의학 박사학위 논문
A Novel Mouse Model of Asthma
Induced by Cockroach
아 주 대 학 교 대 학 원
의 학 과
2
-A Novel Mouse Model of -Asthma
Induced by Cockroach
by
Yoo Seob Shin
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF PHILOSOPHY
Supervised by
Hae Sim Park, M.D., Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
신유섭의 의학 박사학위 논문을 인준함.
심사위원장 박 선 인
심사위원 박 해 심 인
심사위원 이 수 영 인
심사위원 박 중 원 인
심사위원 남 동 호 인
아 주 대 학 교 대 학 원
2008 년 6 월 23 일
4
-Acknowledgement
I am very grateful to many teachers and friends who kindly contributed their time and effort to help me make this manuscript as useful as possible. I would particularly like to express my deepest thanks and respect to Dr. Hae-Sim Park and Dr. Jung-Won Park, who gave me an opportunity to get involved in this experiment, thoroughly reviewed the manuscripts, and always encourage me. I do appreciated Dr. Soo-Young Lee, Dr. Dong-Ho Nahm, and Dr. Sun Park for their kind advice in reviewing this manuscript and for constructive comments. I would like to express sincere gratitude to Dr. Yong-Won Lee, Jung-Ho Sohn, and Joo-Young Kim for their assistance in my experiment.
Finally, I particularly want to acknowledge the great moral support and contributions of my family, wife, lovely son and parents.
Specially, I would like to dedicate this manuscript to my son, Seung-Jun.
August 2008 Yoo Seob Shin
- ABSTRACT -
A Novel Mouse Model of Asthma Induced by Cockroach
Purpose; Cockroach (CR) is an important inhalant allergen and can make a mouse asthma
model. However, mechanism underlying the CR-induced mouse model of asthma is not clear and the role of endotoxin contaminated in CR allergen remains controversial. Therefore, we evaluated the role of endotoxin and toll like receptor-4 (TLR-4) for the induction of asthma in mouse model.
Methods; CR allergen was delivered intranasally to BALB/C mouse, TLR-4 mutant (C3H/HeJ)
or wild type (C3H/HeN) mouse without any adjuvant. Asthma was induced by CR, CR with endotoxin added (10,000 EU/mouse) or endotoxin-depleted CR, respectively.
Results; CR asthma model can be produced by intranasal sensitization of CR allergen, dose
dependently. CR can induce airway hyper-responsiveness (AHR) to methacholine, eosinophilic and neutrophilic inflammation, and airway mucus gland hyperplasia. Endotoxin added in CR attenuated eosinophilic inflammation, IL-13 in BAL fluid, and goblet cell hyperplasia of respiratory epithelium, however, did not affect AHR. Endotoxin-depleted CR also attenuated eosinophilic inflammation and lymphocytosis in BAL fluid, but did not affect AHR and IL-13 levels in BAL fluid compared to CR group. AHR, eosinophilic inflammation, and goblet cell hyperplasia induced by CR were more exacerbated in TLR-4 mouse than in wild type mouse.
ii
Conclusion; CR can induce allergic asthma model presenting increased AHR, eosinophilic and
neutrophilic inflammation, and goblet cell hyperplasia. However, endotoxin in the CR dose not appears to affect the development of AHR, eosinophilic inflammation, and epithelial goblet cell hyperplasia in CR-induced mouse model of asthma.
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· v
LIST OF TABLES ··· vi
Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 4
A. Materials ··· 4
1. Animals ··· 4
B. Methods ··· 4
1. Allergen preparations ··· 4
2. Sensitization ··· 5
3. Measurement of airway hyper-responsiveness ··· 6
4. Bronchoalveolar lavage fluid analysis ··· 7
5. Cytokines analysis ··· 7
6. Pulmonary pathology analysis ··· 7
7. Statistical analysis ··· 7
Ⅲ. RESULTS ··· 9
A. Dose-dependency for development of CR induced mouse model of asthma ··· 9
B. The effects of endotoxin on the development of CR induced mouse model of asthma ··· 9
iv
C. Experiment with TLR-4 mutant mouse ··· 10
Ⅳ. DISCUSSION ··· 12
Ⅴ. CONCLUSION ··· 16
REFERENCES ··· 28
LIST OF FIGURES
Fig. 1 Protocols of cockroach sensitization ··· 17
Fig. 2 Cockroach induced asthma model in BALB/C mouse ··· 18
Fig. 3 Effect of endotoxin added to cockroach induced asthma model in BALB/C mouse ··· 19
Fig. 4 Endotoxin-depleted cockroach induced asthma model in BALB/C mouse ··· 20
Fig. 5 PAS staining of cockroach induced asthma model in BALB/C mouse ··· 21
Fig. 6 Trichrome staining of cockroach induced asthma model in BALB/C mouse ··· 22
Fig. 7 AHR and differential cell count in BAL fluid in TLR-4 mutant mouse exposed to cockroach ··· 23
Fig. 8 The level of cytokines in TLR-4 mutant mouse exposed to cockroach ··· 24
Fig. 9 PAS staining of TLR-4 mutant mouse exposed to cockroach ··· 25
vi
LIST OF TABLES
I. INTRODUCTION
Asthma morbidity continues to rise, especially among inner-city children. Recent data indicate that the disproportionate increase of asthma in densely populated urban areas appears to be due to increment of allergen exposure. In particular, cockroach (CR) allergen exposure has demonstrated a significant correlation with the rise of adolescent asthma in the more densely crowded inner cities, where large numbers of CR can be found.(Campbell et al., 1998) Rosenstreich et al. reported that the exposure to high levels of CR allergen may explain the frequency of asthma-related health problems in inner-city children, (Rosenstreich et al., 1997) and Busse et al. published that sensitization to CR was associated with the admissions and emergency room visit due to asthma for both adults and children.(Busse et al., 2007)
As an important inhalant allergen to human, CR allergen has also been used in animal model of asthma. For the last 10 years, mouse model of asthmas has been developed to study the mechanisms of asthma.(Bice et al., 2000) Among them, OVA and purified indoor allergens such as CR and dust mite are commonly used allergens for murine models.(Clarke et al., 1999; de Siqueira et al., 1997; Zhou et al., 1998) Though asthma model by OVA with adjuvant is the standard prevailing animal asthma model, OVA model has many limitations. It has been shown that continuous respiratory exposure to OVA does not necessarily lead to chronic airway inflammation, but instead results in inhalation tolerance, and OVA model always needs the adjuvant to establish airway inflammation.(Johnson et al., 2004; Swirski et al., 2002) Moreover, the allergens used for these animal models may not represent the same conditions to which allergic patients are exposed throughout their daily life in terms of quality and quantity of
2
-allergens. Although allergens represent an important component of allergy, other initiating factors such as endotoxin are certainly present in the environment.(Kim et al., 2001)
Endotoxin is a component of the outer layer of the outer cell membrane of all Gram-negative bacteria, and influences the disease severity by acting as a natural adjuvant to allergic
inflammation. A significant amount of data has shown that exposure to endotoxin in the
environment or as a component of cigarette smoke leads to exacerbation of pre-existing asthma.(Hasday et al., 1999; Reed et al., 2001) However, other recent studies contradict the notion that endotoxin’s influence is a negative one.(Braun-Fahrlander et al., 2002; Matricardi et al., 1998; Strachan, 2000) Epidemiologic associations of environmental endotoxin exposure with allergy and asthma prevention are consistent with hygiene hypothesis which associates other microbial exposures or infections with a lower incidence of allergic disease. A large amount of endotoxin is present as contaminated in indoor allergens such as house dust mite and CR, therefore, we should first rule out the participation of endotoxin in such allergen experiment.
In this study, we produced a more physiologic animal model of asthma using CR allergen. CR allergen sensitizes via respiratory system, leads to chronic inflammation rather than tolerance, needs no adjuvant, and contains some endotoxin, therefore, it is a good example for physiologic model. Although the role of CR allergens in asthma has previously been established and studies using CR allergen in a mouse model of allergic asthma have established that this allergen is capable of inducing a significant pulmonary response that mimics human disease, underlying mechanism of mouse CR animal model of asthma is not clear and the role of endotoxin contaminated in CR allergen remains controversial.(Campbell et al., 1998)
and toll like receptor-4 (TLR-4) in the CR-induced mouse model of asthma by using TLR-4 mutant mouse.
4
-II. MATERIALS AND METHODS
A. Materials 1. Animals
Female BALB/C (6~8 weeks old), TLR-4 mutant (C3H/HeJ), and TLR-4 wild type (C3H/HeN) mouse were purchased from Jackson Laboratories (Seoul, Republic of Korea). All mice were housed under specific pathogen free conditions, the American Association for the Accreditation of Laboratory Animal Care-approved facilities, and maintained on a 12 hour light-dark cycle with food and water ad libitum. All experiments described in this study were approved by the Animal Research Ethics Board of Yonsei University (Seoul, Republic of Korea).
B. Methods
1. Allergen preparations
German CR extract; We prepared crude German CR extract as previous mentioned.(Lee et al., 2007) Thirty grams of live or frozen Blattela germanica were pulverized in liquid nitrogen. The sample was then defatted in 200 mL of 1 : 1 ethyl ether/ethyl acetate and extracted overnight with slow stirring at 4℃ in phosphate-buffered saline (PBS) (pH 7.4) containing 6mM 2-mercaptoethanol and 1mg/mL 1-phenyl-3-(2-thiazolyl)-2-thiourea to prevent melanization. The extract was then centrifuged at 9300 g for 30 min at 4℃, and the supernatant was filtered through a 0.22 mm filter. The level of endotoxin in the extract was 8,510 EU/mg by the QCL-1000 chromogenic LAL kit (Cambrex, East Rutherford, NJ,
USA), and total protein in the extract was 3.60 mg/mL, as evaluated with a Bradford assay reagent (Bio-Rad, Hercules, CA, USA).
Endotoxin free CR extract; Endotoxin was eliminated from the above crude CR extract by
a detoxi-gel endotoxin removing columns (Thermo, Rockford, IL, USA). Before use, detoxi-gel resin was washed sequentially with five resin-bed volumes of 1% sodium deoxycholate, followed by 3-5 resin-bed volumes of pyrogen-free buffer or water to remove the detergent. The crude CR extract was applied to the column, and the column was incubated for one hour before collecting the sample. After storing the sample, the level of endotoxin was found to be 0.0254 EU/mg by the QCL-1000 chromogenic LAL kit (Cambrex, East Rutherford, NJ, USA).
2. Sensitization
(A) Dose dependent experiment of CR allergen (Fig. 1A)
BALB/C mouse was sensitized twice per week with an intranasal saline, CR allergen (18.8 ㎍, 75 ㎍, or 300 ㎍ of CR/mouse, respectively) without any adjuvant during five weeks under anesthesia with isoflurane.
(B) Experiment for endotoxin added CR allergen (Fig. 1B)
BALB/C mouse was sensitized twice per week with an intranasal saline, CR allergen (300 ㎍ of CR/intranasal dose) or CR allergen plus additional endotoxin (10 ㎍) without any adjuvant during five weeks under isoflurane anesthesia.
(C) Experiment for endotoxin-depleted CR allergen (Fig. 1C)
6
-(300 ㎍ of CR/intranasal dose) or endotoxin-depleted CR allergen without any adjuvant during five weeks under isoflurane anesthesia.
(D) Experiment with TLR-4 mutant mouse (Fig. 1D)
To investigate the function of TLR-4, TLR-4 mutant mouse (C3H/HeJ) and TLR-4 wild type mouse (C3H/HeN) were used. These mice were sensitized twice per week with an intranasal saline or CR allergen (300 ㎍ of CR/intranasal dose) without any adjuvant during five weeks under isoflurane anesthesia.
3. Measurements of airway hyper-responsiveness (AHR)
Forty-eight hours after the last challenge, AHR to methacholine (MCh) was measured in the mouse intubated with 18 gause steel tube. Mouse was ventilated with a flexiVent 5.1® small animal ventilator (SCIREQ, Montreal, PQ, Canada) at 120 breaths per minute and a tidal volume of 30 ml/kg. A positive end expiratory pressure was set at 2 cmH2O. The
ventilation rate was set above the normal breathing rate to suppress spontaneous breathing during measurements.
Mouse was challenged with a saline control aerosol followed by increasing concentrations of MCh (Sigma-Aldrich, MO, USA; 3.1, 6.25, 12.5, 25, and 50 mg/ml). Aerosols were generated with an ultrasonic nebulizer and delivered to the inspiratory line of the flexiVent using a bias flow of medical air. Each aerosol was delivered for 12 seconds during which time regular ventilation was maintained. Two measurements were made at one-minute intervals following each aerosol.
4. Analysis of bronchoalveolar lavage fluid (BALF)
To collect BALF, the lungs were lavaged with 1 ml of Hank's balanced salt solution (HBSS) via intubated tube. Total cell numbers were counted with a hemocytometer. After that procedure, BALF was centrifuged at 1.5 rcf for 3 minutes at 4 ℃, and then smears of bronchoalveolar lavage (BAL) cells were prepared by cytocentrifugation (cytospin3, Thermo, MA, USA) at 1000 rpm for 3 minutes. All smears were stained with Diff Quick staining. Differential cell counts in BAL cells were done at least 200 leukocytes, using standard hemocytologic procedure to classify the cells as either neutrophils, eosinophils, lymphocytes, or other mononuclear leukocytes (macrophages and monocytes).
5. Cytokine analysis
ELISA kits were purchased from R&D Systems (Minneapolis, MN). Interleukin (IL)-5 and 13 for Th2 cell function, and interferon-gamma (IFN-γ) for Th1 cell function in the homogenate of lung tissue were detected by sandwich ELISA according to manufacturer’s recommendation.
6. Pulmonary pathology analysis
After BAL, lungs were inflated with 10% formalin at a standard pressure. Tissues were then embedded in paraffin, and 3 ㎛ thick sections were cut and stained with H&E, trichrome for fibrosis and Periodic Acid-Schiff (PAS) for goblet cell hyperplasia.
8
-Statistical differences in the mean values among groups were determined by using a one-way ANOVA. The paired Student t test was used to compare the mean between two groups. Differences in AHR were determined by F test analysis that compared values over the entire curve between each treatment group. From this F score, a value for p was generated. In all cases, a value for p < 0.05 was considered statistically significant.
III. RESULTS
A. Dose-dependency for development of CR-induced mouse model of asthma
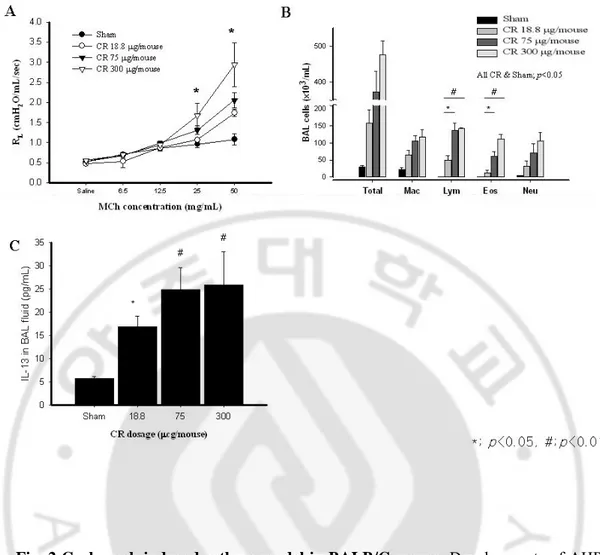
We evaluated the dose-dependency for development of CR asthma model in BALB/C mouse. (Table 1) Mouse sensitized with any concentrations of CR demonstrated significant increase of AHR at 25 and 50 mg/ml of methacholine concentration compared to the sham animal (p<0.05), and AHR achieved had a tendency to increase in dose dependent manner. (Fig. 2A)
Analysis of BAL fluid revealed significant differences in the number of total cells, macrophages, lymphocytes, eosinophils, and neutrophils between the mice sensitized with any concentrations of CR compared with sham mice. Numbers of these cells in BAL fluid were dose-dependently increased. (Fig. 2B) We also observed that the expression of IL-13 in BAL fluid was significantly higher in the mouse administered with 18.8~300 μg of CR extract than sham mice. The levels of IL-13 in BAL fluid were also dose-dependently increased, but with no statistical significance. (Fig. 2C) We observed no evidence of inflammation in the lungs of sham mice. However, the lungs of CR sensitized mouse exhibited remarkable perivascular and peribronchial inflammation, especially eosinophil, with clear evidence of goblet cell hyperplasia. (Fig. 2D)
B. The effects of endotoxin on the development of CR-induced mouse model of asthma
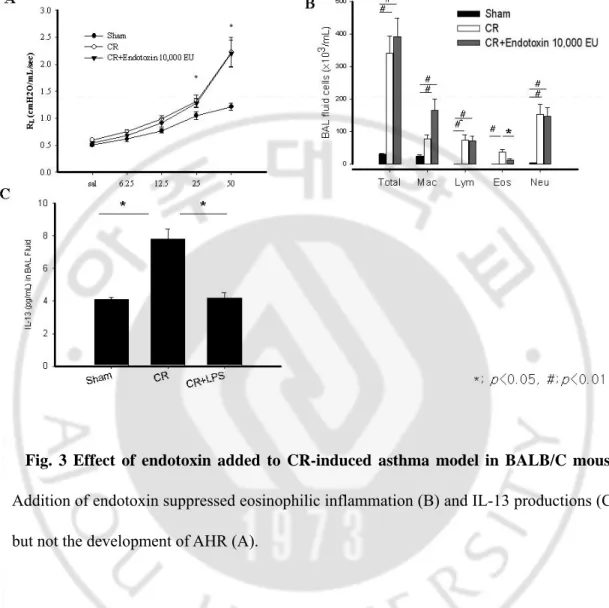
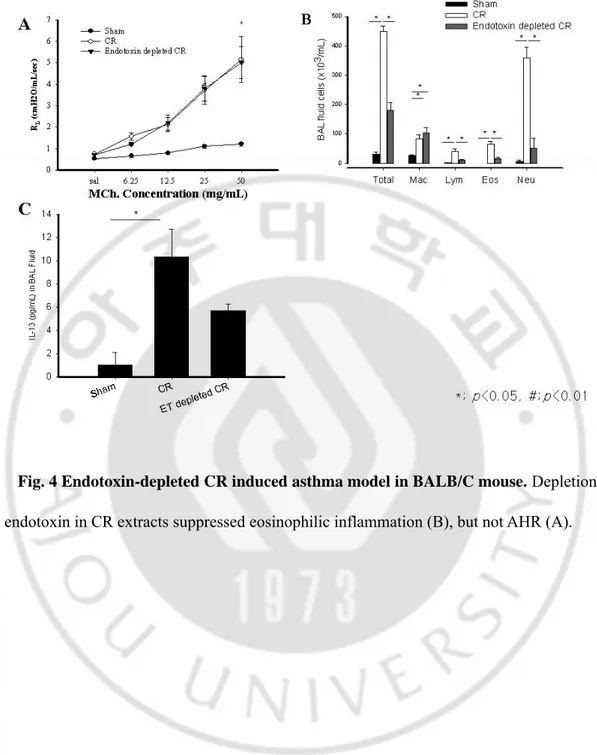
We evaluated the effects of endotoxin on the development of CR asthma model in BALB/C mouse. Addition of endotoxin (10,000 EU) to 300 μg of CR extract did not affect the development of AHR, and endotoxin-depleted CR extracts also developed AHR that was not
10
-different from AHR induced by unmanipulated CR. (Fig. 3A and 4A) Analysis of BAL fluid revealed significant differences in the number of total cells, macrophages, lymphocytes, and neutrophils between the mouse sensitized with endotoxin-added CR and sham mouse, except eosinophils (p<0.05). The number of eosinophil in endotoxin-added CR was significantly lower than that of CR without endotoxin addition (p<0.05). (Fig. 3B) The mouse sensitized with endotoxin-depleted CR extract had significantly less eosinophilic, lymphocytic and neutrophilic inflammation in BAL fluid than those of unmanipulated CR (p<0.05). (Fig. 4B)
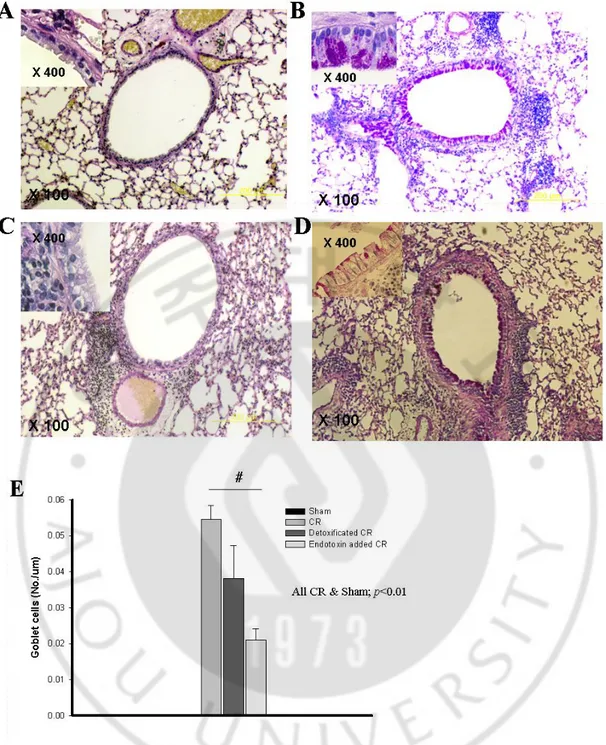
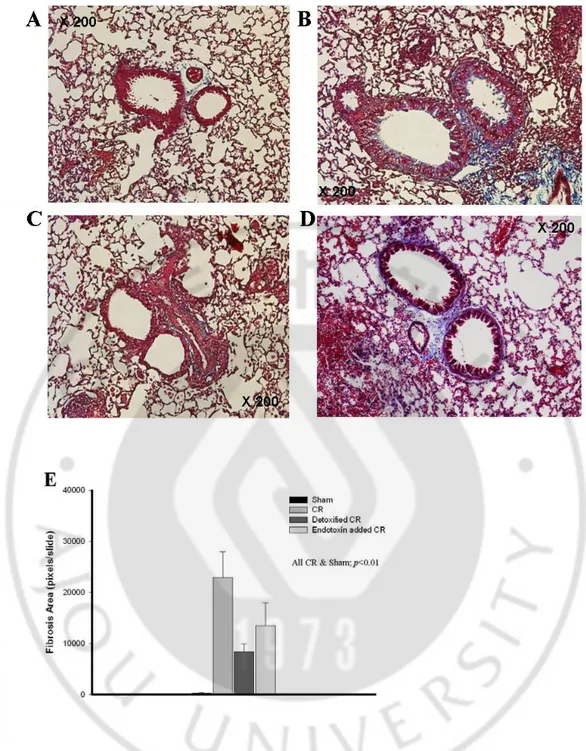
The level of IL-13 in endotoxin-added CR was significantly lower than that of CR (p<0.05), whereas the level of IL-13 in endotoxin-depleted mouse was not significantly different. (Fig. 3C and 4C) We observed remarkable perivascular and peribronchial inflammation in CR, endotoxin-added CR, and endotoxin depleted CR mouse. However, the goblet cell hyperplasia in respiratory epithelium was markedly diminished by the addition of endotoxin, nevertheless, the perivascular and peribronchial inflammatory cell infiltrations were prominent in this mouse. (Fig. 5 and 6)
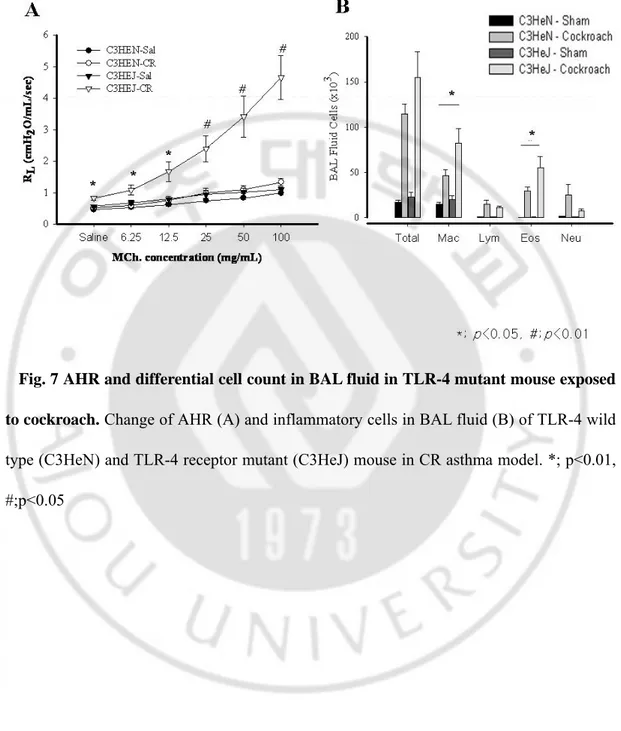
C. Experiment with TLR-4 mutant mouse
We evaluated the effects of 4 on the development of CR-induced asthma model in TLR-4 wild type mouse (C3H/HeN) and TLR-TLR-4 mutant mouse (C3H/HeJ). The mutant mouse developed AHR at all methacholine concentrations (p<0.05), but AHR of wild type mouse was not different from that of sham mice. (Fig. 7A) There were significantly increased numbers of total cells, macrophages, lymphocytes, eosinophils and neutrophils in BAL fluid of the C3H/HeN and C3H/HeJ mouse sensitized to CR, compared to sham mice. However, eosinophil
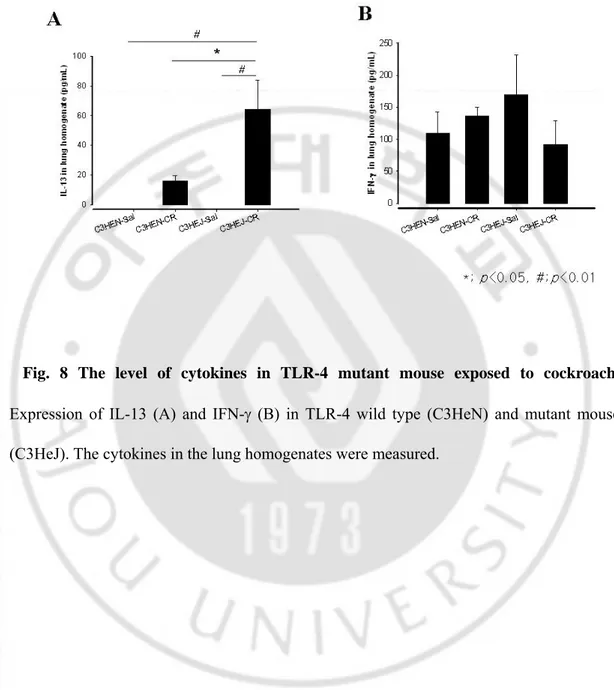
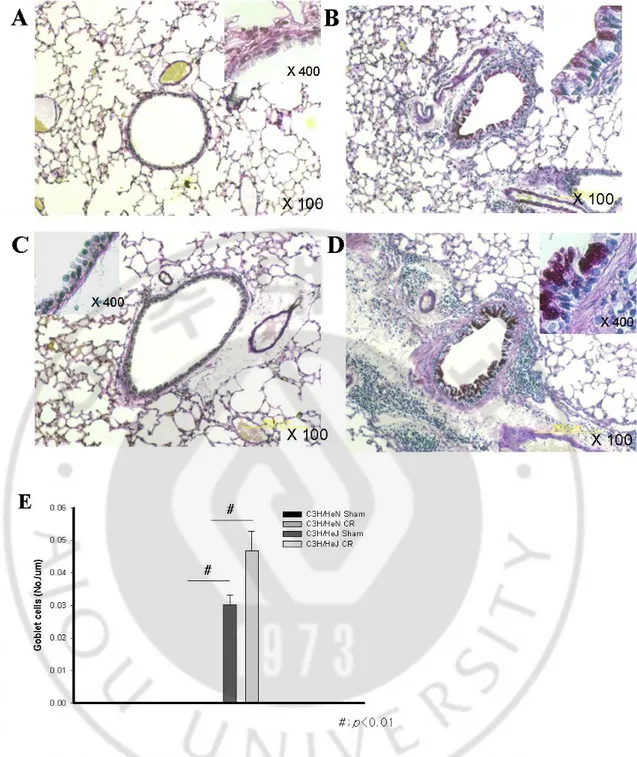
count in BAL fluid was more prominent in the mutant mouse than the wild type mouse (p<0.05). (Fig. 7B) IL-13 expression in the TLR-4 mutant mouse was significantly higher than that of TLR-4 wild type mouse (p<0.05). However, the expression of IFN-γ was not affected by TLR-4 mutation and sensitization of CR extract. (Fig. 8) We also observed remarkable perivascular and peribronchial inflammation in CR-sensitized mouse compared to both C3H/HeN and C3H/HeJ mice. Perivascular and peribronchial inflammatory cell infiltration were more apparent in the TLR-4 mutant mouse. (Fig. 9 and 10)
12
-IV. DISCUSSION
Several animal models of allergic asthma, including those which utilized parasite, OVA, and plant extracts as allergens, have been developed.(Mapp et al., 1985; Pritchard et al., 1983) Among these models, OVA asthma model with aluminum hydroxide as adjuvant is the standard animal model and contributed greatly to the understanding of many important mechanisms of asthma and basic processes that occur during allergic airway responses. Nevertheless, the OVA model is somewhat different from those of human asthma: Allergic diseases in human have a chronic nature resulting from intermittent or continuous exposure to allergen, and structural changes such as airway remodeling are characteristics. On the other hand, however, animal models using OVA are inadequate to evaluate some of the processes involved in airway remodeling.(Blyth et al., 1996; Leigh et al., 2002)
Therefore, many investigators attempted to develop more physiologic asthma model resembling that of human. CR is not only important human allergen, but also potent allergen in mouse. Exposure to CR is closely correlated to asthma severity, and it can sensitize host via respiratory system without any adjuvant.(Arruda et al., 2001; Hong et al., 2004) In the present study, we used CR allergen only with intranasal sensitization, and our results showed that CR allergen dose dependently induced airway hyper-reactivity, eosinophilic and neutrophilic inflammation, and goblet cell hyperplasia of respiratory epithelium. Such presentations resemble those that have been observed in human asthmatic patients.
Although some asthma animal models using CR have been introduced, there are some critical problems in these models.(McKinley et al., 2004; Zhou et al., 1998) These studies did
not explain the function of endotoxin in CR allergen: Crude CR contains a relatively large much amount of endotoxin, (Hong et al., 2004) and the role of endotoxin contaminated in CR allergen is controversial. Some investigators have attempted to explain the function of endotoxin by challenging mouse with endotoxin, however, the clinical relevance of this model is not clear. It has been suggested that exposure to low levels of endotoxin may exacerbate asthmatic responses, whereas exposure to high levels might be protective. This is based on two animal studies with opposite findings: Wan et al. showed that mouse exposed to 40 ng/ml aerosolized endotoxin prior to OVA challenge resulted in higher specific IgE levels and inhibited tolerance to OVA compared to the mouse exposed to saline prior to OVA challenge.(Wan et al., 2000) On the other hand, Tulic et al. found that exposure to 50 mg/ml concentration endotoxin prior to OVA challenge or up to 6 days after OVA challenge was protective and prevented the IgE response in rats.(Tulic et al., 2002)
The lack of a good animal model of endotoxin has so far made it difficult for investigators to explain the relationship between endotoxin, allergen and asthmatic response. However, we developed a novel mouse model of asthma induced by CR allergen that is characterized by airway hyper-responsiveness and pulmonary inflammation. The results, obtained with this model showed that the endotoxin added (15,810 EU/mL) did not improve AHR induced by CR allergen, however rather decreased in eosinophilic inflammation in BAL fluid, IL-13, and goblet cell hyperplasia compared with those induced by crude CR endotoxin (5,810 EU/mL). These data are in good agreement with previous studies, and indicate that high dose of endotoxin is protective in allergic disease progression. However, AHR, Th2 cytokine (IL-13), and goblet cells in airway were not changed in endotoxin-depletion experiment.
14
-In the present study, we also used TLR-4 mutant (C3H/HeJ) and TLR-4 wild type (C3H/HeJ) mouse to evaluate the function of endotoxin in the induction of CR animal model of asthma. TLR-4 functions as the signal transducing receptor for endotoxin in the cells of immune systems including macrophages and dendritic cells.(Medzhitov, 2001) Our results showed that AHR, eosinophil count in BAL fluid, IL-13 in lung homogenate, and goblet cell hyperplasia induced in C3H/HeJ mouse by crude CR were more severe than in wild type mouse (C3H/HeN). These results agree with those of added endotoxin BALB/C mouse: Endotoxin added to crude CR had protective effects on the features of allergic asthma, allergic inflammation, goblet hyperplasia and Th2 responses in BALB/C mouse. These features were aggravated also in TLR-4 non-functioning mouse, suggesting that endotoxin in CR may have a protective role in the development of allergic asthma. These results are also concordant with human epidemiologic study, which showed that endotoxin levels in samples of dust from the child'smattress were inversely related to the occurrence of hay fever, atopic asthma, and atopic sensitization, and that cytokine production by leukocytes was inverselyrelated to the endotoxin level in the bedding.(Braun-Fahrlander et al., 2002)
In this study, endotoxin in CR was found to have limited roles in the development of CR asthma, nevertheless, CR itself can induce allergic asthma. CR allergen contains several important allergens, including Bla g 1, Bla g 2, Bla g 4, and Bla g 5. Among these allergens, Bla g 2 is a potent allergen that elicits hypersensitivity response in 60~80% of CR allergic patients, and is a 36-kD protein that shows primary sequence homology to aspartic protease.(Arruda et al., 2001; Arruda et al., 1995) However, the strong capacity of Bla g 2 to stimulate IgE production is unrelated to aspartic protease activity: Pomes et al. reported that aspartic protease activity of the
allergen is not a prerequisite of allergenicity, and other enzyme such as serine protease of CR could contribute to inflammation although aspartic protease is not required for proteins to elicit IgE response.(Pomes et al., 2002) Kheradmand et al. reported that proteases may be generally required to overcome the allergen tolerance and develope allergic inflammation.(Kheradmand et al., 2002) Especially, serine protease has a specific receptor system that is protease activating receptor-2 (PAR-2), on a variety cell type including macrophage. PAR-2 activation in the airways concurrently with exposure to inhaled OVA induces allergic sensitization via dendritic cell activation, whereas exposure to OVA alone induces allergen tolerance.(Ebeling et al., 2007) Therefore, CR allergen sensitizes host without adjuvant and causes chronic inflammation.
In present study, we studied the characteristics of a mouse model of asthma in response to CR and the roles of endotoxin. CR exposure can elicit asthma-like pulmonary inflammation characterized by AHR, eosinophilia, elevation of Th2 cytokine and goblet cell hyperplasia, and these features are related to the amount of exposed CR during sensitization. Higher dose of endotoxin inhibited eosinophilic inflammation, the elevation of Th2 cytokine level and goblet cell hyperplasia induced by CR allergen. Endotoxin contaminated in CR allergen may have limited roles in the development of CR asthma model and even have protective roles in the development of asthma. This novel mouse model of asthma induced by CR represents a valuable tool for further study and may allow dissecting of the allergen-endotoxin interactions which seem to be important in the pathogenesis of asthma.
16
-V. CONCLUSION
CR can induce an allergic asthma presenting increased AHR, eosinophilic and neutrophilic inflammation, and goblet cell hyperplasia of respiratory epithelium. Endotoxin contaminated in CR has limited roles or protective in the development of CR asthma and does not affect the development of AHR in CR asthma model.
Fig. 1 Protocols of cockroach sensitization: CR allergen dose dependent experiment (A),
CR allergen and addition of endotoxin experiment (B), CR allergen and endotoxin-depletion experiment (C), TLR-4 dependent experiment (D)
18
-Fig. 2 Cockroach induced asthma model in BALB/C mouse. Developments of AHR
(A), eosinophilic inflammation (B), IL-13 secretion (C) and goblet cell hyperplasia of respiratory epithelium (D) are dose-dependent on the CR extracts administered.
Fig. 3 Effect of endotoxin added to CR-induced asthma model in BALB/C mouse.
Addition of endotoxin suppressed eosinophilic inflammation (B) and IL-13 productions (C), but not the development of AHR (A).
20
-Fig. 4 Endotoxin-depleted CR induced asthma model in BALB/C mouse. Depletion of
Fig. 5 PAS staining of cockroach induced asthma model in BALB/C mouse. Sham (A),
CR (B), endotoxin added to CR (C) and endotoxin depleted CR (D). The results of PAS staining were quantified in E.
22
-Fig. 6 Trichrome staining of cockroach-induced asthma model in BALB/C mouse.
Sham (A), CR (B), endotoxin added to CR (C) and endotoxin-depleted CR (D). The results of Trichrome staining were quantified in E.
Fig. 7 AHR and differential cell count in BAL fluid in TLR-4 mutant mouse exposed
to cockroach. Change of AHR (A) and inflammatory cells in BAL fluid (B) of TLR-4 wild
type (C3HeN) and TLR-4 receptor mutant (C3HeJ) mouse in CR asthma model. *; p<0.01, #;p<0.05
24
-Fig. 8 The level of cytokines in TLR-4 mutant mouse exposed to cockroach.
Expression of IL-13 (A) and IFN-γ (B) in TLR-4 wild type (C3HeN) and mutant mouse (C3HeJ). The cytokines in the lung homogenates were measured.
Fig. 9 PAS staining of TLR-4 mutant mouse exposed to cockroach. Wild type (sham -
A, CR - B) and mutant (sham - C, CR - D) mouse. Goblet hyperplasia of respiratory epithelium was quantified in E.
26
-Fig. 10 Trichrome staining of TLR-4 mutant mouse exposed to cockroach. Wild type
28
-References
1. Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD: Cockroach allergens and asthma. J Allergy Clin Immunol 107:419-428, 2001
2. Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, Vedvick TS, Hayden ML, Chapman MD: Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2. Sequence homology to the aspartic proteases. J Biol Chem 270:19563-19568, 1995
3. Bice DE, Seagrave J, Green FH: Animal models of asthma: potential usefulness for studying health effects of inhaled particles. Inhal Toxicol 12:829-862, 2000
4. Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D: Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol 14:425-438, 1996
5. Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E: Environmental exposure to endotoxin and its relation to asthma in school-age children.
6. Busse WW, Mitchell H: Addressing issues of asthma in inner-city children. J Allergy
Clin Immunol 119:43-49, 2007
7. Campbell EM, Kunkel SL, Strieter RM, Lukacs NW: Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J
Immunol 161:7047-7053, 1998
8. Clarke AH, Thomas WR, Rolland JM, Dow C, O'Brien RM: Murine allergic respiratory responses to the major house dust mite allergen Der p 1. Int Arch Allergy Immunol 120:126-134, 1999
9. de Siqueira AL, Russo M, Steil AA, Facincone S, Mariano M, Jancar S: A new murine model of pulmonary eosinophilic hypersensitivity: contribution to experimental asthma.
J Allergy Clin Immunol 100:383-388, 1997
10. Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H: Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol 179:2910-2917, 2007
11. Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W: Bacterial endotoxin is an active component of cigarette smoke. Chest 115:829-835, 1999
12. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM: German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J
30
-13. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M: Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care
Med 169:378-385, 2004
14. Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB: A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol 169:5904-5911, 2002
15. Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG: Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol 167:2808-2815, 2001
16. Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH: Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp
Allergy 37:1364-1373, 2007
17. Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD: Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 27:526-535, 2002 18. Mapp C, Hartiala J, Frick OL, Shields RL, Gold WM: Airway responsiveness to inhaled
antigen, histamine, and methacholine in inbred, ragweed-sensitized dogs. Am Rev
19. Matricardi PM, Franzinelli F, Franco A, Caprio G, Murru F, Cioffi D, Ferrigno L, Palermo A, Ciccarelli N, Rosmini F: Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol 101:439-444, 1998
20. McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG: Reproducibility of a novel model of murine asthma-like pulmonary inflammation. Clin Exp Immunol 136:224-231, 2004
21. Medzhitov R: Toll-like receptors and innate immunity. Nat Rev Immunol 1:135-145, 2001
22. Pomes A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V: Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit
Care Med 165:391-397, 2002
23. Pritchard DI, Eady RP, Harper ST, Jackson DM, Orr TS, Richards IM, Trigg S, Wells E: Laboratory infection of primates with Ascaris suum to provide a model of allergic bronchoconstriction. Clin Exp Immunol 54:469-476, 1983
24. Reed CE, Milton DK: Endotoxin-stimulated innate immunity: A contributing factor for asthma. J Allergy Clin Immunol 108:157-166, 2001
25. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F: The role of cockroach allergy
32
-and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 336:1356-1363, 1997
26. Strachan DP: Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax 55 Suppl 1:S2-10, 2000
27. Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR: Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J
Immunol 169:3499-3506, 2002
28. Tulic MK, Holt PG, Sly PD: Modification of acute and late-phase allergic responses to ovalbumin with lipopolysaccharide. Int Arch Allergy Immunol 129:119-128, 2002 29. Wan GH, Li CS, Lin RH: Airborne endotoxin exposure and the development of airway
antigen-specific allergic responses. Clin Exp Allergy 30:426-432, 2000
30. Zhou D, Chen G, Kim JT, Lee LY, Kang BC: A dose-response relationship between exposure to cockroach allergens and induction of sensitization in an experimental asthma in Hartley guinea pigs. J Allergy Clin Immunol 101:653-659, 1998
- 국문요약 -
바퀴알레르기항원을 이용한 새로운 마우스 천식 모델
아주대학교 대학원의학과 신 유 섭 (지도교수: 박 해 심) 목적: 바퀴는 주요한 흡입성 알레르기항원으로써 마우스에서 천식을 유발시킨다. 그러나 바퀴가 천식을 일으키는 기전은 아직 명확하지 않으며, 바퀴 알레르기항원 속의 내독소의 역할에 대해서는 논쟁의 여지가 있다. 우리는 바퀴로 천식을 유발시키는 과정에서 내독소와 TLR-4 의 역할에 대해서 알아보고자 하였다. 재료 및 방법: BALB/C 마우스, TLR-4 변이체인 C3H/HeJ 마우스와 TLR-4 야생형인 C3H/HeN 마우스에게 코를 통하여 바퀴 알레르기항원을 보조제 없이 주입하였다. 바퀴 알레르기항원은 각각 조항원과 내독소 없는 항원과 조항원에 내독소가 10,000 EU/mL 추가로 들어간 바퀴 알레르기항원을 사용하였다. 결과: 바퀴 천식 모델은 코를 통하여 주입한 바퀴 알레르기항원에 용량 의존적으로 생성되고, 바퀴 알레르기항원은 기도에서 호산구성 염증반응, 호중구성34 -염증반응, 기도과민성, 점액 샘의 증식을 일으켰다. 바퀴 알레르기항원에 추가로 내독소를 주입한 마우스에서는 기관지의 호산구성 염증과 기관지폐포액 속의 인터루킨-13, 술잔 세포의 증식이 감소되는 소견을 보였으나 기도과민성에는 영향을 미치지 않았다. 또한 내독소가 없는 바퀴 알레르기항원도 일반적인 바퀴항원에 비하여 기관지폐포액 속의 호산구성 염증과 임파구를 감소시켰으나 기도과민성과 기관지폐포액 속의 인터루킨-13에는 영향을 미치지 않았다. TLR-4가 없는 마우스에서도 바퀴 알레르기항원은 호산구성 염증, 술잔 세포의 증식과 기도과민성의 증가를 나타냈다. 결론: 바퀴는 마우스에서 호산구성 염증, 호중구성 염증, 기도과민성과 술잔 세포 증식이 있는 천식을 유발한다. 비록 바퀴 알레르기항원내의 내독소가 호산구성 염증에 대하여 양면성이 있지만, 바퀴 천식 모델의 발생에 영향은 미미하며, 바퀴 천식 모델의 기도과민성 발생에는 영향을 미치지 않는다.