5 and 71.5% at 3, 6, 12 and 24 hr post incubation, respectively. And then, the amount of cytokines released from microglial cells in co-culture systems at 3, 6 and 12 hr were 121.6, 90.4 and 81.0 pg/ml of TNF-α, 88.5, 92.7 and 190.1 pg/ml of IL-1β, and 298.8, 414.9 and 251.1 pg/ml of IL-6, respectively.
Thus, this present study shows that the microglial cells may be involved in the inflammation stage of N. fowleri infection as secreting various inflammatory cytokines.
VI. REFERENCES
1. Adams DO, Kao KJ, Farb R, Pizzo SV: Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol 124: 293- 300, 1980
2. Adams LB, Hibbs Jr JB, Taintor RR, Krahenbuhl JL: Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol 144: 2725-2729, 1990
3. Amber IJ, Hibbs Jr JB, Parker CJ, Johnson BB, Taintor RR, Vavrin Z: Activated macrophage conditioned medium: identification of the soluble factors inducing cytotoxicity and the L-arginine dependent effector mechanism. J Leukocyte Biol 49: 610-620, 1991
4. Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP: TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4: 1116–1122, 2004
5. Benedetto N, Folgore A, Carratelli CR, Galdiero F: Effects of cytokines and prolactin on the replication of Toxoplasma gondii in murine microglia. Fur Cytokine Netw 12: 348-358, 2001
6. Benveniste EN: Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med 75: 165–173, 1997
7. Bottone EJ: Free-living amebas of the genera Acanthamoeba and Naegleria: an overview and basic microbiologic correlates. Mt Sinai J Med 60: 260-270, 1993
8. Brown T: Observations by light microscopy on the cytopathogenicity of Naegleria
fowleri in mouse embryo cell cultures. J Med Microbiol 11: 249-259, 1978
9. Brown T: Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med
Microbiol 12: 363-371, 1979
10. Carbonell WS, Murase S, Horwitz AF, Mandell JW: Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci 25: 7040–7047, 2005
11. Carter RF: Primary amoebic meningoencephalitis: An appraisal of present knowledge.
Trans R Soc Trop Med Hyg 66: 193-213, 1972
12. Chang SL: Etiological, pathological, epidemiological and diagnostical considerations of primary amoebic meningoencephalitis. CRC Crit Rev. Microbiol 3: 135-159, 1974
13. Chang SL: Pathogenesis of pathogenic Naegleria amoeba. Folia Parasito 26: 195-200, 1979
14. Chao CC, Gekker G, Hu S, Peterson PK: Human microglial cell defense against
Toxoplama gondii: The role of cytokines. J Immunol 152: 1246-1252, 1994
15. Christensen RN, Ha BK, Sun F, Bresnahan JC, Beattie MS: Kainate induces rapid redistribution of the actin cytoskeleton in ameboid microglia. J Neurosci Res 84: 170– 181, 2006
16. Cho MS, Jung SY, Park S, Kim KH, Kim KI., Sohn S, Kim HI,. Im KI, Shin HJ: Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic
Nagleria fowleri. Clin Diagn Lab Immunol 10: 954-959, 2003
17. Clark IA, Cowden WB, Butcher GA: TNF and inhibition of growth of Plasmodium
falciparum. Immunol Lett 25: 175-178, 1990
18. Culbertson CG: The pathogenicity of soil amebas. Annu Rev Microbiol 25: 231-254, 1971
19. Cursons RTM, Brown TJ: Use of cell cultures as an indicator of pathogenicity of free-living amoebae. J Clin Pathol. 31: 1-11, 1978
20. Dasgupta S, Jana M, Liu X, Pahan K: Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem 278: 22424–22431, 2003
21. DeTitto ER, Catterall JR, Remington JS. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanasoma cruzi. J Immunol 137: 1342-1345, 1986
22. Ding AH, Nathan CF, Stueher DJ: Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 141:2407-2412, 1988
23. Elder MJ, Kilvington S, Dart JK: A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 35: 1059–1064, 1994
24. Feldman MR: Naegleria fowleri: Fine structural localizaion of acid phosphatase and heme proteins. Ex Parasitol 41: 290-306, 1977
25. Ferrante A, Bates EJ: Elastase in the pathogenic free-living amebae Naegleria and
26. Fowler N, Carter RT: Acute pyogenic meningitis probably due to Acantamoeba sp: a preliminary report. Br Med J 2: 740–742, 1965
27. Fulford DE, Bradley SG, Marciano-Cabral F: Cytopathogenicity of Naegleria fowleri for cultured rat neuroblastoma cells. J Protozool 32: 176-180, 1985
28. Giulian D, Baker TJ: Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6: 163-178, 1986
29. Gonzalez MM, Gould E, Dickinson G : Acquired immunodeficiency syndrome associated with Acanthameba infection and other opportunistic organisms. Arch Pathol
Lab Med 110: 749-51, 1986
30. Green SJ, Nacy CA, Meltzer MS: Cytokine induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens.
J Leukocyte Biol 50: 93-103, 1991
31. Hadas E, Mazur T: Proteolytic enzymes of pathogenic and non-pathogenic strains of
Acanthamoeba spp. Trop Med Parasitol 44: 197-200, 1993
32. Ichinose Y, Bakouche O, Tsao JY, Fidler IJ: Tumor necrosis factor and IL-1 associated with plasma membranes of activated human monokines lyse monokine-sensitive but not
monokine-resistant tumor cells whereas viable activated monocytes lyse both. J Immunol 141: 512-518. 1988
33. Im KI., Shin HJ: Pathogenic free-living amoebae in Korea. Korean J Parasitol 42: 93-119, 2004
34. James SL, Hibbs, Jr JB: The role of nitrogen oxides as effector molecules of parasitic killing. Parasitol Today 6: 303-305, 1990
35. Jana M, Dasgupta S, Saha RN, Liu X, Pahan K: Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem 86: 519–528, 2003
36. John DT: Primary amebic meningoencephalitis and the biology of Naegleria fowleri.
Ann Rev Microbiol 36: 101-123, 1982.
37. John DT. Opportunistically pathogenic free-living amebae. In: Kreier JP, Baker JR, editors. Parasitic protozoa 2nd ed, vol 3. San Diego (Calif.): Academic Press Inc; 1993. p. 143-246.
38. Jeong SR, Kang SY, Lee SC, Song KJ, Im KI, Shin HJ: Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria
fowleri. Korean J Parasitol 42: 35-40, 2004
39. Kang SY, Song KJ, Jeong SR, Kim JH, Park S, Kim K, Kwon MH, Shin HJ: Role of the Nfa1 protein in pathogenic Naegleria fowleri treated with CHO target cells. Clin Diagn
Lab Immunol 12: 873-876, 2005
40. Keene WE, Hidalgo ME, Orozco E, McKerrow JH: Correlation of the cytophatic effect of virulent trophozoites of Entamoeba histolytica with the secretion of a cysteine proteinases. Exp Parasitol 71: 199-206, 1990
41. Khan NA, Jarrol EL, Panjwani N, Cao Z, Paget TA: Proteases as markers for differentiation of pathogenic and non-pathogenic species of Acanthamoeba. J Clin
Microbiol 43: 391-395, 2000
42. Kilvington S, Beeching J: Development of PCR for identification of Naegleria fowleri from the environment. Appl Environ Microbiol 61: 3764–3767, 1995
43. Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends
Neurosci 19: 312–318, 1996
44. Lachman LB, Dinareilo CA, Llansa ND, Fidler LJ: Natural and recombinant human interleukin 1-1 is cytotoxic for human melanoma cells. J Immunol 136: 3098-3102, 1986
45. Last-Barney K, Homon CA, Faanes RB, Merluzz VJ: Synergistic and overlapping activities of tumor necrosis factor-α and IL-1. J Immunol 141:527-530, 1988
46. LeBlanc PA, Heath LS, Um HD: Activated macrophages use different cytolytic mechanisms to lyse virally infected or a tumor target. J Leukocyte Biol 48: 1-6, 1990
47. Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY: Mannose induces the release of cytopathic factors from Acanthamoeba Keratitis. Infet Immun 66: 5-10, 1988
48. Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA: Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A 102: 9936–9941, 2005
49. Liew FY, Li Y, Millot S: Tumor necrosis factor α synergizes with IFN γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol 145: 4306- 4310, 1990
50. Lin JY, Chadee K: Macrophage cytotoxicity against entamoeba trophozoites is mediated by nitric oxide from L-arginine. J Immunol 148: 3999-4005, 1992
51. Lockwood BC, North MJ, Scoot KI, Bremner AF, Coombs GH: The use of a highly sensitive electrophoretic method to compare the proteinases of trichomonads. Mol
Biochem Parasitol 24: 89-95, 1987
52. Ma P, Visvesvara GS, Martinez AJ, Thedore FH, Daggett PM, Sawyer TK: Naegleria and Acanthamoeba infection: Review. Rev Infect Dis 12: 490-513, 1990
53. Marciano-Cabral F: Biology of Naegleria spp. Microbiol 52: 144-33, 1988
54. Marciano-Cabral F, Ludwick CR, Puffenbarger A, Cabral GA: Differential stimulation of microglial pro-inflammatory cytokines by Acanthamoeba culbertsoni versus
Acanthamoeba castellanii. J Eukaryot Microbiol 51: 472-479, 2004
55. Marciano-Cabral F, Fulford DE: Cytopathology of pathogenic and nonpathogenic Naegleria species for cultured rat neuroblastoma cells. Appl Environ Microbiol 51: 1133-1137, 1986
56. Marciano-Cabral F, Patterson M,. John DT, Bradley SG: Cytopathogenecity of
Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J Parasitol 68: 1110-1116, 1982
57. Marshall MM, Naumovitz D, Ortega Y, Sterling CR: Waterborne protozoan pathogens.