Effects of EGCG from green tea on IL-1
ββββ
-induced expression of MMP-1 and MMP–13,
and production of PGE
2
and NO
on human IVD cells in vitro
Ji Ae Jun
Department of Medical Science
Effects of EGCG from green tea on IL-1
ββββ
-induced expression of MMP-1 and MMP–13,
and production of PGE
2
and NO
on human IVD cells in vitro
Directed by Professor Seong-Hwan Moon
The Master’s Thesis
submitted to the Department of Medical Science
the Graduate School of Yonsei University
in partial fulfillment of the requirements for the degree of
Master of Medical Science
Ji Ae Jun
This certifies that the Master’s Thesis
of Ji Ae Jun is approved.
---
[Thesis Supervisor : Seong-Hwan Moon]
---
[Hwan-Mo Lee : Thesis Committee Member#1]
---
[Keung-Nyun Kim : Thesis Committee Member#2]
The Graduate School
Yonsei University
Acknowledgements
The years have passed all too soon and I would like to leave a paper. I'd like to express my heartfelt thanks to everyone who have helped me.
First of all, I appreciate professor, Seong-Hwan Moon who is my adviser. Although I couldn't meet him frequently because he has been very busy, I always thank him and wish all the best for him. And I also appreciate professor, Hwan-Mo Lee and Hak-Sun Kim. Although I haven't often contacted them, I will never forget and I'll always pray for them.
I appreciate all of my laboratory colleagues. First and foremost, I really appreciate our oldest member, Hyang Kim, who has lots of experience and rich knowledge about research. She has helped me a lot and I have learned how to experiment well and many other things. I couldn't forget her for a long time. I also would like to thank Un-Hye Kwon as my senior and a friend. It was a short time to be close, but she was a good colleague in the lab. I really appreciate her kindness. I have another colleague Kwang-Il Lee, whom I gave thanks to. He always kindly responded when I asked anything. I really appreciate that. I thank Jung-Wook Lee, who is my only younger colleague. I didn’t spend time a lot with him, but I believe he’ll carry everything through in the lab. There is another colleague, Sun-Young Kong, whom I thank in the room. Although she was not in my part, she was a good company.
Coming to the end of the master course, I can naver thank my parents enough. They supplied the huge registration fee even though I’m a grown-up daughter. Otherwise, I couldn’t pass the degree. And also I thank my sister, Ji-Su Jun, and my younger brother, Young-Uk Jun. They are always a supporter of my body and soul.
My parents-in-law have always cheered me up and gave me allowances whenever I met them. It really helped me a lot. Thank you for everything. And also I appreciate my sister-in-law
and brother-in-law. Even though I’m younger than them, they have always respected and cared about me. I will do the best in the future. Lastly, my darling, Dae-Hee Kim has always been helpful to me and encouraged me to be mature. I love you, darling.
i
CONTENTS
ABSTRACT………….………...…………..v
Ⅰ
Ⅰ
Ⅰ
Ⅰ. INTRODUCTION………..1
Ⅱ
Ⅱ
Ⅱ
Ⅱ. MATERIALS AND METHODS………4
1. Materials……….………...4
2. Human Intervertebral disc cell culture………….………..4
3. Incorporation of isolated cells into alginate beads………...5
4. Depolymerization of alginate beads ………...6
5. Stimulation of cytokine and EGCG ………..………...…...6
6. Cytotoxicity ……..……….……..………..………6
7. DNA synthesis ...………..7
8. Newly synthesized proteoglycan ………..……7
9. RT-PCR analysis ...…....……….8
10. ELISA for MMP-1 and MMP-13 ……..…….………...8
11. Total PGE
2and NO Immunoassay ………..………...…9
12. Statistical analysis ..………..10
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS………13
1. Cytotoxicity of EGCG in human disc cells ………..…13
2. DNA synthesis………..…14
3. Newly synthesized proteoglycan ………..……….……14
4. mRNA expression of aggrecan, collagen type I, II, COX-2, and iNOS
………17
ii
6. Total NO and PGE
2Immunoassay ………21
Ⅳ
Ⅳ
Ⅳ
Ⅳ. DISCUSSION……….…..23
Ⅴ
Ⅴ
Ⅴ
Ⅴ. CONCLUSION……….…24
REFERENCES………...25
ABSTRACT (In Korean)………30
iii
LIST OF FIGURES
Figure 1. Effects of EGCG on cytotoxicity in human IVD
cells ………....13
F i g u re 2 . E f f e c t o f E G C G o n D N A s y n t h e s i s i n h u ma n
IVD cells ……….…15
F i g u r e 3 . E f f e c t o f E G C G o n N e w l y s y n t h e s i z e d
p r o t e o g l y c a n n o r m a l i z e d b y D N A s y n t h e s i s
in human IVD cells ………….…………..………...16
Figure 4. RT-PCR of beta-actin, aggrecan, collagen type I, II, COX-2,
and iNOS ………….………...………..18
Figure 5. Densitometry of aggrecan, collagen type I, II,
C O X - 2 , a n d i N O S m R N A e x p r e s s i o n i n
human IVD cells ……..………….………..19
F i g u re 6 . E f f e c t o f E G C G o n I L - 1
β-induced expression
o f M M P - 1 a n d M M P - 1 3 i n h u m a n I V D c e l l s
……….………...………..20
F i g u re 7 . E f f e c t o f E G C G o n e x p re s s i o n o f P G E 2 a n d
NO in human IVD cells ………..22
iv
LIST OF TABLES
Table 1. Sequences of the RT-PCR primers used ………..…11
Table 2. RT-PCR conditions ………..12
v
ABSTRACT
Effects of EGCG from green tea on IL-1
ββββ
-induced expression of
MMP-1 and MMP–13, and production of PGE
2and NO
on human IVD cells in vitro
Ji Ae Jun
Department of Medical Science
The Graduate School, Yonsei University
(Directed by Professor Seong-Hwan Moon )
Study design: In vitro experimental study.
Objectives: To investigate the effect of EGCG, on human IVD cells in terms of cellular
proliferation, mRNA expression of matrix components and inflammatory mediators, protein expression of MMPs and catabolic mediators PGE2 and NO.
Summary of literature review: Tea is the most popular and widely consumed beverage in the
world, after water, and is reported to possess beneficial health effects for human. It is well known that polyphenols in green tea are potent antioxidants, with the majority of beneficial effects elicited by epigallocatechin-3-gallate (EGCG), one of the main constituents of green tea.
Materials and Methods: Human IVD tissues were obtained from patients undergoing surgical
vi
were culture in three dimensional alginate beads. They were serum-starved overnight and then treated with IL-1β (10ng/㎖) and IL-1β + EGCG (10-200µM) for 24h. After 24 days culture periods, the cytotoxicity was measured by MTT assay and the DNA synthesis by [3 H]-thymidine incorporation and newly synthesized proteoglycan by [35S]-sulfate incorporation. Reverse transcription-polymerase chain reactions for the mRNA expressions of aggrecan, collagen types I, II, COX-2 and inducible NOS were performed. MMP-1, MMP-13 and PGE2, NO expression were detected by enzyme linked immono-sorbent assays.
Results: Human IVD cells with various concentrations of EGCG (10, 100, 200µM) showed no
cytotoxicity and also no discernible differences in DNA synthesis. However newly synthesized proteoglycan showed slightly increase in human IVD cells treated with EGCG. Human IVD cells in alginate bead with IL-1β and/or EGCG showed similar patterns of mRNA expression of aggrecan, collagen type II, COX-2 and PGE2 compared to control cultures except collagen type I. EGCG down-regulated IL-1β-induced increase in expression of MMP-1 and MMP-13 in dose dependent manner. However human IVD cells with various concentration of EGCG inhibited slightly the IL-1β-mediated induction of PGE2 in a dose-dependent manner, while showed no discernible differences in NO production.
Conclusion: There were no cytotoxicity in human IVD cells treated EGCG and EGCG
inhibited IL-1β-induced expression of MMP-1 and MMP-13. In addition, ECGC also inhibited slightly IL-1B-induced production of PGE2. In conclusion, EGCG showed anti-resorptive and anti-inflammatory effect without cytotoxicity in human IVD cells.
- 1 -
Effects of EGCG from green tea on IL-1
ββββ
-induced expression of
MMP-1 and MMP–13, and production of PGE
2and NO
on human IVD cells in vitro
Ji Ae Jun
Department of Medical Science
The Graduate School, Yonsei University
(Directed by Professor Seong-Hwan Moon)
Ⅰ
Ⅰ
Ⅰ
Ⅰ. INTRODUCTION
Tea is the most popular and widely consumed beverage in the world, after water, and is reported to possess beneficial health effects for humans.1,3-5,13,24,25 These beneficial health effects are attributed to green tea polyphenols, mostly catechins, and have attracted considerable attention in recent years for preventing oxidative stress-related diseases, including cancer and cardiovascular and neurodegenerative diseases.1,2,5,6,9,20,24,25,27 Such polyphenols as catechins have attracted attention as a functional food with various bioactivities, for example anti-cancer, anti-mutagenic, anti-microbial, and anti-viral activities.2,6-10,12-14, 19,20,28
The polyphenols, especially the catechins, are the most significant group of flavanols. The major catechins in green tea are epigallocatechin gallate (EGCG), epicatechin (EC), epigallocatechin (EGC) and epicatechin gallate (ECG), and catechin (C).8,14 Among these polyphenols, EGCG is the major component of green tea.26 It is well known that polyphenols in green tea are potent antioxidants, with the majority of beneficial effects elicited by
- 2 -
epigallocatechin-3-gallate(EGCG), one of the main constituents of green tea.27 Several epidemiologic and experimental observations have confirmed an association between green tea consumption and the prevention of both cancer development and atherosclerosis progression.7-8, 14
Proinflammatory cytokines such as interleukin-1β(IL-1β) affect extracellular matrix(ECM) turnover in terms of accelerating the degradation of cartilage matrix, and inducing chondrocytes apoptosis.3-5,15,17,18,21 Additionally, IL-1β induces high levels of prostaglandins E2(PGE2) and nitric oxide(NO) production via enhanced expression of enzymes cyclooxygenase-cyclooxygenase-2(COX-2) and inducible nitric oxide synthase(iNOS) in the synovium and chondrocytes. 4,16,21,29-37,43 In addition, EGCG inhibits activation of the cytokine and inhibits the activation and expression of cytokine-activated matrix metalloproteinases. 8-10,19,38-42
MMPs are a large group of enzymes that play a crucial role in tissue remodeling as well as in the destruction of cartilage and bone in an arthritic joint due to their ability to degrade a wide variety of extracellular matrix components. Production and release of MMPs is micro-environmental and induced by several factors including the pro-inflammatory cytokine IL-1β. 3,10,19,38,39
IL-1 is produced in the degenerate IVD, and it is synthesized by native disc cells, and treatment of human disc cells with IL-1 induces an imbalance between catabolic and anabolic events, responses that represent the changes seen during disc degeneration.11,16,22,23 Furthermore, EGCG has been shown to be an equally or better effective antioxidant against both hydrophilic and hydrophobic free radicals and can cross blood-brain barrier and penetrate the cell membrane with partition reaction reaching equilibrium.11,16
Recently, a study suggests that EGCG affords protection against IL-1β-induced inflammatory effects in human chondrocytes by regulating the expression and catalytic activity
- 3 -
of their respective enzymes. Nevertheless the effect of EGCG on intervertebral disc (IVD) cells was not studied before. Accordingly, the objective of this study is to investigate the effect of EGCG, on human IVD cells in terms of cellular proliferation, mRNA expression of matrix components, mRNA expression of MMPs, and catabolic mediators NO and PGE2.
- 4 -
Ⅱ
Ⅱ
Ⅱ
Ⅱ. MATERIALS AND METHODS
All experimental protocols were approved by Institutional Review Board and Animal Experimentation Committee of the institution.
1.
Materials
Lumbar and cervical IVD tissues were obtained from ten patients (age range : 21 to 67 years) during surgical disc procedures. Classification of the IVD of each patient as grade of degeneration was performed based on magnetic resonance images of each disc as described in the literature. Grade Ⅲ and Ⅳ degenerations were included in this study to minimize the effect of degeneration grades in the expression of phenotype and matrix synthesis. An attempt was made by the operating surgeon (SHM, HML) to carefully obtain tissues from the central aspect of the disc to optimize harvest of only nucleus pulposus and transitional zone. The disc tissue specimens were washed with Hank’s balanced salt solution(HBSS, Gibco-BRL, Grand Island , NY, USA) to remove blood and bodily fluid contaminants, and were then transported in sterile HBSS to the laboratory, less than 20minutes following surgical removal.
2.
Human intervertebral disc cell culture
Any obvious granulation tissue, dense outer anulus, and cartilaginous endplate were removed carefully from the disc tissue specimens. Human disc cells were then isolated from the inner annulus and nucleus pulposus as described before. Briefly the dissected specimens were minced with a scalpel into pieces of approximately two cubic millimeters in volume. Disc tissues from the nucleus pulposus and inner annulus were digested in Ham’s F-12 medium
(F-- 5 (F--
12, Gibco-BRL, Grand Island, NY) containing 1% (v/v) penicillin, streptomycin, nystatin (all antibiotics from Gibco-BRL, Grand Island, NY), 0.4% (w/v) Protease, 0.004% (w/v) deoxyribonuclease II type Ⅳ (DNase, Sigma, St. Louis, MO, USA) for an hour at 37℃ under gentle agitation. The tissues were then washed 2 times with Ham’s F-12 medium and digested in Ham’s F-12 containing 1% (v/v) antibiotics, 0.025% (w/v) collagenase type II, 0.004% (w/v) DNase for 2 hours under the same conditions. The digested tissues were filtered through a sterile cell strainer (Falcon, Franklin Lakes, NJ) with a pore size of 70㎛. The filt rated was centrif uged at 1,500 r pm f or 5 mi nut es t o separate the cells.
Primary cultures were sustained for 2 to 3 weeks in Dulbecco’s modified eagle medium and Hams F-12 medium (DMEM/F-12, Gibco-BRL,
Grand Island, NY) supplemented with 10% heat activated fetal bovine serum (FBS, Gibco-BRL, Grand Island, NY), 1% (v/v) antibiotic, and 25㎍/㎖ ascorbic acid at 37℃ in 5% CO2 incubator with humidity. Culture medium was changed twice a week.
3. Incorporation of isolated cells into alginate beads
The preparation of human IVD cells in alginate beads was performed as described elsewhere. Briefly, cells isolated from primary culture with trypsinization were resuspended in sterile growth medium containing 1.2 % low-viscosity alginate (Sigma, St. Louis, MO, USA) at a density of two million cells per milliliter, and then slowly expressed through a 22-gauge needle in a drop-wise fashion into 102mM CaCl2 solution. After that, the beads were allowed to polymerize further for a period of 10 minutes in the CaCl2 solution. And then they were washed in DMEM/F-12 medium three times. The beads were finally placed in complete culture medium. Ten beads were cultured in each well of a 24-well plate.
- 6 -
4. Depolymerization of alginate beads
To remove cells from the alginate beads, beads were rinsed twice with PBS and then incubated with 28mM EDTA/0.15M NaCl buffer at 37℃ for 10 minute in 5% CO2 incubator with humidity.
5. Stimulation of cytokine and EGCG
After incubation at 37℃ in 5% CO2 incubator with humidity for over 30 hours, each cells were washed with DPBS (Gibco-BRL, Grand Island, NY) and then serum-less medium(DMEM/F-12, 0.5% FBS, 1% (v/v) antibiotic, and 25㎍/㎖ ascorbic acid). After that, the cells were cultured with serum-less medium including 10ng/㎖ of IL-1β and/or a various dose of EGCG for 24 hours. The control group was only cell seeded without anything.
6. Cytotoxicity
Cells were plated in 96 well plates and cell survi val was measured by t h e M T T ( 3 - ( 4 , 5 - d i m e t h y l t h i a z o l - 2 - y l ) - 2 , 5 - d i p h e n y l t e t r a z o l i u m b r o m i d e ) a s sa y. MT T w as di sso l ve d at 5 ㎎/ ㎖ i n D P B S a n d s t e r i l i ze d b y p a s sa ge through a 0.2 ㎛ Millipore filter (Nalgene, Rochester, NY). 10 ㎕ of MTT solution was transferred to each well to yield a final assay volume of 100 ㎕/well. The plate was incubated for 4 hours at 37℃ and 5% CO2. After incubation, supernatants were removed, and 100 ㎕ each of DMSO was added. The plates are placed on an orbital shaker for 5min, and the absorbance was recorded at 570nm. The percent viability was expressed as absorbance in the presence of test compound as a percentage of that in the control.
- 7 -
7. DNA synthesis
DNA synthesis was measured by the [3H]-thymidine incorporation. 5u Ci/㎖ of [3 H]-thymidine (Amersham Biosciences, Uppsala, Sweden; 25Ci/mmol specific activity) was added to control and treated cultures for 24h. The medium was then discarded and the beads were depolymerized with 28mM EDTA/0.15M NaCl buffer at 37℃ for 10 minute in 5% CO2 incubator with humidity. The dissolved cells were filtered onto glass fiber filters (Whatman GF/C; Maidstone, England), and transferred to scintillation vial. Filters were dried and counted in 3㎖ of scintillation cocktail solution (Beckman Coulter Inc. USA) in a Packard scintillation counter (Packard #1900 TR, Mariden, CT). The results of each experiment, expressed as cpm/well, are the means of three parallel cultures.
8. Newly synthesized proteoglycan
10 µ Ci/㎖ of [35S]-sulfate (Amersham Biosciences, Uppsala, Sweden; 25Ci/mmol specific activity) was added to control and treated cultures for 4h. At the end of culture, the medium was collected and the beads were dissolved with 28mM EDTA/0.15M NaCl. The cells were then placed in an extraction medium (4M guanidine HCl solution, 5mM sodium acetate (pH5.8), proteinase inhibitor) at 4℃ for 48hours. Aliquots (200 ㎕) of the cell extracts were eluted on Sephadex G-25M in PD-10 columns (Amersham Biosciences, Uppsala, Sweden) under dissociative condition. Fractions (1 ㎖) were colleted in scintillation vial and mixed with 6 ㎖ scintillation cocktail solution (Beckman Coulter Inc. USA). Five fractions were collected p er sa mp l e, an d t h r ee mi d dl e f r a ct i o ns we r e c o un t e d i n a Pa cka r d l i q ui d scintillation counter (Packard #1900 TR, Meriden, CT).
- 8 -
9. Reverse transcription-polymerase chain reaction for aggrecan, collagen type I, II, COX-2 and iNOS mRNA
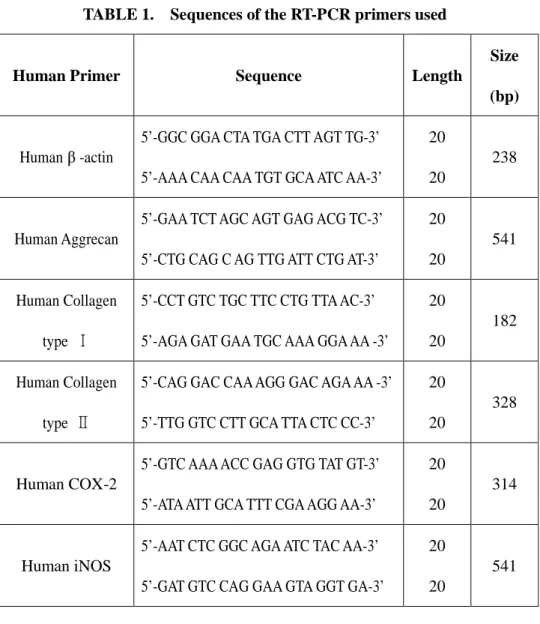
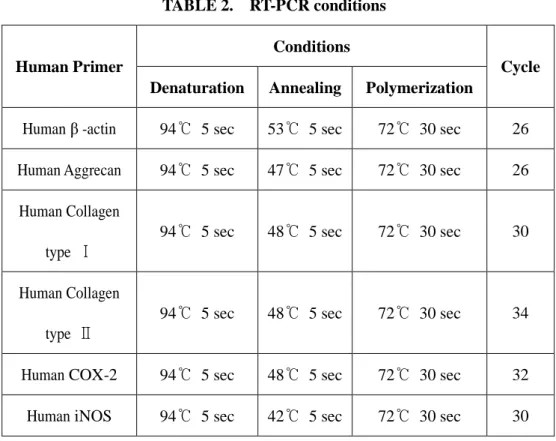
Total RNA was eluted by selective binding to a silica gel-based membrane using an RNeasy mini kit(QIAGEN, GmbH, Germany). Reverse transcription of RNA into cDNA was performed incubating 1㎕ of RNA in a reaction mixture containing 0.5㎎/㎖ cDNA reaction product and was used as the template to co-amplify β-actin, aggrecan, collagen type I, collagen type II, COX-2 and iNOS. PCR was performed using a DNA thermal cycler. The same reaction profile was used for all primer sets: an initial denaturation at 94℃ for 1 minute, followed by 26~34 cycles of: 94℃ for 5 seconds; 42~53℃ for 5 seconds; and 72℃ for 30 seconds; and an additional 2 min extension step at 72℃ after the last cycle. Amplification reactions specific for the following cDNAs were performed: β-actin, aggrecan, collagen type I, collagen type II, COX-2 and iNOS. Primer sequence of each cDNA was listed on Table 1 and PCR conditions listed on Table 2. PCR products (4㎕) were analyzed by electrophoresis in 1.5 % agarose gel, and detected by staining with ethidium bromide. The intensity of the products was quantified using the BioImage Visage 110 system (BioRad, Hercules, CA, USA).
10. Enzyme-linked immunosorbent assay for MMP-1 and MMP-13
To determine the effect of EGCG on MMP-1 and MMP-13 in culture supernatants, human IVD cells were plated in 48-well plates and serum-starved for overnight. The medium was replaced with fresh medium containing recombinant human IL-1β (10ng/ml), IL-1β + EGCG (10-200µM), or only EGCG (200µM) and incubated for 24h at 37°C and 5%CO2. At the end of incubation, medium was collected and used to analyze the cellular level of of
MMP-- 9 MMP--
1 and MMP-13 using a commercially available kit(R&D Systems, MN, USA). MMP-1 and MMP-13 were quantitated following the manufacturer’s ELISA protocol.
Briefly, the cell culture supernatants were diluted in buffer and added to 96-well ELISA plates coated with each antibody. The plates were incubated for 2 hours at room temperature on a shaker (250 rpm), washed three times with wash buffer and then detection antibodies were added. After subsequent three times washing, the ELISA microtiter plates were developed with substrate solution. The reaction was stopped by the addition of 2N sulfuric acid. ELISA microtiter plates were read by an ELISA reader set to 450nm. Components of the extracellular matrix were quantitated using recombinant human components as standards. The sensitivity of the immunoassays was 0.021ng/㎖ for MMP-1 and 7.7pg/㎖ for MMP-13.
11. Total Prostaglandin E2 and Nitric Oxide Immunoassay
To determine the effect of EGCG on IL-1β-induced release of PGE2 and NO in culture supernatants, IVD cells were plated in 48-well plates and serum-starved for overnight. The medium was replaced with fresh medium containing recombinant human IL-1β (10ng/㎖), IL-1β + EGCG (10-200µM), or only EGCG (200µM), and incubated for 24h at 37°C and 5%CO2. At the end of incubation, medium was collected and used for the estimation of PGE2 and nitrite accumulation using a commercially available kit (R&D Systems, MN, USA). PGE2 and NO were quantitated following the manufacturer’s ELISA protocol. ELISA microtiter plates were read by an ELISA reader set to 570nm. Catabolic mediators were quantitated using sodium nitrate solution for NO and PGE2 in buffer for PGE2 as standards. The sensitivity of the immunoassays was 13.4pg/㎖ for PGE2 and 1.35nmol/L for NO.
- 10 -
12. Statistical analysis.
The numerical data from each experiment were the average from at least triplicate samples. The same experiments were repeated three times to ensure the repeatability of the methods used. One-way analysis of variance and Fisher’s protected LSD post-hoc test, power analysis were performed to test difference in densitometric data, [3H]-thymidine labeled DNA, and [35S]-sulfate labeled proteoglycan. Significance level was set as p<0.05.
- 11 -
TABLE 1. Sequences of the RT-PCR primers used
Human Primer Sequence Length
Size (bp)
Human β -actin
5’-GGC GGA CTA TGA CTT AGT TG-3’ 5’-AAA CAA CAA TGT GCA ATC AA-3’
20 20
238
Human Aggrecan
5’-GAA TCT AGC AGT GAG ACG TC-3’ 5’-CTG CAG C AG TTG ATT CTG AT-3’
20 20 541 Human Collagen type Ⅰ 5’-CCT GTC TGC TTC CTG TTA AC-3’ 5’-AGA GAT GAA TGC AAA GGA AA -3’
20 20
182
Human Collagen type Ⅱ
5’-CAG GAC CAA AGG GAC AGA AA -3’ 5’-TTG GTC CTT GCA TTA CTC CC-3’
20 20
328
Human COX-2
5’-GTC AAA ACC GAG GTG TAT GT-3’ 5’-ATA ATT GCA TTT CGA AGG AA-3’
20 20
314
Human iNOS
5’-AAT CTC GGC AGA ATC TAC AA-3’ 5’-GAT GTC CAG GAA GTA GGT GA-3’
20 20
- 12 -
TABLE 2. RT-PCR conditions Conditions Human Primer
Denaturation Annealing Polymerization
Cycle
Human β -actin 94℃ 5 sec 53℃ 5 sec 72℃ 30 sec 26 Human Aggrecan 94℃ 5 sec 47℃ 5 sec 72℃ 30 sec 26
Human Collagen type Ⅰ
94℃ 5 sec 48℃ 5 sec 72℃ 30 sec 30
Human Collagen type Ⅱ
94℃ 5 sec 48℃ 5 sec 72℃ 30 sec 34
Human COX-2 94℃ 5 sec 48℃ 5 sec 72℃ 30 sec 32 Human iNOS 94℃ 5 sec 42℃ 5 sec 72℃ 30 sec 30
- 13 -
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS
1. Cytotoxicity of EGCG in human disc cells
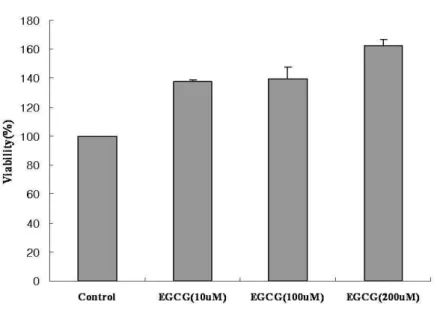
Human IVD cells were cultured with various concentrations of EGCG (10, 100, 200 µM) for 24h. Human IVD cells treated with EGCG showed significant increase in cell proliferation compared to control culture without EGCG. The cells cultured with high dose of EGCG (200µM) were strongly increased up to 60% in cell viability compared to the control (Figure. 1) (p<0.05).
Figure 1. Effects on cytotoxicity in human IVD cells. The target cells (1.5 X 104cells per well) were placed in each well of 96-well plates and then added various concentrations of EGCG (10, 100, 200µM) for 24h. The percentage of cell viability of all groups was
- 14 -
2. DNA synthesis
Human IVD cells were cultured in three-dimensional alginate beads with IL-1β (10ng/㎖) and/or EGCG (100µM). Human IVD cells cultured in IL-1β showed no discernible differences in DNA synthesis, but in IL-1β and/or EGCG were rather reduced in DNA synthesis compared to control culture without anything (Figure 2) (p<0.05).
3.Newly synthesized proteoglycan normalized by DNA synthesis
Human IVD cells in three-dimensional alginate beads were cultured with IL-1β β (10ng/㎖) and/or EGCG (100µM). Human IVD cells cultured in IL-1β and EGCG showed slightly increase in newly synthesized proteoglycan, but in IL-1β were no discernible differences compared to control culture without anything (Figure 3) (p<0.05).
- 15 -
Figure 2. Effects of EGCG on DNA synthesis in human IVD cells. The target cells in three-dimensional alginate beads were placed in each well of 24-well plates and then added IL-1β (10ng/㎖㎖㎖㎖) and/or EGCG (100µM) for 24h. The percentage of all groups was calculated versus the control. Results were measured by [3H]-thymidine incorporation. Values shown were mean ± SD of three independent experiments and each run in triplicate (p<0.05).
- 16 -
Figure 3. Newly synthesized proteoglycan of human IVD cells treated IL-1β(10ng/㎖㎖㎖㎖) and/or EGCG(100µM). The target cells in three-dimensional alginate beads were placed in each well of 24-well plates and then added IL-1β and/or EGCG for 24h. The percentage of all groups was calculated versus the control. Results indicated as percentage of newly synthesized proteoglycan normalized by DNA synthesis. Values shown were mean ± SD of three independent experiments and each run in triplicate (p<0.05).
- 17 -
4.
mRNA expression
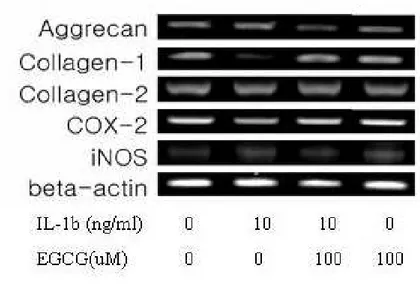
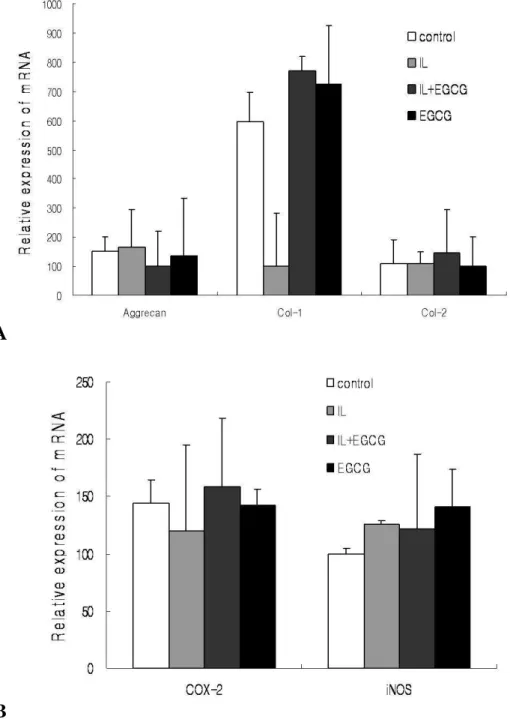
for aggrecan, collagen type-I, collagen type-II, COX-2 and iNOSHuman IVD cells in three-dimensional alginate beads were cultured with IL-1β (10ng/㎖) and/or EGCG (100µM). The values obtained were normalized to the level of β-actin mRNA in the samples. Human IVD cells treated with IL-1β reduced mRNA level of collagen type I, while showed no discernible differences in aggrecan, collagen type II, COX-2, and iNOS. In addition, collagen type I mRNA levels showed slightly increase in the samples treated with IL-1β and EGCG or EGCG. However, human IVD cells cultured in with IL-1β and/or EGCG showed no statically significant changes in mRNA expression of aggrecan, collagen type II, COX-2, and iNOS (Figure 4, 5) (p<0.05).
- 18 -
Figure 4. RT-PCR of beta-actin, aggrecan, collagen type I, II, COX-2, and iNOS. Total RNA was isolated from human IVD cells and subjected to RT-PCR. The target cells (1 X 105cells per well) were placed in each well of 12-well plates and then added IL-1β(10ng/㎖㎖㎖㎖) or EGCG(100µM) for 24h. The PCR products were separated on 1.5% agarose gels containing ethidium bromide, and then observed on an ultraviolet transilluminator.
- 19 -
A
B
Figure 5. Densitometry of A; extracellular matrix components (aggrecan, collagen type I, II), B; inflammatory mediators (COX-2 and iNOS) mRNA expression. The expression of each PCR band was quantified using an image analyzer. The results are presented as the percentage of the mRNA level relative to beta-actin for each band.
- 20 -
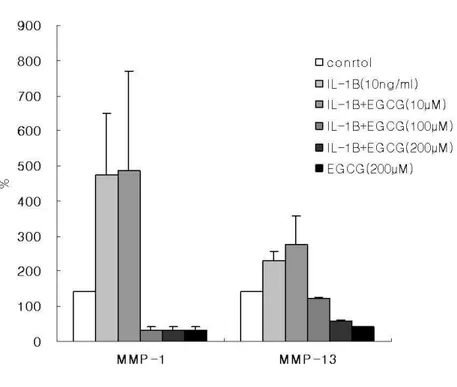
5. Enzyme-linked immunosorbent assay for MMP-1 and MMP-13
Human IVD cells were cultured with IL-1β or various concentrations of EGCG (10, 100, 200 µM) for 24h. As shown in figure 6, stimulation with IL-1β (10ng/㎖) significantly increased the expression of MMP-1 and MMP-13 in human IVD cells when compared to untreated controls. Interestingly, human IVD cells treated with high dose of EGCG (100, 200µM) caused inhibition in the expression of MMP-1 and MMP-13. But the cells at a dose of 10µM EGCG showed no significant effect (Figure. 6) (p<0.05).
Figure 6. Effect of EGCG on IL-1β-induced expression of MMP-1 and MMP-13 in human IVD cells. Human IVD cells were stimulated with IL-1β (10ng/㎖㎖㎖㎖) alone or in the presence of EGCG (10, 100, 200µM) or EGCG (200µM) alone for 24h. Expression of MMP-1 and MMP-13 was measured using an ELISA kit and values were obtained from a
- 21 -
standard curve. Values shown were mean ± SD of three independent experiments, each run in triplicate and performed with age- and sex-matched samples (p<0.05).
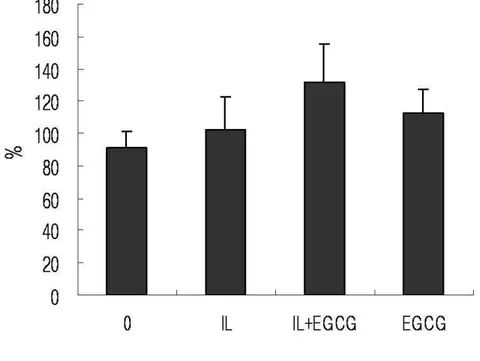
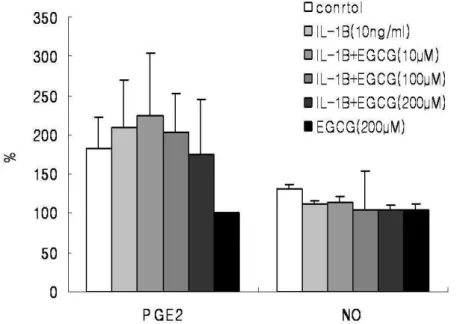
6. Total Prostaglandin E2 and Nitric Oxide Immunoassay
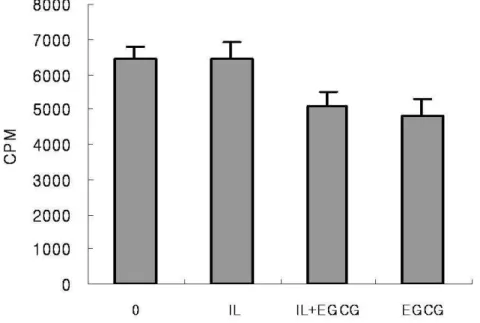
Human IVD cells were cultured with IL-1β and/or various concentrations of EGCG (10, 100, 200 µM) for 24h. Human IVD cells treated with EGCG (100, 200 µM) down-regulated IL-1β-induced increase in PGE2 in dose dependent manner. The cells cultured with high dose of EGCG (200uM) alone were strongly down-regulated production of PGE2 compared to the control (Figure. 7) (p<0.05). However, human IVD cells treated with IL-1β showed no up-regulation in NO production and cells treated with EGCG showed no discernible differences in NO production (Figure 7) (p<0.05).
- 22 -
Figure 7. Human IVD cells were stimulated with IL-1β (10ng/㎖㎖㎖㎖) alone or in the presence
of EGCG (10, 100, 200µM) or EGCG (200µM) alone for 24h. Production of PGE2 and NO
was measured using an ELISA kit and values were obtained from a standard curve. Values shown were mean ± SD of three independent experiments, each run in triplicate and performed with age- and sex-matched samples(p<0.05).
- 23 -
Ⅳ
Ⅳ
Ⅳ
Ⅳ. DISCUSSION
Green tea has been consumed as a beverage for thousands of years in the world, and beneficial health effects of tea have been demonstrated in animal experiments and some human studies supporting that frequent consumption of green tea is inversely associated with the risk of several types of human cancer and other chronic diseases. Today, it is well known for the chemopreventive and chemoprotective effects of green tea attributed to its polyphenols. Recently, a study suggests that EGCG affords protection against IL-1β-induced inflammatory effects in human chondrocytes by regulating the expression and catalytic activity of their respective enzymes. Although there are many studies of EGCG, there have been few studies of EGCG on human IVD cells. Therefore, this study systematically examined biological effect of EGCG on human IVD cells; cytotoxicity, DNA synthesis, mRNA levels of extracellular matrix (ECM) components, and protein levels of MMPs and catabolic mediators.
This study utilized human IVD cells cultured in three-dimensional alginate beads in order to maintain the phenotype of IVD cells. As shown in the results, there was no cytotoxicity in the human IVD cells treated with EGCG, but no significant effects in DNA synthesis. In proteoglycan synthesis, human IVD cells showed no discernible stimulation in IL-1β, but 40% increase in IL-1β and EGCG compared to control group without anything. In mRNA expression of ECM and proinflammatory mediators, there was no significant effect except collagen type I. Human IVD cells stimulated IL-1β showed strongly decrease in collagen type I mRNA and significantly increase in EGCG. In addition, high doses of EGCG stimulated IL-1β-induced expression of MMP-1 and MMP-13. However, it stimulated slightly in PGE2, but no in NO. In summary, polyphenol EGCG derived from green tea appears to be a potent inhibitor of IL-1β-mediated matrix metalloproteinases in human IVD cells.
- 24 -
expression of MMP-1 and MMP-13 is not clear, it is possible that this effect of EGCG provides a new molecular event for the inhibition of MMP-1 and MMP-13 in human IVD cells. Alternatively, EGCG from green tea could potentially be used as a nutritional supplement in patients with a problem of disc. However, further research is necessary to elucidate the possible benefit for EGCG supplementation in disc degeneration.
Ⅴ
Ⅴ
Ⅴ
Ⅴ. CONCLUSION
There were no cytotoxicity in human IVD cells treated EGCG and EGCG inhibited IL-1β-induced expression of MMP-1 and MMP-13 in human IVD cells . In addition, ECGC also inhibited slightly IL-1B-induced production of PGE2. In conclusion, EGCG showed anti-resorptive and anti-inflammatory effect without cytotoxicity in human IVD cells.
- 25 -
REFERENCES
1. Ahmed S, Rahman A, Hashain A, et.al. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β- induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Elsevier Sci. Inc. 2002; 33: 1097-1105.
2. Katiyar S. Tea consumption and cancer. World Rev. Nutr. Diet. 1996;79:154-184.
3. Ahmed S, Wang N, Lalonde M, et.al. Green tea polyphenol epigallocatechin-3-gallate(EGCG) differentially inhibits interleukin-1β-induced expression of matrix metallopreteinase-1 and -13 in human chondrocytes. J. Pharm. and Exp. Therap. 2003;308 : 767-773.
4. Singh R, Ahmed S, Islam N, et.al. Epigallocatechin-3-gallate inhibits interleukin-1β-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes. Arthritis and Rheumatism 2002;46: 2079-2086.
5. Singh R, Ahmed S, Malemud CJ, et.al. Epigallocatechin-3-gallate selectively inhibits interleukin-1β-induced activation of mitogen activated protein kinase subgroup c- Jun N- terminal kinase in human osteoarthritis chondrocytes. J Ortho. Res 2003;21: 102-109. 6. Han DW, Kim HH, Lee MH, et.al. Protection of osteoblastic cells from freeze/thaw
cycle-induced oxidative stress by green tea polyphenol. Spinger. 2005; 9: 655-660.
7. Park YH, Han DW, Suh H, et. al. Protective effects of green tea polyphenol against reactive oxygen species-induced oxidative stress in cultured rat calvarial osteoblast. Cell Bio. Toxic. 2003; 19; 325-337.
8. Cheng XW, Kuzuya M, Kanda S, et. al. Epigallocatechin-3-gallate binding to MMP-2 inhibits gelatinolytic activity without influencing the attachment to extracellular matrix proteins but enhances MMP-2 binding to TIMP-2. Arch. Bioch. Bioph. 2003 ; 415 :126-132. 9. Garbisa S, Sartor L, Biggin S, et. al. Tumor gelatinases and invasion inhibited by the green
- 26 -
10. Yun JH, Pang EK, Kim CS, et. al. Inhibitory effects of green tea polyphenol(-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Perio. Res. 2004 ; 39 : 300-307.
11. Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis. Res. Ther. 2005 ; 7 : R732-745.
12. Axanova L, Morre DJ, Morre DM. Growth of LNCaP cells in monoculture and coculture with osteoblasts and response to tNOX inhibitors. Cancer Let. 2005 ; 225 : 35-40.
13. Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obesity Res. 2005 ; 13 : 982-990.
14. Adcocks C, Collin P, Buttle DJ. Catechins from green tea(Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type II collagen degradation in vitro. J. Nutr. 2002 ; 132 : 341-346.
15. Martin G, Andriamanalijaona R, et. al. Comparative effects of IL-1β and hyarogen peroxide(H2O2) on catabolic and anabolic gene expression in juvenile bovine chondrocytes. OsteoArthritis and Cartilage. 2005 ; 10 : 915-924.
16. Miyamoto H, Saura R, Doita M, et. al. The role of cyclooxygenase-2 in lumbar disc herniation. Spine. 2002 ; 27 : 2477-2483.
17. Ijima Y, Kobayashi M, Kubota E. Role of interleukin-1 in induction of matrix metalloproteinases synthesized by rat temporomandibular joint chondrocytes and disc cells. Eur. J Oral Sci. 2001 ; 109 : 50-59.
18. Shikhman AR, Kuhn K, et. al. N-acetylglucosamine prevents IL-1β-mediated activation of human chondrocytes. J. Immun.. 2001 ; 166 : 5155-5160.
19. Demeule M, Brossard M, Page M, et. al. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta. 2000 ; 1478 : 51-60.
- 27 -
20. Garbisa S, et.al. Tumor invasion : molecular shears blunted by green tea[letter]. Nat. Med. 2000 ; 5 : 1216.
21. Andriamanalijaona R. Comparative effects of 2 antioxidants, seleno-methionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J. Rheumatol. 2005 ; 32 : 1958-1967.
22. Declan B, et.al. Discs degenerate before facets. Spine. 1990; 15: 111-113.
23. Buckwalter JA, et.al. Aging and degeneration of the human IVD. Spine. 1995 ; 20 : 1307-1314.
24. Raymond C, James M, Dorothey M. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. Jour. Althe. And Comp. Med . 2005 ; 11 : 521-528
25. Bevely AW, Lametta SS, Gary RB, et al. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001 ; 131 ; 1731-1737.
26. Chou FP, Chu YC, Hsu JD, et al. Specific induction of glutathione S-Transferase GSTM2 subunit expression by epigallocatechin gallate in rat liver. Biochem. Pharmacol. 2000; 60; 643-650.
27. Hsu TC, Zhao YJ, Wang RY, et al. Comparative efficacy as antioxidants between ascorbic acid and epigallocatechin gallate on cells of two human lymphoblastoid lines. Can. Gene. And Cyto. 2001; 124; 169-171.
28. Sato T, Miyata G. The nutraceutical benefit, part I: green tea. Nutr. Pharma. 2000; 16; 315-317.
29. Ralston SH, Grabowski PS, Mechanisms of cytokine induced bone resorption : role of nitric oxide, cyclic guanosine monophosphate, and prostaglandins. Bone. 1996; 19; 29-33. 30. Murakami M, Naraba H, Tanshihiro T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthease that acts in
- 28 -
31. Min YK, Rao Y, Okada Y, et al. Regulation of prostaglandin G/H synthase-2 expression by interleukin-1 in human osteoblast-like cells. Jour. Bone. Miner. Res. 1998; 13; 1066-1075. 32. Hardy MM, Seibert K, Manning PT, et al. Cyclooxygenase 2-dependent prostaglandin E2
modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arth. Rheu. 2002; 46; 1789-1803.
33. Fritsche E. Baek SJ. King LM, et al. Functional characterization of cyclooxygenase-2 polymorphisms. JPET. 2001; 299; 468-476.
34. Xu JQ, Cissel DS, Varghese S. et al. Cytokine regulation of adult human osteoblast-like cell prestaglandin biosynthesis. Jour. Cell. Biochem. 1997; 64; 618-631.
35. Jakibsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E cynthase : a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Biochem. 1999; 96; 7220-7225.
36. Vane JR, Mitchell JA, Appleton I, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Pharma. 1994; 91; 2046-2050.
3 7 . S a m a d TA , M o o r e K A , S a p i r s t e i n A , e t a l . I n t e r l e u k i n - 1β-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001; 410; 471-475.
38. Sadowski T, Steinmeyer J. Effects of non-steroidal anti-inflammatory drugs and dexamethasone on the activity and expression of MMP-1, MMP-3 and tissue inhibitor of MMP-1 by bovine articular chondrocytes. Osteoarthritis and cartilage. 2001; 9; 407-415. 39. Corps AN, Curry VA, Buttle DJ, et al. Inhibition of interleukin-1β-stimulated collagenase
nad stromelysin expression in human tendon fibroblasts by epigallocatechin gallate ester. Matrix Biology. 2004; 23; 163-169.
40. Ludwig A, Lorenz M, Grimbo N, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells.
- 29 -
BBRC. 2004; 316; 659-665.
41. Han MK, Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic β-cell damage. 2003; 35; 136-139.
42. Wheeler DS, Cartravas JD, Odoms K, et al. Epigallocatechin-3-gallaate, a green tea-derived polyphenol, inhibits IL-1β-dependent pro-inflammatory signal transduction in cultured respiratory epithelial cells. J. Nutr. 2004; 134; 1039-1044.
43. Andriamanalijaona R. Felisaz N, Kim SJ, et al. Mediation of interleukin- 1β-induced transforming growth factor β1 expression by activator protein 4 transcription factor in primary cultures of bovine articulara chondrocytes. Arth. Rheuma. 2003; 48; 1569-1581.
- 30 -
ABSTRACT (In Korean)
녹차로부터
녹차로부터
녹차로부터
녹차로부터
추출된
추출된
추출된 EGCG
추출된
EGCG
EGCG
EGCG
가
가
가
가
인간
인간
인간
인간
추간판
추간판
추간판
추간판
세포
세포
세포
세포
에서의
에서의
에서의
에서의
항
항
항
항
흡수
흡수
흡수
흡수
효과와
효과와
효과와
효과와
항
항
항
항
염증
염증
염증
염증
효과에
효과에
효과에
효과에
미치는
미치는
미치는
미치는
영향
영향
영향
영향
<
<
<
< 지도교수
지도교수
지도교수
지도교수 ;
;
; 문
;
문
문
문
성
성
성
성
환
환
환
환 >
>
>
>
연세대학교
연세대학교
연세대학교
연세대학교
대학원
대학원
대학원
대학원
의과학과
의과학과
의과학과
의과학과
전
전
전
전
지
지
지
지
애
애
애
애
연구계획 연구계획 연구계획 연구계획 : 실험자 연구 연구목적 연구목적 연구목적 연구목적 : 인간 척추 추간판 세포에 녹차로부터 추출된 EGCG 를 처리하여 세포 독성, 세포 외 기질(ECM)과 기질 분해 효소 발현 및 catabolic mediator 인PGE2와 NO 등에 어떠한 영향을 미치는지를 등을 알아보고자 한다. 연구 연구 연구 연구 배경배경배경배경: 녹차가 인간의 건강에 도움을 준다는 연구가 보고 되고 있고, 이러한 녹차의 유효 성분인 폴리페놀은 항암 작용과 항균 작용 같은 다양한 효과를 가진 기능성 식품이며, 그 중에서도 녹차에 많이 함유되어 있는 EGCG 가 지금까지의 연 구 결 과 들 중 에 가 장 효 과 가 좋 다 고 알 려 져 왔 고 , 최 근 보 고 에 의하면 녹차 성분 중 하나인 EGCG 가 연골에 대해 보호 효과를 발휘하는 것으로 알려져 있다. 대상 대상 대상 대상 및및및 및 방법방법방법방법: 인간 추간판 세포를 분리하여 alginate bead 를 이용한 3 차원 배양을 하였다. 배양한 추간판 세포에 10ng/㎖ 의 IL-1β와 10µM-200µM 의 EGCG 를 첨가하여 37℃, 5%의 CO2 하에서 24 시간 추가 배양한다. 세포 독성은 MTT assay,
당단백 생성은 [35S]-sulfate incorporation, DNA 생성은 [3H]-thymidine
- 31 - 발현은 RT-PCR 을 통해 분석하였다. 기질 분해 효소와 PGE2, NO 의 단백질 발현 등은 ELISA 를 통하여 측정하였다. 결과 결과 결과 결과 : EGCG 를 처리한 결과, 세포 독성은 나타나지 않았다. EGCG 에 의한 세포 증식력은 증가하지 않았으나, DNA 합성으로 정량화한 당단백은 증가하였다. 또한, 제 1 형 교원질의 mRNA 발현은 IL-1β 투여된 인간 추간판 세포 배양군 보다 EGCG 에 의해 증가하였다. IL-1β에 의해 증가된 MMP-1 과 MMP-13 의 단백질 발현은 고농도의 EGCG 처리에 의해 아무것도 투여되지 않은 대조군 보다도 확연히 감소하였고, PGE2의 경우도 감소하는 경향을 보였다. 결론 결론 결론 결론 : EGCG 는 인간 추간판 세포에 있어 세포 독성을 나타내지 않고, 항 흡수 효과를 나타낸다. -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- 핵심되는 핵심되는 핵심되는