Th e Eff ect of N a-K-Cl cotran sp orter
in h ib iti on on o ste oclastog en e si s i n du ce d by
1α,25-D ihy droxy v itam i n D
3Eu n-You n g Kim
Th e Gradu ate Sch o o l
Yo n se i U n iv ersity
Th e Eff ect of N a-K-Cl cotran sp orter
in h ib iti on on o ste oclastog en e si s i n du ce d by
1α,25-D ihy droxy v itam in D
3A d esser tation
su bm itted to th e Dep ar tm en t of Den tistry
an d th e Gra d u ate Sch ool of Yon sei Un iv er sity
in p ar tial fu lfillm en t of th e
requ irem en ts for th e d egree of
Doctor of Ph ilosop h y
Eu n -You n g Kim
Th is cer tifies th at th e d issertation of
Eu n -You n g Kim is ap p rov ed
____________________
Th esis Sup ervisor : Jon g-Gap Lee, D .D .S., Ph . D .
________________________
Th esis Com m ittee Mem ber : H eu n g-Kyu Son, D .D .S., Ph . D .
________________________
Th esis Com m ittee Mem ber : Byun g-Jai Ch oi, D .D .S., Ph . D .
________________________
Th esis Com m ittee Mem ber : Hyun g-Jun Ch oi, D .D .S., Ph . D .
________________________
Th esis Com m ittee Mem ber : Syn g-Ill Lee, D .D .S., Ph . D.
The Gradu ate Sch ool Yonsei University
A ck n ow le dg e m e nts
First of all, I w ou ld like to app reciate Professor Jon g-Gap Lee on his kin d concern an d w arm su p p ort throu gh ou t m y course of gradu ate school years. His acad em ic kn ow led ge and advices have alw ays been helpfu l in accom plishing m y d octoral d egree.
My sincere gratitu d es w ou ld h ave to go to Professor Syn g-Ill Lee. With ou t his th ou ghtfu l su pp ort an d gu id e, it w ould have been im p ossible for m e to finish the th esis. I adm ire his p assion an d d evotion tow ard th e basic science researchin g w hich encou raged m e to stu dy an d research m ore.
My d eep est ap preciation s to Professor H eun g-Kyu Son, Byu ng-Jai Ch oi an d Hyu ng-Ju n Choi for th eir kin d su pp orts. Also, m y gratitu d es to Research Assistant Professor Seu n g-H o Ohk an d Assistant Hyu n-Ah Lee for th eir sacrifices and encou ragem ents.
I w ould like to th ank m y m oth er, for ju st bein g there w h en I w as in trou ble. She gave m e th e ju st right advices w hen ever I n eed ed th em . Also, m y loving hu sban d an d sw eet d au ghter, So-You n, d eserves m uch ap preciation s. Th ey gave m e th e strength to w ithstan d th e hardtim es th at I had to go throu gh . An d fin ally I d evote this th ese to m y secon d child abou t to be born n ext April w ith all m y love.
Tab le of Co nte nts
List of Figu res
Abstract iii
I. Introduction 1
II. Materials an d Method s 9
1. TRAP staining of osteoclasts 9
2. In du ction of osteoclasts in coculture 11
3. Viability test (MTT assays) 11
4. Measurem ent of m ean cell volu m e 12
5. Reverse transcrip tase-p olym erase chain reaction (RT-PCR) 12
6. Western blot 13
7. Data analysis 14
III. Results 15
1. Effect of bum etanid e on 1α,25(OH)2D3-indu ced osteoclast
form ation 15
2. Evalu ation of ch an ges in cell viability by bum etanid e 15 3. Decrease of cell volu m e by bu m etanid e 18 4. Ch an ges in m RN A expression profile in RAN KL, OPG, an d
M-CSF 21
5. Ratio of RAN KL/ OPG m RN A in resp on se to bu m etanid e 21 6. Th e effect of bu m etanid e on t-JN K and p -JN K 26
IV. Discu ssion 28
V. Conclu sion 33
VI. References 35
Li st of Figu re s
Fig. 1. General ou tline of bon e rem od elin g 2 Fig. 2. Sch em atic diagram of osteoclast differentiation 4 Fig. 3. Mechanism s of osteoclastogen esis and osteoclastic bon e
resorp tion 6
Fig. 4. Hyp othetical m od el for m odu lation of osteoclastogen esis 7 Fig. 5. Cocultu re system of osteoblastic cell an d bone m arrow 10 Fig. 6. Inhibition of osteoclast differentiation by 10 μM
bu m etanid e 16
Fig. 7. Ch anges in VitD3-in du ced TRAP p ositive cells in resp on se
to bum etanid e 17
Fig. 8. Effect of bum etanid e on cell proliferation in cocu lture 19 Fig. 9. Effect of bum etanid e on osteoblastic cell volu m e 20 Fig. 10. Effect of bu m etanid e on th e expression of RAN KL
m RN A in osteoblastic cell 22
Fig. 11. Effect of bu m etanid e on th e expression of M-CSF m RN A
in osteoblastic cell 23
Fig. 12. Effect of bu m etanid e on th e expression of OPG m RN A in
osteoblastic cell 24
Fig. 13. Relative exp ression of RAN KL and OPG m RN A in
osteoblastic cells 25
Fig. 14. Effect of bu m etanid e on th e activation of JN K 27 Fig. 15. Prop osed m od el for the alteration of osteoclastogen esis
A b stract
Th e Ef f ect of N a-K-Cl cotran sp o rter inh ib itio n o n o ste o clasto g en e s is in du ce d by 1α,25- D ihy dro xy v itam i n D3
Kim, Eu n-Yo u n g D ep artm e nt of D e ntistry , Th e G radu ate Sch o o l, Yo n se i U n iv ers ity
(Directed by Prof. Jon g-Gap Lee, D.D.S., Ph .D)
N a/ K/ Cl cotran sp orters (N KCC1) h ave been rep orted in nu m erou s, diverse tissu es, an d several cellu lar fu nction s. N KCC1 is involved in ion tran sp ort across th e secretory an d absorp tive ep ith elia, regulation of cell volum e, and p ossibly m odu lation of cell grow th and d evelopm ent . Fu rtherm ore, recent stu dies sh ow ed th at N KCC1 is p resent in osteoblasts. In this stu dy physiological role of N KCC1 in th e p rocess of osteoclastogen esis w as exploited in coculture system . 10 an d 100 μM bum etanid e, a sp ecific inhibitor of N KCC1, d ecreased the nu m ber of tartrate-resistant acid p hosph atase (TRAP) p ositive m u ltinu cleated cells. One m icrom olar bum etanid e did not show any effect on the 1α,25(OH)2D3-indu ced osteoclast differentiation . To clarify th e role
of bu m etanid e on the inhibition of osteoclastogen esis, m RN A exp ression of RAN KL and OPG w as analyzed by RT-PCR.
Exp osu re of osteoblastic cell to th e bu m etanid e treated m ediu m resu lted in th e reduction of RAN KL m RN A expression indu ced by 1α,25(OH)2D3, being d ep en d ent on the bu m etanid e
concentration . On the other h an d, the expression of OPG m RN A, a n ovel TN F receptor fam ily m em ber w as increased . Th ese resu lts im p ly th at 10 μM bum etanid e inhibits osteoclast differentiation via inhibition of th e RAN KL m RN A expression an d enh ancem ent of th e OPG m RN A expression in osteoblastic cell. H ow ever the exp ression of M-CSF m RN A w hich has been kn ow n to be a su rvival factor of osteoclasts w as n ot ch anged at tested concentration s (1, 10, 100 μM). Also, w e exam in ed th e expression an d p hosph orylation of c-Ju n N H2-term in al kin ase (JN K). Th e
p h osp horylation of JN K w as redu ced . Fu rth erm ore, bu m etanid e cau sed the d ecrease in the relative cell volum e u p to 36% of th e original volum e. Taken all togeth er, these resu lts su ggest th at N KCC1 in osteoblastic cell h as an im p ortant role in the osteoclast differentiation an d the inhibition of N KCC1 activity reduced th e osteoblastic cell volum e ch an ge, w hich in tu rn resu lts in th e inhibition of osteoclastogen esis, d ecreasing th e ph osph orylation of JN K.
______________________________________________________________ Key w ord s : N KCC1, bu m etanid e, osteoclast, RAN KL, OPG, JN K
I. Intro du ctio n
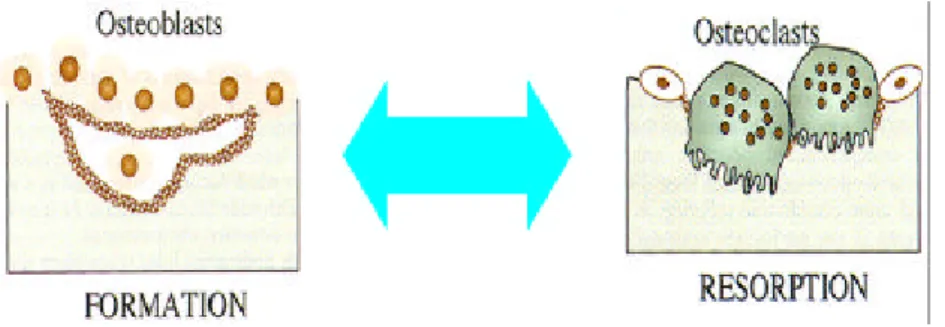
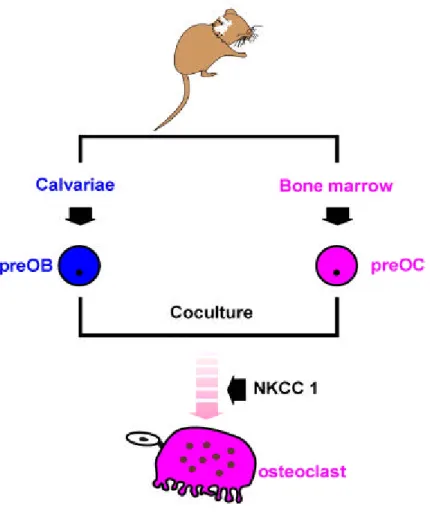
Bone rem od eling is regu lated by th e activity of bon e-form in g osteoblasts an d bone-resorbing osteoclasts. Both osteoblasts an d osteoclasts are regulated by a variety of h orm ones and local factors. Osteoblasts stem s from m esenchym al stem cells, w hereas osteoclasts arise by th e differentiation of osteoclasts p recu rsors of m on ocyte/ m acroph age lin eage and their fu nction is requ ired n ot only for the d evelopm ent of th e skeleton, but also for m in eral h om eostasis an d n orm al rem od elin g of bon e (Raise, L., 1998). An im balance betw een bone form ation an d bon e resorption cau ses su ch m etabolic bone diseases like osteop etrosis and osteop orosis (Au bin, J.E., 1998). Therefore, osteoblasts an d osteoclasts are kn ow n to h ave close relation ship s in th e process of rem od elin g an d the relation ship is also continu ou s (Fig. 1).
A cocu ltu re system of sp leen cells w ith osteoblastic cell or bone m arrow strom al cells h as been establish ed to produ ce osteoclasts (Takah ashi, N . 1999). In th e cocu lture system , osteoclast-like cells are form ed from spleen cell in th e p resence of su ch stim ulators of bon e resorption as interleu kin 6 (IL-6), IL-11, p arathyroid horm one (PTH), p rostaglandin E2 (PGE2), and
1α,25-dihydroxyvitam in D3 (Fu ller, K. et al., 1998). Th erefore,
m ature osteoclasts are produ ced by th e action of osteoblastic cells as a resu lts of m any stim ulators. Th e cell-to-cell interaction betw een osteoblasts/ strom al cells and osteoclast p rogenitors in th e
Fig . 1. General outlin e of bone rem od elin g. Bone rem od elin g is
regu lated by th e activity of bon e form in g osteoblasts and bone resorbing osteoclasts.
cocu ltu res has been foun d to be essential for the osteoclast form ation (Fig. 2).
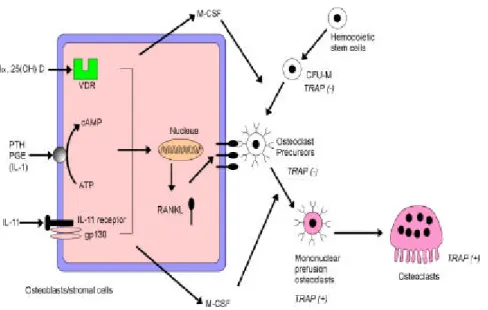
In th e p rocess of d eveloping m atu re osteoclasts, certain kin d s of signal m olecu les w hich are exp ressed from the osteoblasts h ave m ajor role in osteoclastogen esis. Recently, m any researchers h ave tried to elu cid ate th e role of th ese sign al m olecu les in the p rocess of osteoclastogen esis. As a resu lt of stim u lation of osteotropic factors such as p arathyroid horm one, sign al m olecules seem to act on osteoblast/ strom al cells to in du ce osteoclastogen esis hyp oth esized th at a m em bran e-boun d, d esignated as "recep tor activator of N F-κB ligand (RAN KL)," is exp ressed on osteoblasts/ strom al cells in resp on se to osteotrop ic factors, an d th at it tran sdu ces, to osteoclast progenitors, a sign al essential for osteoclastogenesis, throu gh cell-to-cell interaction (Jim m i, E. et al., 1999). It is now kn ow n th at in ord er to differentiate m atu re osteoclasts from osteoclast p rogenitors, RAN KL w hich is p roduced from osteoblast an d m acrop hage-colony stim u latin g factor (M-CSF) are required also. M-CSF is know n to be th e survival factor of the osteoclastogen esis and is essential for the m acroph age to be tran sform ed into osteoclasts. H ow ever, osteoprotegerin (OPG), a d ecoy receptor of RAN KL is released from osteoblasts to inhibit th e osteoclastogen esis. OPG is a secreted m em ber of th e tu m or n ecrosis factor receptor (TN FR) fam ily, an d it inhibits osteoclastogen esis stim ulated by 1,25(OH)2D3, PTH, or IL-11
Fig . 2. Sch em atic diagram of osteoclast differentiation . Mature osteoclasts are form ed from m acroph age in th e presence of stim ulators as 1α,25(OH)2D3, PTH, PGE2 or IL-11.
(Takah ashi, N . et al., 1999). It is believed th at RAN KL, M-CSF an d OPG w hich express from osteoblasts p lay an essential roles in regu lation of osteoclastogen esis an d osteoblast is the m ajor factor in th e process of bone rem od elin g (Fig. 3).
Recently, it is rep orted th at N a/ K/ Cl cotransp orter (N KCC1) is p resent in osteoblasts. Sim ply, it m ay be p ossible th at N KCC1 is involved in th e bone rem od elin g process. So, ou r qu estion w as w hat th e role of N KCC1 is in osteoblast . Of cou rse, N KCC1 is n ow kn ow n to be p resent in num erou s, diverse tissu es from a w id e variety of anim al sp ecies, an d their fu nction s are ion tran sp ort across secretory and absorp tive ep ith elia, m ainten ance an d regulation of cell volu m e an d ion gradients, an d p ossibly m odu lation of cell grow th an d d evelop m ent (H aas, M., Forbu sh, B., 1998). It can be hyp othesized th at N KCCl m ay cencern osteoclastogen esis in resp ect to th e RAN KL, OPG, M-CSF m RN A exp ression . Becau se there are several facts to be assu m ed that N KCC1 is involved in osteoclastogenesis, th ose are 1) N KCCl cotran sp orter exists in osteoblasts, 2) N KCCl transp orts th e ion inw ard and ou tw ard across th e epith elia and play an im p ortant role in regu latin g th e cell size an d volu m e, and 3) N KCCl affects th e d evelopm ent an d grow th of th e cells (Ru ssell, J.M. et al., 2000). N everth eless there is no plau sible exp lanation for th e role of N KCC1 in osteoblast in concern w ith osteoclastogen esis (Fig. 4). Therefore, w e aim ed at revealin g th e role of N KCC1 in osteoblast, inhibiting th e N KCC1 activity u sing bu m etanid e w hich
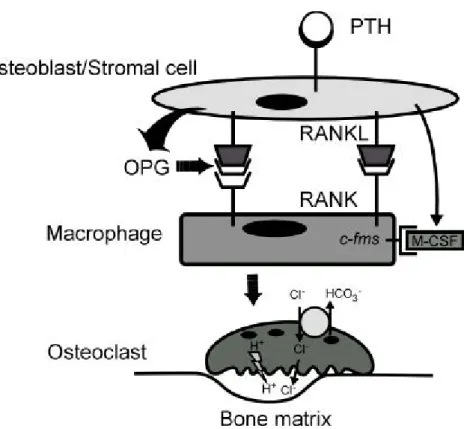
Fig . 3. Mechanism s of osteoclastogenesis an d osteoclastic bone
resorption . Strom al cells/ osteoblasts express RAN KL an d M-CSF, w hich are up -regulated by osteoclastogenic m olecules su ch as PTH . PTH also blu nts expression of OPG. RAN KL an d M-CSF, interacting w ith th eir receptors on m onocyte-m acroph age cells, indu ce osteoclast differentiation, a p rocess inhibited by OPG. The differentiated osteoclast p olarizes on th e bon e surface. After form ation of th e ru ffled m em brane, the osteoclast acidifies an extracellular m icroenvironm ent by m ean s of p roton pu m p . Intracellu lar p H is m aintain ed by H CO3
-/ Cl
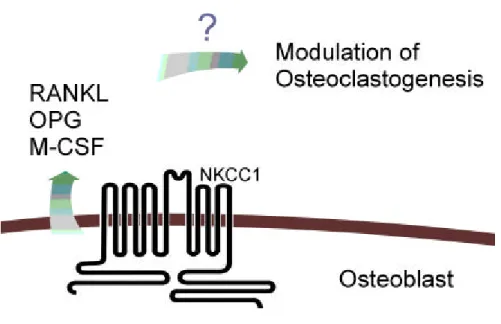
Fig . 4 . Hyp oth etical m od el for m odulation of osteoclastogen esis.
The above m od el for N KCC1 an d th e osteoclastogenesis w as inferred from th e evid ences th at N KCC1 is exist in osteoblast, an d p lay an im p ortant role in regu latin g th e cellu lar size an d volum e. Therefore, blockin g th e N KCC1 m ight be a p otent regu lator of both bon e form ation an d bone resorption .
is a sp ecific inhibitor of N KCC1. Un exp ectedly, w e fou n d that bum etanid e inhibited the osteoclastogenesis in cocu ltu re system , osteoblast/ strom al cell. This fact give u s th e new clu es th at N KCC1 is p robably resp on sible for th e d ow n-regu lation of osteoclastogen esis. Th erefore, in this stu dy w e app lied osteoblast/ strom al cell cocu ltu re system to evalu ate 1) Effect of bum etanid e on 1α,25(OH)2D3-in du ced osteoclast form ation, 2)
Decrease in cell volum e by bum etanid e, 3) Ch an ges in m RN A exp ression p rofile in RAN KL, OPG, an d M-CSF, 4) Effect of bum etanid e on t-JN K an d p-JN K.
II. M aterials an d M eth o ds
1. T RA P stain in g of o ste oclasts
osteoblastic cells w ere isolated from 1~2 d ay-old n ew born m ice. 30~50 calvariae w ere subjected to digestions u sing 10 m l enzym e solu tion containing 0.2% collagen ase (Wako, Jap an) an d 0.1% disp ase (GiBCO BRL, U .S.A) for 20 m inutes at 37。C in a sh akin g w ater bath . Th e su p ern atant w as discard ed and 10 m l enzym e solu tion w as ad d ed . After shakin g at 37。C for 20 m inu tes, th e su p ern atant w as then collected carefu lly an d tran sferred into a new tube. This digestion of calvariae w ith collagenase-disp ase w as rep eated three tim es. Collected su p ern atant (30 m l) w as app lied to centrifu ge at 1,500×g for 10 m inu tes to collect th e osteoblastic cells. Collected cells w ere resu sp end ed in α-MEM containin g 10% fetal calf serum (FCS) an d cultu red in 10 cm cu ltu re dish es at a concentration of 105 cells/ dish to confu lence. Cells w ere th en d etach ed from cultu re dish es by tryp sin-EDTA, su sp en d ed in α-MEM w ith 10% FCS an d u sed for cocu lture as osteoblastic cells (Lacey, D . et al., 1998). Fem orial and tibial bone m arrow cells w ere collected from 4-w eek-old m ice. Tibiae and fem ora w ere rem oved and dissected free of adhering tissu es. The bone en d s w ere rem oved and th e m arrow cavity w as flu sh ed by slow ly injectin g m edia in at one en d u sin g a 25-gau ge n eedle. Th e collected calvariae an d bon e m arrow cells w ere w ashed an d u sed for th e cocu ltu re (Fig. 5).
Fig . 5. Cocu ltu re system of osteoblastic cell an d bone m arrow .
Mou se calvarial cells (1 × 104
cells/ w ell) w ere cocu ltu red w ith bone m arrow cells (1 × 105 cells/ w ell) in α-MEM containing 10% fetal calf seru m in 48-w ell plates. All cu ltu res w ere m aintained at 37。C in a hu m idified atm osp h ere of 5% CO2 in
air . After treatm ent, the cells w ere su bjected to tartrate-resistant acid p hosph atase (TRAP, a m arker enzym e of osteoclasts) stainin g.
2. In du ctio n o f o ste o clasts in co cu ltu re
Mou se osteoblastic cells (1 × 104 cells/ w ell) w ere cocu ltu red w ith bon e m arrow cells (1 × 105 cells/ w ell) in α-MEM containin g 10% fetal calf seru m in 48-w ell plates (Cornin g Inc., Corning, N Y). Cu ltu re volum e w as m ad e u p to 400 μl p er w ell w ithα-MEM su pp lem ented w ith 10% fetal calf seru m (FCS), in th e p resence of 1 ,25(OH)2D3 (10 nM), w ith or w ith out
bum etanid e. All cu ltures w ere m aintained at 37。C in a hu m idified atm osp here of 5% CO2 in air . After treatm ent, the
cells w ere su bjected to tartrate-resistant acid ph osp hatase (TRAP, a m arker enzym e of osteoclasts) stainin g.
3. V iab ility te st (M TT as say s)
MTT (3-〔4,5-dim ethylthiazol-2-yl-〕-2,5-diph enyltetrazoliu m brom id e) test u ses th e p rinciple w hich tetrazoliu m salts be redu ced by reducin g enzym e (succin ate d ehydrogen ase) of m itoch on dria so th at th e toxicity of viable cells an d th e cellu lar differentiation s can be m easured . MTT w as dissolved in p h osp hate-bu ffered salin e (PBS) at 5 m g/ m l an d filtered to rem ove any in solu ble residu es (Slad ow ski, D. et al., 1993). Th e MTT solution w as ad d ed directly to the assay p lates at a rate of 10 μl to 100 μl cell cu ltu re m edium an d th en th e cells w ere incubated for a furth er 4 hou rs at 37。C. Th e p urp le form azan
crystals form ed w ere dissolved by th e ad dition of acidic isop rop an ol (100 μl 0.04 N H Cl inisop rop an ol) follow ed by th orou gh m ixin g. The plates w ere subsequ ently read on a sp ectroph otom eter at 570nm and 630 nm .
4 . M e asu re m e nt o f m e an ce ll v o lu m e
Poly-L-lysin e coated cover glass on w hich cells w ere cu ltu red w as m ou nted on a th erm al stage (37。C), w hich w as set on th e m ech anical stage of an inverted m icroscop e connected to a vid eo-im agin g system . The cells w ere p erfu sed w ith the 0.01% w / v-L-lysin e solu tion (McCarty, N .A ., O' N eil, R., 1991). Th e im ages of th e optical m icroscop e w ere continu ou sly record ed w ith a vid eo-im agin g system . To estim ate cell volum e, th e area of osteoclasts in th e vid eo im age (A ) w as m easu red . Th e averaged valu e of area m easu red in th e first 2 m in w as u sed as th e control (A0). Th e relative cell volu m e, V/ V0 = (A/ A0)
1 .5
w as estim ated, w h ere V is the volum e, A is th e area, an d subscript 0
is th e valu e of the control.
5. Rev erse tran scrip tase-p o ly m erase ch ain re actio n (RT- PCR)
cDN A can be p olim erized from m RN A throu gh reverse tran scription p rocedu re an d then am p lified by p olym erase ch ain reaction (PCR). RT-PCR is u sed n ot only in th e qu alitative but
also in the qu antitative an alysis of m RN A exp ressed in cells. RT-PCR exp erim ent con sists of RN A sep aration, cDN A p olym erization and PCR am p lification .
Trizole reagent, chloroform , isop ropyl and ethan ol w ere u sed to sep arate RN A from cells. Qu antitative an alysis of extracted tRN A for the p urity an alysis w as d on e. N ext step is to p rim e th e m RN A for cDN A synth esis. In the first, a 3' (antisen se) gen e-sp ecific p rim er is ann ealed to the m RN A and extend ed w ith reverse transcriptase. This generates a cDN A tem p late for th e 5' (sen se) p rim er . Wh en p rim in g cDN A w ith a gen e-sp ecific prim er, a nu m ber of exp erim ental p aram eters m ay n eed to be op tim ized, inclu din g prim er concentration an d ann ealin g tem p eratu re. Five to ten m icroliter dilu te cDN A is u sed for each 50 μl PCR reaction . In ord er to com p are the resu lts easily, the d ata w ere ru n throu gh d en stom ery. In th at w ay, th e d en sity of resu lts of RT-PCR can be easily distingu ish ed .
6. W e stern b lo t
Th e p rotein extracts from osteoblastic cells w ere prep ared . The bon e m arrow cell w ere resu sp end ed in th e p rew arm ed cracking bu ffer (80 m M Urea, 0.05% SDS, 0.4 % 10 m M Tris-H Cl, 1 μM EDTA, 40 μg/ m l Brom oph en ol blu e, 10 μl/ m l β -Mercap toeth anol, 70μl/ m l protease inhibitor solu tion, 50 μι 100× PMSF, 60。C), and tran sferred to a tu be containing glass
bead s (425-600 μm ; Sigm as #G-8772) to disrup t th e bon e m arrow cells. Th e cell resu sp ension s w ith glass bead s w ere lysed by h eatin g at 70。C for 10 m in follow ed by vigorou s vortexin g for 1 m in . Th e lysates as p rotein extracts w ere sep arated from u nbroken cells by centrifu gation at 14,000 rpm for 5 m in . For a w estern blot analysis, p rotein extracts w ere resolved by SDS-p olyacrylam id e gel electrop horesis (SDS-PAGE) an d transferred to a p olyvinylid ene diflu orid e m em brane (PVDF, Bio-Rad). Th e blot w as incu bated in a blocking solu tion containin g 4 % bovin e seru m album in (BSA) an d 10% norm al goat seru m (N GS) in p hosph ate buffered salin e plu s 0.15% Tw een-20 (PBS-T) at RT for 2 hrs. Th e blot w as th en probed w ith the p rim ary antibody (m on oclon al antibody of p-JN K or t-JN K, Clontech) for overnight at 4。C, follow ed by d etection w ith a h orseradish p eroxid ase-linked goat anti-m ou se IgG antibody (Jackson Im m u n oResearch Laboratories, Inc) an d enhanced chem ilum in escence (ECL) system (Am ersh am Ph arm acia Biotech).
7. D ata an aly sis
Com p arison betw een group s w as p erform ed by th e Stu d ent' s
t-test . Significant differences from each oth er w ere d eterm ined at P < 0.05.
III. Re su lts
1. Eff ect of b u m etan i de o n 1α ,25 (O H )2D3-in du ce d o steo clast
f o rm atio n
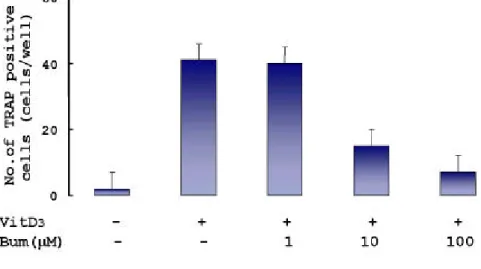
To investigate th e effect on th e differentiation of osteoclast, 1, 10, and 100 μM bu m etanid e w ere ad d ed to th e osteoblastic cell/ bone m arrow cocu ltu re w ith 1α,25(OH)2D3. 1α,25(OH)2D3
w as u sed to indu ce osteoclast differentiation . Ten nan om olar 1 α,25(OH)2D3 w as ad d ed to th e cocu ltu re, an d 42 TRAP p ositive
m u ltinucleated cells w ere form ed w hereas n o TRAP p ositive cell w as d etected w h en cocu ltu re w as incubated in m edia only . The ad dition of 10 or 100 μM bu m etanid e d ecreased th e nu m ber of TRAP p ositive m u ltinu cleated cells u p to 15 or 8 cells p er w ell, resp ectively . H ow ever, 1 μM bu m etanid e did not ch an ge 1 α,25(OH)2D3-in duced osteoclast differentiation (Figs. 6, 7). Th e
effect of bu m etanid e on differentiation of osteoclasts sh ow ed in d ose d ep en d ent m ann er .
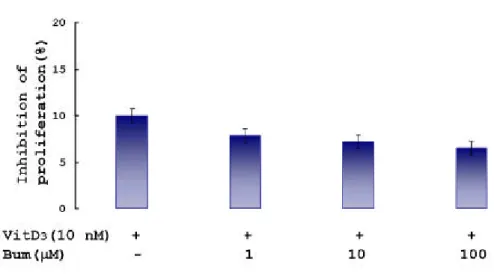
2. Ev alu atio n o f ch an g e s in ce ll v iab ility by b u m etan i de
To d em on strate the effect of bu m etanid e on th e cell p roliferation, MTT assay w as p erform ed accordin g to the m ethod of Ferrari et al. 1989. One, ten, and a hu ndred m icrom olar bum etanid e w as ad d ed to th e cocu ltu re an d incu bated for 3 d ays
10 nM 1α,25(OH)2D3 10 nM 1α,25(OH)2D3 m edia only
+ 10 μM bu m etanid e
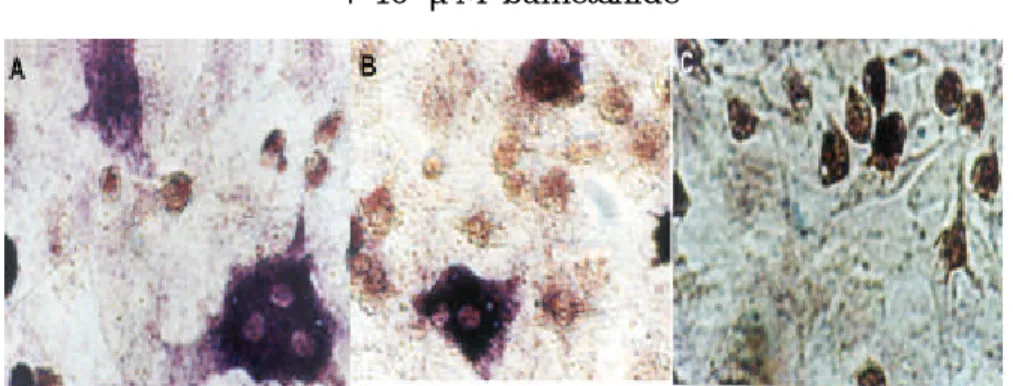
Fig . 6. Inhibition of osteoclast differentiation by 10 μM bum etanid e. Ten m icrom olar bu m etanid e w as ad d ed to osteoblastic cell/ bon e m arrow coculture to block N KCC1. Cells w ere treated w ith bu m etanid e an d / or 1α,25(OH)2D3. After
incubation for 4 d ays, cells w ere stained w ith TRAP staining m ethod . Pan els A, 10 nM 1α,25(OH)2D3; B, 10 nM 1α,25(OH)2D3
Fig . 7. Ch anges in VitD3-in duced TRAP p ositive cells in resp on se
to bu m etanid e (Bum ). Bum etanid e (1, 10, 100 μM) w as ad d ed to th e osteoblastic cell/ bon e m arrow cocu ltu re in th e presence of 10 nM 1α,25(OH)2D3. After incubation for 4 d ays, cells w ere stained
by TRAP stainin g m eth od . TRAP p ositive m ultinu cleated cells th at h ave m ore than 3 nu cleu s w ere counted .
(Fig. 8). Th e inhibition s of cellu lar p roliferation by bu m etanid e w ere less than 10% of control w hich w as incu bated w ith m ediu m only . This im p lies th at bu m etanid e did n ot sh ow toxic effect w hen ad d ed up to 100 μM to th e cocu ltu re. Th erefore, th e resu lts m ean th at th e effect of bu m etanid e on differentiation w as cau sed by controllin g N KCC1, n ot by its toxic effects on the cell.
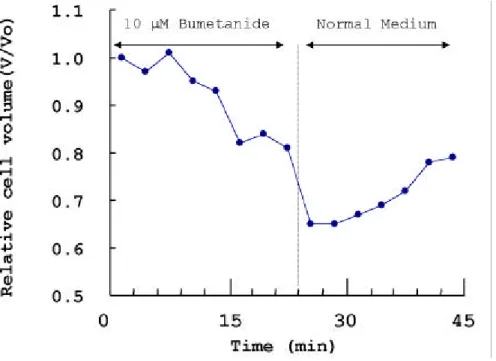
3. D ecre ase o f cel l v o lu m e by b u m etan i de
Ten m icrom olar bum etanid e redu ced th e relative cell volu m e to th e half level (Fig. 9). Du rin g th e first 24 m inutes, n orm al m edia w as p erfu sed throu gh th e cover glass for stabilization of osteoclasts, and th en du rin g th e n ext 20 m inu tes, 10 μM bum etanid e w as p erfu sed . Th e relative cell volu m e w as reduced from on e to 0.6. The original volu m e of osteoclasts w as rep resented as V0 an d testing volu m e, as V. V/ V0 w as less th an
1, it w as regard ed as th e redu ction of cell volum e. As a result, th e relative cell volum e w as redu ced from 1 to abou t 0.6 level, su ggestin g that th e bu m etanid e reduced the relative volu m e of th e osteoclasts. Du rin g th e last 20 m inutes norm al m edia w as p erfu sed again for th e recovery of cell volum e. Un d er th e circu m stance of cell p erfu sed w ith n orm al m edia, cell volu m e w as not com pletely recovered to the origin al volu m e. H ow ever it is obscure w h at hap p en s in the cell volu m e regu lation before an d after bu m etanid e treatm ent .
Fig . 8. Effect of bu m etanid e on cell p roliferation in coculture.
Bu m etanid e (1, 10, 100 μM) w as ad d ed to th e osteoblastic cell/ bone m arrow cocu ltu re in the p resence of 10 nM 1α,25(OH)2D3. After incu bation for ad dition al 4 d ays, MTT assays
w ere carried out accordin g to th e m eth od d escribed in m aterials an d m eth od s.
Fig . 9. Effect of bu m etanid e on osteoblastic cell volu m e. Ten m icrom olar bu m etanid e w as treated to osteoblastic cell. In th e p resence of bu m etanid e, cell volu m e w as d ecreased su ggestin g th at th e blockad e of N KCC1 activity cau sed th e d ecrease in osteoblastic cell volu m e. After exp osin g th e osteoblastic cell to the n orm al m edia for 30 m in, 10 μM bu m etanid e w as treated to osteoblastic cell.
4 . Ch an g e s i n m RN A exp re ss io n p ro f ile in RA N KL, O PG, an d M -CSF
Osteoclastogenesis is kn ow n to be m ediated an d m odu lated by th e secretion of RAN KL, OPG, an d M-CSF by osteoblastic cells (Figs. 10, 11, 12). Therefore, bu m etanid e w as ad d ed to th e osteoblastic cell cu lture an d incubated for 3 d ays. Total RN A w as isolated an d th e exp ression s of RAN KL, OPG, and M-CSF w ere m onitored by RT-PCR. As th e bu m etanid e concentration ad d ed to th e osteoblastic cell cu ltu re increased, the expression of RAN KL m RN A indu ced by 1α,25(OH)2D3 w as d ow n-regu lated . Th e
d ecrease of RAN KL expression w as d ep en d ent on the increase in bum etanid e concentration . Moreover, the expression of OPG m RN A, a novel TN F receptor fam ily m em ber w as increased . These findings in dicate th at bu m etanid e inhibits osteoclast differentiation via inhibiting th e exp ression of RAN KL m RN A an d enh ancin g th e exp ression of OPG m RN A. H ow ever th e expression of M-CSF, a su rvival factor of osteoclasts, w as n ot chan ged regardless of bu m etanid e concentration . β-actin w as u sed as the control of th e m RN A exp ression in th e osteoblastic cells.
5. Ratio o f RA N KL/ O PG m RN A in re sp o n se to b u m etan i de
Ratio of RAN KL/ OPG m RN A w as illu strated in Figu re 13. As th e bu m etanid e concentration w as increased, th e ratio of
A B Vit D3 + + + + Vit D3 + + + + (10 n M ) (10 n M ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM )
Fig . 10. Effect of bu m etanid e on th e expression of RAN KL m RN A in osteoblastic cell. Panels A, RAN KL; B, β-actin . Bu m etanid e (1, 10, 100 μM) w ere ad d ed to the osteoblastic cell in the presence of 10 nM 1α,25(OH)2D3. After incubation for 2
d ays, total RN A w as isolated and m onitored RAN KL m RN A u sin g RT-PCR.
A B Vit D3 + + + + Vit D3 + + + + (10 n M ) (10 n M ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM )
Fig . 11. Effect of bu m etanid e on th e expression of M-CSF m RN A
in osteoblastic cell. Pan els A, M-CSF; B, β-actin . Bu m etanid e (1, 10, 100 μM) w ere ad d ed to th e osteoblastic cell in th e p resence of 10 nM 1α,25(OH)2D3. After incu bation for 2 d ays, total RN A
A B Vit D3 + + + + Vit D3 + + + + (10 n M ) (10 n M ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM ) Bu m - 1 10 100 Bu m - 1 10 100 (μM ) (μM )
Fig . 12. Effect of bu m etanid e on th e exp ression of OPG m RN A in
osteoblastic cell. Panels A, OPG; B, β-actin . Bu m etanid e (1, 10, 100 μM) w ere ad d ed to th e osteoblastic cell in th e presence of 10 nM 1α,25(OH)2D3. After incubation for 2 d ays, total RN A w as
Fig . 13. Relative exp ression of RAN KL and OPG m RN A in osteoblastic cells. Agarose gel electroph oresis w as carried ou t and d en sities of th e ban d s w ere analyzed w ith d ensitom eter . Relative ratio of th e RAN KL and OPG m RN A w as calcu lated based on th e d ensity of each ban d .
RAN KL/ OPG m RN A w as d ecreased . This m eans that bu m etanid e cau sed th e ch anges in m RN A exp ression (RAN KL an d OPG) of signal m olecu les w hich are closely linked to th e osteoclastogen esis. Con sequ ently, bum etanid e inhibited osteoclastogen esis, leadin g to the alteration of RAN KL, OPG m RN A expression . Also su ch ch anges in expression levels of signal m olecules w ere d ep en d ent on bu m etanid e concentration .
6. Th e eff ect o f b u m etan i de o n t-JN K an d p-JN K
c-Ju n N H2-term in al kinase (JN K), on e of th e m itogen activated
p rotein kin ase (MAPK) fam ily, has been kn ow n to exhibit activation by hyp er osm olarity an d cellu lar volu m e chan ge (Janet
et. al., 1999). To investigate th e effect of bu m etanid e on the
exp ression an d ph osp h orylation of JN K (p -JN K), bu m etanid e w as ad d ed to the osteoblastic cells an d incubated for 3 d ays.
Bu m etanid e did n ot h ave any effect on the exp ression of JN K. Total am ou nt of JN K (t-JN K) w as rem ained con stant in th e ran ge of bu m etanid e concentration to be u sed . H ow ever, bu m etanid e au gm ented the ph osp horylation of JN K in the d ose d ep en d ent m ann er, in dicatin g that bum etanid e activated th e JN K in th e p rocess of osteoclastogenesis. (Fig. 14).
Fig . 14 . Effect of bu m etanid e on th e activation of JN K. Osteoblastic cells w ere exp osed to 10 μM bum etanid e for indicated tim e. Anti-p h osp ho-JN K or anti-JN K antibody w as u sed as a p rim ary antibody for th e Western blot.
IV . D iscu ssio n
In th e process of d evelop in g m ature osteoclasts, th e fu nction s of osteoblasts an d osteoclasts are intim ately related . Du rin g skeletal d evelop m ent an d bon e rem od elin g process, osteoblasts synth esize an d secrete sign al m olecu les th at control osteoclast differentiation (Ducy et al., 2000) an d cell-to-cell interaction s are n ecessary during th e w h ole procedure. This is a direct and crucial interaction th at has been w ell established in vivo (Bu rgess
et al., 1999). Thu s osteoblasts p lay a cru cial role in differentiatin g
th e m acroph age/ m onocyte to m ature osteoclasts. H ow ever, it has been only 4-5 year since clear m ech anism of m aturation of m acrop hages into osteoclasts has been id entified . There are tw o m olecules th at are essential and su fficient to prom ote osteoclastogen esis: M-CSF an d RAN KL (Teitelbau m , S., 2000). M-CSF bin d s to its recep tor, c-fm s, on m acrop h age to provid e signals requ ired for their survival and proliferation . OPG is kn ow n to be a solu ble "d ecoy " receptor th at com p etes w ith RAN K for RAN KL. It h as been d escribed th at th e balance betw een th e expression of the stim ulator of osteoclastogenesis, RAN KL, an d of th e inhibitor, OPG, is the cru cial factor th at governs the am ou nt of bone resorbed .
In th e conju ction of m achin eries in osteoblast, it h as been rep orted th at N a/ K/ Cl cotran sp orter (N KCC1) is present in osteoblasts recently . We still d o n ot kn ow the role of N KCC1 in
osteoblast . N evertheless, it m ay be p ossible to hyp oth esize that th e N KCC1 is involved in th e bon e rem od eling p rocess, ju d gin g from th e facts that 1) N KCC1 cotran sp orter exists in osteoblasts, 2) N KCC1 tran sp orts the ion inw ard an d outw ard across th e ep ith elia and p lay an im p ortant role in regu latin g th e cell size an d volu m e, and 3) N KCC1 affects the d evelopm ent an d grow th of the cells (Ru ssell, J.M. et al., 2000). H ow ever, th ere is n o p lau sible exp lan ation for th e role of N KCC1 in osteoblast in concern w ith osteoclastogen esis (Fig. 4). Therefore, w e aim ed at revealing th e role of N KCC1 in osteoblast, inhibitin g th e N KCC1 activity u sing bu m etanid e.
Unexp ectedly, w e fou nd that bu m etanid e inhibited th e osteoclastogen esis in cocu ltu re system , osteoblast/ strom al cell. This fact give u s th e new clu es th at N KCC1 is p robably resp on sible for the d ow n-regu lation of osteoclastogenesis. Therefore, in this stu dy w e app lied osteoblast/ strom al cell cocu ltu re system to evalu ate 1) effect of bum etanid e on 1α,25(OH)2D3-indu ced osteoclast form ation, 2) d ecrease in cell
volum e by bum etanid e, 3) chan ges in m RN A exp ression p rofile in RAN KL, OPG, an d M-CSF, 4) Effect of bu m etanid e on t-JN K an d p-JN K.
As sh ow n in Fig 6 an d 7, w h en 10 an d 100 μM bum etanid e w as ap plied to the cocu ltu re system , th e num ber of TRAP p ositive cells w ere m arkedly reduced in a d ose d ep en d ent m ann er com p are to th e control group . H ow ever, 1 μM
bum etanid e did not ch ange 1α,25(OH)2D3-in duced osteoclast
differentiation . Moreover, cell viability test sh ow ed th at bum etanid e did n ot sh ow toxic effect w hen ad d ed u p to 100 μ M to th e cocu ltu re (Fig. 8). It is clear th at bu m etanid e has som e effects on form ation of TRAP p ositive cell w ith no toxic effects. So, cell volu m e w as m easu red to d eterm ine w heth er bu m etanid e h as any effect on cell volu m e since N KCC1 plays a m ajor role in controllin g th e volu m e of cells. As sh ow n in Fig. 9, 10 μM of bum etanid e redu ced th e relative cell volum e to th e half level.
Osteoclastic bon e resorp tion con sists of m u ltip le step s such as th e differentiation of osteoclast p recu rsors into m on onuclear p refu sion osteoclasts (pOC), the fu sion of p OCs to form m u ltinucleated osteoclasts, an d the activation of osteoclasts to resorb bone. Several factors related in th e differentiation, m aturation and activation of osteoclasts h ave been rep orted, w hich inclu d e RAN KL, OPG and M-CSF. Since osteoclast differentiation h as been rep orted to be m ediated by th ese factors, m RN A exp ression s w ere investigated . Th e exp ression of RAN KL m RN A in duced by 1α,25(OH)2D3 w as d ow n-regulated w ith the
increase of bu m etanid e concentration . Moreover, the expression of OPG m RN A, a n ovel TN F receptor fam ily m em ber w as increased . These findings in dicate th at bu m etanid e inhibits osteoclast differentiation via inhibiting th e exp ression of RAN KL m RN A an d enh ancin g the expression of OPG m RN A.
inhibits cellu lar u ptake of N a+
, K+
, and Cl
-ion s, w hich resu lts in th e cellular volum e ch an ge. O'Donnell et. al. d em onstrated th at p h osp hatase inhibition results in th e elevation of en d othelial cell volum e, thu s su ggestin g th at restin g en d oth elial cell volum e is also d eterm in ed by th e integrated activities of kin ases an d p h osp hatases. The stim ulation of co-tran sp orter by cell shrinkage w as n ot enhanced by ph osp h atase inhibition, in dicating th at the effects of shrinkage an d p h osp hatase inhibition w ere n ot ad ditive, su ggestin g th at shrinkage inhibits th e p hosph atase. In this stu dy, bum etanid e did not h ave any effect on the expression of JN K. Total am ou nt of JN K (t-JN K) w as rem ained con stant in th e ran ge of bu m etanid e concentration to be u sed bu t th e activated form of JN K, ph osp horylated JN K (p -JN K), w as d ecreased accordin g to th e increase of bu m etanid e concentration . JN K is on e of the m itogen activated p rotein kinase (MARK) fam ily . It h as been kn ow n to exhibit activation by hyp er-osm olarity and cellu lar volum e ch an ge. Th e d ecrease of p -JN K in dicates th at th e cell volum e ch an ge of osteoclasts are du e to activation of JN K in osteoclasts.
From th ese resu lts, it m ight be inferred that cellu lar shrinkage of osteoblastic cell in du ced by th e ad dition of bu m etanid e, w hich resu lts the inhibition of N KCC1 activity, is an im p ortant m odu lator in regulating osteoclastogen esis.
Fig . 15. Prop osed m od el for th e alteration of osteoclastogen esis
via N KCC1 m odu lation . Th e osm otic shock on osteoblastic cell resu lts in m odu lation of N KCC1. It is p roved that th e shirinkage in osteoblasts resu lt in d ecrease of osteoclast activity.
V . Co n clu sio n
In th e process of osteoclastogenesis, th ere are several factors th at inhibit or stim u late th e w h ole process. The m atu re osteoclasts are produ ced in th e presence of osteoblasts and osteoclast p recu rsor cell togeth er w ith cell-to-cell interaction . Several sign al m olecu les, kn ow n as RAN KL, OPG, an d M-CSF w hich exp ressed from osteoblasts h ave im p ortant fu nctions in th e process of osteoclastogen esis.
Th ere are several w ays to control osteoblasts in th e p rocess of osteoclastogen esis but th e role of N a-K-Cl cotran sp orter of osteoblasts related to osteoclast differenciation h as not been clarified . Since it is rep orted that th e presence of N KCC1 in th e m em brane of osteoblasts an d the fact th at it controls the ion tran sp orts an d cell volum e, p ossibilities of N KCC1 en gaged in th e p rocess of osteoclastogenesis m ight be p resu m ed .
Th erefore, bu m etanid e, a sp ecific inhibitor of N KCC1, w as u sed in th e cocu ltu re system of osteoblasts and osteoclasts to evalu ate th e effect of N KCC1 on osteoclastogen esis,. When 10 an d 100 μM of bu m etanid e w as u sed, inhibition of osteoclasts w as n oted . Th e resu lts w ere run throu gh MTT test to evalu ate th e toxic effect of bu m etanid e on osteoclasts an d proved th at bum etanid e concentration low er th an 100 μM d oes n ot effect the cell p roliferation .
resu lted from th e effect of bum etanid e w ere m easu red . The resu lts show ed th at th e relative cell volum e w as redu ced to th e h alf level. To confirm the cell volum e ch an ges of osteoclasts, th e m odu lator for cell volu m e ch an ge, the JN K of osteoclasts w as an alyzed . Th e resu lt sh ow ed th at th e activated form of JN K w as increased accordin g to increase of bum etanid e concentration . Moreover, the m RN A exp ression of the sign al m olecu les, kn ow n as RAN KL, OPG, an d M-CSF w ere m easu red . The results sh ow ed that the m RN A expression of RAN KL an d M-CSF w ere d ecreased and th at of OPG w as increased .
Th ese resu lts su ggest th at w h en bu m etanid e, a sp ecific inhibitor of N KCC1, is u sed, th e p rocess of osteoclastogen esis is inhibited by controlling the m RN A expressions of sign al m olecules su ch as RAN KL an d OPG in osteoblasts an d also by redu cing the relative cellu lar volu m e of osteoclasts.
V I. Ref ere n ce s
1. Al-H abori, M . Macrom olecu lar crow din g an d its role as intracellular sign allin g of cell volu m e regu lation . Inter J of Biochem & Cell Bio 33: 844-864, 2001
2. Akatsu , T., Takahashi, N ., Ud agaw a, N ., Sato, K., N agta, N ., Moseley, J.,Martin, J., Su d a, T. Parathyroid h orm on e-related protein is a p otentstim u lator of osteoclast-like m ultinucleated cell form ation to th e sam e extent as PTH in m ou se m arrow cu ltu res. En d ocrinology 125: 20-27, 1989
3. Au bin, J.E. Bon e stem cells. J of Cell Bioch em su pp lem ents 30: 73-82, 1998
4. Bu rgess, T., Kau fm an, S., Ring, B., Van, G., Capp arelli, C., Kelley, M., H su , H ., Boyle, W ., Du nstan, C., Hu , S., Lacey, D . Th e ligand for osteoprotegerin (OPGL) directly activates m atu re osteoclasts. J of Cell Bio 145: 527-538, 1999
5. Du cy, P ., Shinke, T., Karsenty, G. Th e osteoblast : a sop histicated fibroblast u nd er central su rveillance. Science 289: 1501-1507, 2000
6. Ferrari, M., Fornasiero, M.C., Isetta, A .M. MTT colorim etric assay for testing m acroph age cytotoxic activity in vitro. J of Im m u n Method s 131: 165-172, 1990
7. Fu ller, K., Won g, B., Fox, S., Ch oi, Y., Cham bers, T. TRAN CE is n ecessary and su fficient for osteoblast-m ediated activation of bon e resorption in osteoclasts. J Biol Ch em 272: 25190-25194,
1998
8. H aas, M., Forbu sh, B. The N a-K-Cl cotran sp orters. J of Bioen erg & Biom em b 30: 161-172, 1998
9. Jim i, E., Akiyam a, S., Tsu rukai, T., Okah ashi, N ., Kobayashi, K., Ud agaw a, N ., N ishihara, T., Takah ashi, N ., Su d a, T. Osteoclast differentiation factor acts as a m ultifu nctional regu lator in m u rin e osteoclast differentiation an d fu nction . J of Im m u n 163: 434-442, 1999
10. Krieger, N .S. Parathyroid h orm on e, prostaglan din E2, and
1,25-dihydroxyvitam in D3 d ecrease th e level of N a +
-Ca2 +
exchan ge protein in osteoblastic cells. Calcif Tissu e Int 60: 473-478, 1997
11. Kon g, Y.Y., Yoshid a, H ., Sarosi, I., Tan, H ., Tim m , E., Capp arell, C. OPGL is a key regu lator of osteoclastogen esis, lym p hocyte d evelopm ent an d lym ph-n od e organogen esis. N atu re 397: 315-323, 1999
12. Lacey, D ., Tim m s, E., Tan, H ., Kelley, M., Dun stan, C., Burgess, T., Elliott, R., Colom bero, A ., Elliott, G., Scu lly, S., H su , H ., Su llivban, J., H aw kin s, N ., Davy, E., Cap p arelli, C., Eli, A., Dau fm an, S., Sarosi, I., Sh alh oub, V., Senal, G., Gu o, J., Delan ey, J. Osteop rotegerin lin gan d is a cytokine th at regu lates osteoclast differentiation an d activation . cell 93: 165-176, 1998
13. Maru n aka, Y., N iisato, N ., O' Brod ovich, H ., Post, M ., Tansw ell, A .K. Roles of Ca2 +
in su lin action on cell volum e via N a+
an d K+
channels and N a+/ K+/ 2Cl- cotransp orter in fetal rat alveolar typ e II pn eu m ocyte. J Mem b Biol 168: 91-101, 1999
14. McCarty, N .A ., O' N eil, R. Calcium -d ep en d ent control of volum e regu lation in renal p roxim al tu bu le cells: Roles of dihydrop yridine-sen sitive an d insen sitive Ca2 + entry p athw ays. J Mem b Biol 123: 161-170, 1991
15. N akagaw a, N ., Yasu d a, H ., Yano, K., Shin-ichi, M ., Kobayashi, N ., Fujim oto, H ., Yham agu chi, K., Shim a, N ., Morinaga, T., Higashio, K.Basic fibroblast grow th factor inhibits osteoclast form ation in du ced by 1α,25-dihydroxyvitam in D3 throu gh
su pp ressin g th e produ ction of osteoclast diffentiation factor . Bioch em & Biop hys Res Com m 265: 45-50, 1999
16. Raise, L. Physiology and p athop hysiology of bon e rem od eling. Bone 23: 339-409, 1998
17. Rod an, A.R. Control of bone form ation an d resorp tion : Biological and clinical p ersp ective. J of Cell Bioch em su pp lem ents 30: 55-61, 1998
18. Ru ssell, J.M. Sodium -p otassium -chlorid e cotran sp ort . Physiol Review s. 80: 211-227, 2000
19. Sim on et, W .S., Lacey, D .L., Dun stan, C.R., Kelly, M ., Ch an g, M.S., N gu yen, H ., Wood en, S., Benn ett, L. Boon e, T., Shim am oto, G., DeRose, M . Osteoprotegerin : a n ovel secreted protein involved in th e regulation of bon e d en sity. Cell 89: 309-319, 1997
20. Slad ow ski, D ., Steer, S.J., Clothier, R.H ., Ball, M. An im p roved MTT assay . J of Im m u n Meth od s, 157: 203-207, 1993
21. Su d a, T., Ud agaw a, N ., N akam ura, I., Miyau ra, C., Takah ashi, N . Modulation of osteoclast differentiation by local factors. Bon e 17: 87s-91s, 1995
22. Takahashi, N ., Ud agaw a, N ., Su d a, T. A new m em ber of
tum or n ecrosis factor ligan d fam ily,
ODF/ OPGL/ TRAN CE/ RAN KL, regu lates osteoclast differentiation an d fu nction . Biochem & Biop hy Res Com m 256: 449-455, 1999
23. Tsu d a, E., Goto, M., Mochizuki, S., Yano, K., Kobayashi, F., Morinaga, T., Higashio, K. Isolation of novel cytokin e from hum an fibroblasts that sp ecifically inhibits osteoclastogenesis. Biochem & Biop hy Res Com m 234: 137-142, 1997
24. Tsu kii, K., Shim a, N ., Mochizu ki, S., Yam agu chi, K., Kin osaki, M., Yan o, K., Shibata, O ., Ud agaw a, N ., Yasu d a, H . Osteoclast differentiation factor m ediates an essential signal for bon e resorption in du ced by 1α,25-dihydroxyvitam in D3,
prostaglan din E2, or p arathyroid h orm one in th e
m icroenvironm ent of bon e. Bioch em & Biophy Res Com m 146: 337-341, 1998
25. Yasu d a, E., Shim a, N ., N akagaw a, N ., Yam agu chi, K., Kn osaki, M. Osteoclast differentiation factor is a ligand for osteop rotegerin / osteoclastogenesis-inhibitory factor an d is id entical to TRAN CE/ RAN KL. Proc N atl Acad Sci USA
95:3597-3602, 1998
26. Whisen ant, N ., Zh ang, B., Khad em azad, M., Loessberg, P., Mu allem , S. Regu lation of N a-K-2Cl cotran sp ort in osteoblasts. Am . J of Physiol 257: C433-440, 1991
국 문 요 약
N a- K-Cl cotran sp orter의 비 활 성 화 가 비 타 민 D3에 의 해
유 도 되 는 파 골 세 포 의 분 화 에 미 치 는 영 향
연세대학교 대학원 치의학과 김은영
N a-K-Cl cotransp orter (N KCC1)은 다양한 세포와 조직에 분포 하며 N KCC1의 기능은 세포 내외로 이온을 전달하고 세포의 부피 와 이온의 균형을 이루고 또한 세포의 성장과 발육에 영향을 준다. 이 연구에서는 비타민 D3에 의해 유도되는 파골세포에 N KCC1이 미치는 영향을 검토하였다. N KCC1의 선택적 억제제인 bum etanid e 를 10, 100 μM 첨가하였을 경우 TRAP 양성 세포의 수는 각 w ell 당 평균 42에서 15로 감소하였다. Bu m etanid e 1 μM를 첨가하였 을 경우 비타민 D3에 의해 유도되는 파골세포의 분화에는 영향을 주지 못하였다. 결과적으로 N KCC1의 억제로 인하여 파골세포의 분 화가 억제된 것을 알 수 있다. Bu m etanid e의 억제 효과를 확인하기 위하여 RT-PCR을 통한 RAN KL, OPG 그리고 M-CSF의 m RNA의 발현을 측정한 결과 조골세포에 대한 bu m etanid e의 농도가 높아질 수록 RAN KL과 M-CSF의 발현 정도는 감소하였고 RAN KL의 d ecoy receptor인 OPG의 발현 정도는 증가하였다. 이러한 결과로 부터 조
골세포에 bu m etanid e를 첨가하는 경우 조골세포내의 RAN KL,
OPG, M-CSF의 발현을 조절하여 파골세포로의 분화가 억제됨을 확 인하였다. 또한 bum etanid e를 첨가하여 N KCC1을 억제할 경우 원
래 파골세포의 부피에 비하여 상대적인 파골세포의 부피가 36% 정 도까지 감소하였으며, 이러한 변화의 기전을 밝히기 위하여 세포의 용적 변화의 매개 물질인 JN K를 검사한 결과 약물의 농도가 증가할 수록 활성화된 JN K가 증가하는 것을 확인할 수 있었다.
결과적으로 N KCC1은 파골세포의 분화에 중요한 역할을 가지고 있으며, bum etanid e에 의하여 억제된 N KCC1의 활성은 조골세포의 부피의 변화를 초래하고, 파골세포 분화 조절 인자인 RAN KL, M-CSF의 감소 및 OPG의 증가를 유발한다.
_____________________________________________________________________
핵심되는 말 : N KCC1, bu m etanid e, 파골 세포, RAN KL, OPG, JN K