저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The effect of cytosine
deaminase-expressing mesenchymal stem cells in
orthotopic glioma model of immune
competent mice
By
Ha Vu Hai
Major in Neuroscience
Neuroscience Graduate Program
The Graduate School, Ajou University
The effect of cytosine
deaminase-expressing mesenchymal stem cells in
orthotopic glioma model of immune
competent mice
By
Ha Vu Hai
A dissertation Submitted to The Graduate School of
Ajou University for Degree of Master in Neuroscience
Supervised by
Haeyoung Suh-Kim, Prof.
Major in Neuroscience
Neuroscience Graduate Program
The Graduate School, Ajou University
This certifies that the dissertation of Ha Vu Hai
is approved.
SUPERVISORY COMMITTEE
_______________________________
Haeyoung Suh-Kim
_______________________________
Sung-Soo Kim
_______________________________
Young-Don Lee
The Graduate School, Ajou University
i
The effect of cytosine deaminase-expressing
mesenchymal stem cells in orthotopic glioma model of
immune competent mice
ABSTRACT
Glioblastoma multiforme (GBM) is the tumor common deriving from glia and aggressive primary brain tumor. Current standard therapy provides only modest improvements in progression-free and overall survival of patients. Most of the preclinical studies for glioma utilize immune deficient animals, which limits the assessment of role of immune system in glioma progression and therapy. However, the contribution of innate immune system in glioma progression cannot be excluded in clinical settings.
In this study, an orthotopic glioma mouse model were established using a syngeneic mouse glioma cell line, GL261 in immune competent wild type C57BL/6 mice. GL261 cells (3x104 cells) were transplanted to the striatum and tumor growth kinetics was assessed by MRI imaging and histological analyses. The therapeutic effects of mesenchymal stem cells (MSC) that was previously engineered to express a suicide gene, cytosine deaminase (MSC/CD) was evaluated in this model. MSC/CD with administration of a
ii
prodrug 5-florocytosine (5-FC) was effective to suppress tumor growth. Therapeutic effect of MSC/CD with 5-FC was further enhanced when followed by treatment of temozolomide. Compared to nude mice, the overall survival of the animals treated with MSC/CD and 5-FC was higher in C57BL/6 mice, suggesting contribution of systemic immune system. Histological analysis revealed that more immune cells including macrophage/microglia, T lymphocytes were infiltrated to the tumor sites. The result indicate that combination therapy of MSC/CD with 5-FC and TMZ possess potent anticancer effects by direct chemoablation and subsequent activation of innate immune system.
Keywords: Mesenchymal stem cells, Temozolomide, Cytosine deaminase,
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF FIGURES ...v
ABBREVIATION... vi
INTRODUCTION ...1
Glioblastoma multiforme (GBM) ...1
Anti-cancer therapies for GBM patients ...1
Immunotherapy ...1
Suicide gene therapies...2
Herpes simplex virus thymidine kinase (HSV-tk) with ganciclovir (GCV) 2 5-FC anti-cancer drug and Cytosine Deaminase (CD) gene ...3
Stem cells as cellular vehicle of therapeutic genes ...4
Temozolomide ...6
MATERIALS AND METHODS ...8
Cultivation of syngeneic murine glioma cell lines ...8
Characterization of GL261 cell line ...8
In vitro suicide effect of MSC/CD ...9
In vitro bystander effects ...9
Isobologram analysis...10
In vivo therapeutic effects ...11
iv
MRI analysis of tumor growth ...12
Statistics analysis ...12
RESULTS ...13
5-FU Sensitivity of glioma cells was preserved after reporter gene labeling ...13
In vitro Suicide effects of MSC/CD ...15
In vitro Bystander effects of MSC/CD against GL261/GFP cells ...16
Combination effect of TMZ and 5FU in GL261/GFP cells in vitro ...18
Combination effect of MSC/CD+5-FC and TMZ in GL261/GFP cells ....19
Tumor growth kinetics ...20
In vivo therapeutic effect ...22
Contribution of immune-component factors in preclinical model ...25
Critical function of immune system influent to tumor rejection in preclinical model ...27
DISCUSSION ...29
CONCLUSIONS...32
Supplementary data ...33
v
LIST OF FIGURES
Figure 1. Cytosine deaminase and metabolism of 5-FU and 5-FC ...4 Figure 2. Migratory property of MSC toward tumor site ...5 Figure 3. Cytotoxic effect of TMZ in cancer cells ...7 Figure 4. Characteristics of GL261 cells were not altered by GFP labeling.14 Figure 5. Suicide effect of MSC/CD...15 Figure 6. Bystander effect of MSC/CD on GL261/GFP glioma cells. ...17 Figure 7. Synergistic effects of 5-FU and TMZ on GL261/GFP glioma cells ...18 Figure 8. Synergistic interaction of MSC/CD with 5-FC and TMZ on GL261 glioma cells ...19 Figure 9. Kinetics of tumor growth: ...21 Figure 10. Synergistic efficacy of MSC/CD with TMZ in syngeneic glioma model...23 Figure 11. Inflammatory response of immune system in preclinical model.25 Figure 12. Critical function of immune system synergistically contributed the combination of TMZ and MSC/CD therapy to prolong life-span of glioma-bearing mice ...28
vi
ABBREVIATION
MTT 3,4,5-dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide 5-FC 5-Fluorocytosine 5-FU 5-Fluorouracil CD Cytosine deaminase CI Combination index DMSO Dimethyl sulfoxide FBS Fetal bovine serum GBM Glioblastoma multiforme GFP Green fluorescence protein HSA Human serum albumin I.P Intraperitoneal injectionIC50 The half maximal inhibitory concentration M.S. Median survival
MRI Magnetic resonance imaging MSCs Mesenchymal stem cells O.S. Overall survival
P.O Oral administration TMZ Temozolomide
1
INTRODUCTION
Glioblastoma multiforme (GBM)
Glioblastoma multiforme (GBM) is the most common tumor of malignant brain tumor in the central nervous system. GBM has a poor prognosis and high incidence of recurrence because it infiltrates the brain parenchyma due to suboptimal resection, innate resistance of tumor cells to therapeutic agents, and impermeability of the blood-brain barrier (BBB) to most chemotherapeutic agents. Brain resection followed by a concurrent treatment of chemotherapy and radiotherapy remains the most effective therapy and adopted as a standard of care. However, such standard of care extends the median survival period for glioma patients by 7 to 9 months(Stupp R et al. 2009).
Anti-cancer therapies for GBM patients Immunotherapy
Adoptive therapeutics is a strikingly specialized anticancer therapy that concern both immune cell administration and direct anticancer activity to the cancer-bearing host. Cytokine induced killer (CIK) cells are major histocompatibility (MHC)-unrestricted cytotoxic lymphocytes that can be generated in vitro from peripheral blood mononuclear cells and cultured with the addition of interferon (IFN)-γ, interleukin (IL)-2, and CD3 monoclonal antibody (CD3mAb). Recently, the application of CIK cells has evolved from experimental studies to phase II clinical studies. Since the first clinical trial of adoptive immunotherapy using CIK cells in 1999, a growing number of trials have suggested that CIK therapy is associated with a significantly prolonged mean survival and control of disease progression. Recent studies demonstrated
2
that immunotherapy with CIK cells improved clinical outcome and promoted the quality of life in cancer patients.
These studies supported the contention that immune responses could actually contribute to the success of anticancer therapies and possibly eliminate residual tumor cells resistant to the action of cytotoxic drugs. Results from the group led by Kroemer and Zitvogel (Apetoh L et al. 2007) further supported an essential role for immune responses in the efficacy of some anticancer therapies. Upon dissecting the molecular bases accounting for the remarkable ability of these cytotoxic agents to promote anticancer immune responses(Logg CR et al. 2012; Ghiringhelli FApetoh L 2013). The aforementioned findings may suggest that future anticancer therapies will most probably rely on combinatorial treatments associating chemotherapy with immunomodulation.
Suicide gene therapies
Herpes simplex virus thymidine kinase (HSV-tk) with ganciclovir (GCV)
The suicide gene/prodrug system applied to prostate cancer, ovarian cancer and GBM patients in clinical trials is HSV-tk with GCV (Alvarez RD et al. 2000; Immonen A et al. 2004; Nasu Y et al. 2007). Thymidine kinase can convert GCV to its cytotoxic metabolite, GCV-triphosphate that subsequently inhibits activity of DNA polymerase by utilizing its cytotoxic effects. However, the bystander effects of HSV-tk on cancer cells are restrained to permeate into cellular membrane because GCV-triphosphate could only be transported through gap junction (Portsmouth D et al. 2007).
3
5-FC anti-cancer drug and Cytosine Deaminase (CD) gene
5-Fluorouracil is one of the most commonly used chemotherapy agents for auxiliary therapy of patients with solid tumor such as colon, breast, prostate and liver cancer more than 40 years. 5-FU is conceded to be an effective anticancer drugs on gastric cancer as either mono-therapy or relying on a combinatorial treatment (Ohtsu A et al. 2000; Longley DB et al. 2003). However, 5-FU has shown limited delivery to brain blood barrier which lead to the least effective response in clinical tests of malignant glioma patients. However, the irregular structure of vascular vessel in glioma, eliminating the possibility of poor delivery but rather indicating the heterogeneity of glioma (Schneider et al. 2004). Therapeutic reception of glioma patients relies on individuals even treated same drug. Even for GI-tumors, only 10-30% of patients are positive response to 5-FU, further implying the inherent heterogeneity.
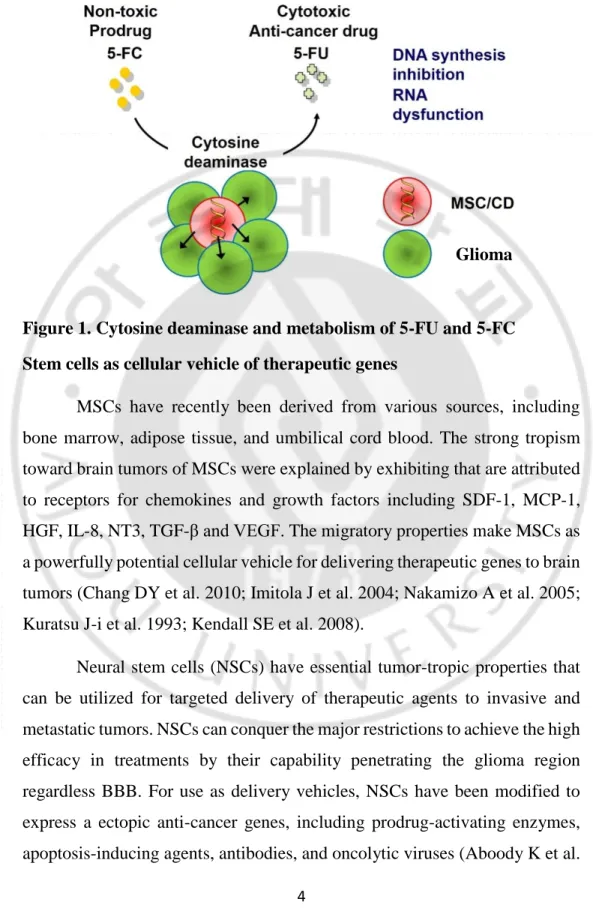
Cytosine deaminase (CD) enzyme converts the prodrug, 5-fluorocytosine (5-FC) into the 5-fluorouracil (5-FU), leading to direct cytotoxicity of the cancer cells. This treatment modality has immense pertinence in various tumor types (Ghiringhelli FApetoh L 2013).
4
Figure 1. Cytosine deaminase and metabolism of 5-FU and 5-FC Stem cells as cellular vehicle of therapeutic genes
MSCs have recently been derived from various sources, including bone marrow, adipose tissue, and umbilical cord blood. The strong tropism toward brain tumors of MSCs were explained by exhibiting that are attributed to receptors for chemokines and growth factors including SDF-1, MCP-1, HGF, IL-8, NT3, TGF-β and VEGF. The migratory properties make MSCs as a powerfully potential cellular vehicle for delivering therapeutic genes to brain tumors (Chang DY et al. 2010; Imitola J et al. 2004; Nakamizo A et al. 2005; Kuratsu J-i et al. 1993; Kendall SE et al. 2008).
Neural stem cells (NSCs) have essential tumor-tropic properties that can be utilized for targeted delivery of therapeutic agents to invasive and metastatic tumors. NSCs can conquer the major restrictions to achieve the high efficacy in treatments by their capability penetrating the glioma region regardless BBB. For use as delivery vehicles, NSCs have been modified to express a ectopic anti-cancer genes, including prodrug-activating enzymes, apoptosis-inducing agents, antibodies, and oncolytic viruses (Aboody K et al.
5
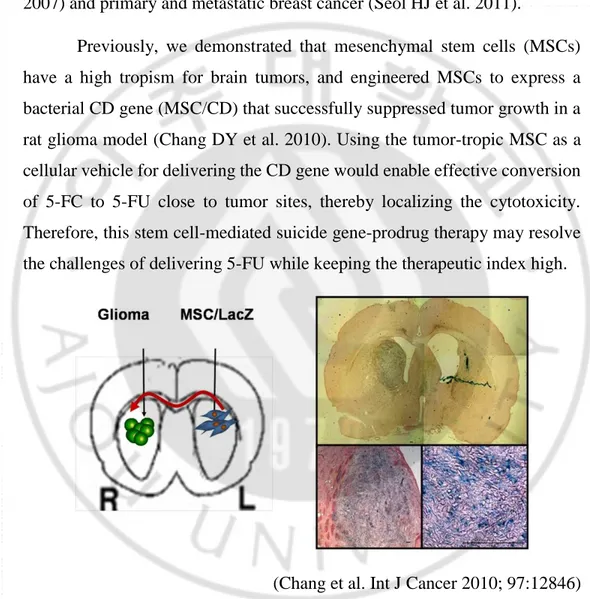
2008; Frank RT et al. 2010). The engineered NSCs were injected intra-cerebrally to exert robust therapeutic efficacy in orthotopic glioma model (Ahmed AU et al. 2011), medulloblastoma (Gutova M et al. 2013), melanoma brain metastases (Aboody KS et al. 2006), neuroblastoma (Danks MK et al. 2007) and primary and metastatic breast cancer (Seol HJ et al. 2011).
Previously, we demonstrated that mesenchymal stem cells (MSCs) have a high tropism for brain tumors, and engineered MSCs to express a bacterial CD gene (MSC/CD) that successfully suppressed tumor growth in a rat glioma model (Chang DY et al. 2010). Using the tumor-tropic MSC as a cellular vehicle for delivering the CD gene would enable effective conversion of 5-FC to 5-FU close to tumor sites, thereby localizing the cytotoxicity. Therefore, this stem cell-mediated suicide gene-prodrug therapy may resolve the challenges of delivering 5-FU while keeping the therapeutic index high.
(Chang et al. Int J Cancer 2010; 97:12846)
6
Temozolomide
Temozolomide (TMZ) is a prodrug that is extraordinarily stable at an acidic pH, resulting in 100% oral bioavailability. In slightly basic pH environment of the blood and tissue, TMZ is spontaneously hydrolyzed into its active metabolite, 5-(3-methyltriazen-1-yl)imidazole-4-carboxamide, which sequentially methylate DNA at the N7 and O6 positions of guanine, and the N3 position of adenine (Nagasawa DT et al. 2012) . The guanine-thymine mismatches created by O6-methylguanine causes DNA breaks if the lesions are not repaired by O6-methylguanine DNA methyltransferase (MGMT), a DNA methylation repairing enzyme. TMZ crosses the BBB with tolerable systemic toxicity, to halt or slow brain cancer cell growth. The standard of care for GBM currently includes surgery followed by fractionated radiotherapy (RT) with adjuvant daily TMZ administration. In a phase III trial, combination of chemotherapy and radiotherapy significantly increased median survival rate and overall survival rate compared to that observed with radiotherapy single treatment (Stupp R et al. 2005). Although the survival advantage of the combined treatment lasted for up to 5 years in the long-term follow-up study, in most patients, the tumors recurred and eventually patients died because of tumor burden. The antitumor effect of TMZ is less efficient by high level of MGMT expression in cancer cells (Damia GD’Incalci M 1998; Stupp R et al. 2006). Inversely, the methylation in promoter region of MGMT gene induces epigenetic silencing that prevents synthesis of MGMT, leading to cancer cells sensitize to TMZ (Hegi ME et al. 2005). The correlation of MGMT expression levels and TMZ sensitivity are essential to develop alternative, multi-modal therapies for cancer patients who cannot receive
7
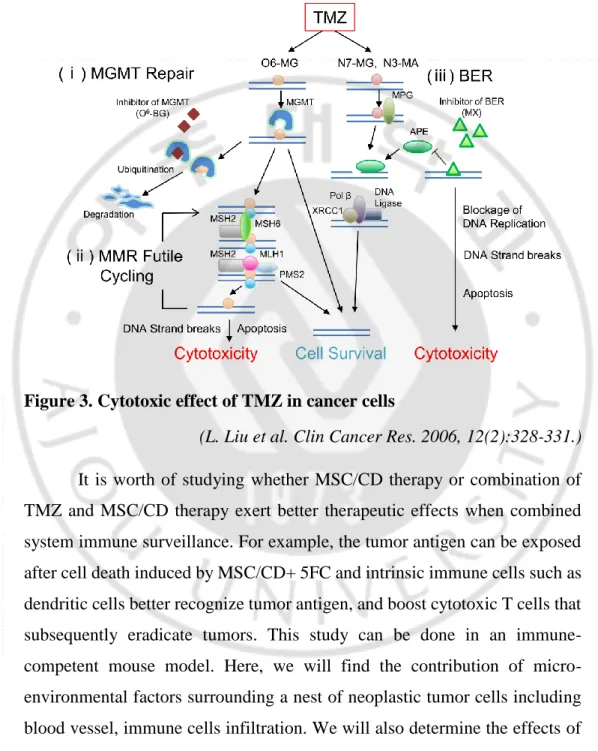
remarkable efficacy from TMZ treatment because of intrinsic resistance to MGMT.
Figure 3. Cytotoxic effect of TMZ in cancer cells
(L. Liu et al. Clin Cancer Res. 2006, 12(2):328-331.) It is worth of studying whether MSC/CD therapy or combination of TMZ and MSC/CD therapy exert better therapeutic effects when combined system immune surveillance. For example, the tumor antigen can be exposed after cell death induced by MSC/CD+ 5FC and intrinsic immune cells such as dendritic cells better recognize tumor antigen, and boost cytotoxic T cells that subsequently eradicate tumors. This study can be done in an immune-competent mouse model. Here, we will find the contribution of micro-environmental factors surrounding a nest of neoplastic tumor cells including blood vessel, immune cells infiltration. We will also determine the effects of those factors with respect to the lifespan of the GBM animals.
8
MATERIALS AND METHODS
Cultivation of syngeneic murine glioma cell lines
GL261 cells were obtained from The Jackson Laboratory#000664 and tested for the absence of mycoplasma infection. GL261 cells were cultured in RPMI medium, supplemented with 10% fetal bovine serum (FBS, Australia, Cat# SH30084.03) 1% L-glutamine (Gibco, Cat#) and 1% pen/strep (Gibco, Cat# 15140-122). All experimental protocols using human MSCs were approved by the Institutional Review Board of the Ajou University Medical Center (Suwon, South Korea). GL261 cells were transduced with lentiviral vector that was produced with pGFP-C-shLenti in 293T cells using a standard method. Briefly, 293T cells were plated in a density of 2x106 cells in 100-mm culture dish. Next day, the cells were transfected with plasmids including: pGFP-C-shLenti (10 µg), pΔ8.9 (8 µg) and pVSV-G (5 µg) by a standard calcium phosphate precipitation method. Eight hours later the medium was replaced with fresh one and the viral supernatant was collected twice at 48 hours and 72 hours after transfection. Viral supernatant was filtered through a 0.45 µm filter and used for transducing GL261 cells with 8 µg/ml polybrene for 8hrs. Viral transduction was repeated one more and the GFP-positive GL261 cells were FACS sorted to enhance the purity.
Characterization of GL261 cell line
GL261 cells were plated 1x104 cells/well in 24 well-plate. Twenty-four hours later, 5FU and TMZ were individually added to culture medium with serial concentration (0; 0.3; 1; 3; 10; 30 µM of 5-FU and 0; 10; 30; 100; 300; 1000 µM of TMZ)The medium was replaced every 2 days with same concentration of 5-FC. Five days later, the medium was aspirated and subject to MTT assays. Briefly, the remaining cells were incubated in PBS containing 0.5 mg/ml 3,4,5-dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide
9
(MTT) for 2 hours at 37oC. After removal of the supernatant, dimethyl sulfoxide was added to the cells and allowed 5 min to dissolve the purple
formazan product. The formazan in the DMSO solvent was transferred to 96 well plates and absorbance was measured at 540 nm. The OD540nm was used to represent the mitochondrial NADPH activity and indirectly surviving cells.
In vitro suicide effect of MSC/CD
Cryopreserved MSC/CD cells were thawed and were plated in a density of 1x104 cells/well in 12 well plates. Twenty-four hours later, 5-FC was added to the culture to a final concentration of 1~1,000 µM. The medium was replaced every 2 days with same concentration of 5-FC. Seven days later, the medium was aspirated and subject to MTT assays. Briefly, the remaining cells were incubated in PBS containing 0.5 mg/ml 3,4,5-dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) for 2 hours at 37oC. After removal of the supernatant, dimethyl sulfoxide was added to the cells and allowed 5 min to dissolve the purple formazan product. The formazan in the DMSO solvent was transferred to 96 well plates and absorbance was measured at 540 nm. The OD540nm was used to represent the mitochondrial NADPH activity and indirectly surviving cells.
In vitro bystander effects
Cryopreserved MSC/CD cells with indicated numbers were simultaneously plated with 1x104 GL261/GFP glioma cells per well in 12 well plates. Twenty-four hours later, various concentrations of 5-FC was added to the culture and medium was replaced every 2 days. Seven days later, remaining cells were rinsed with PBS and lysed in 200 µl 1x Lysis buffer (Promega, Madison, WI, USA) for 10 min at 4oC. The cell lysates were transferred to the centrifuge tubes and centrifuged at 10,000 x g for 10 min at
10
4oC. The supernatant was transferred to 96 cell plates and the fluorescence was measured using fluorometry (Molecular Devices, Sunnyvale, CA, USA)
Isobologram analysis
To assess the interaction of 5-FU and TMZ, the cells were incubated in the presence of various concentrations of 5-FU and TMZ. Five days later, the cells were subject to MTT assays and isobologram analysis. The IC50 value of each drug was determined using dose response assays. The synergism between 5-FU and TMZ was quantified by determining the combination index (CI), which is a quantitative evaluation of a two-drug pharmacological interaction:
CI = C1/S1 + C2/S2
S1, S2 indicated the concentration of 5-FU or TMZ, whereas C1 and C2 were doses of the respective drugs in the combination required to produce the same effect that S1 or S2, the dose of each drug would produce. The combined effect of both drugs can be summarized as, CI = 1, < 1, and > 1 indicate summation (additive with no interaction), synergism, and antagonism, respectively. The CI was calculated using the CompuSyn ver 1.0 program (ComboSyn Inc, Paramus, NJ, USA) for each isobologram point. For the isobologram analysis of combined bystander effect of MSC/CD with 5-FC and TMZ, the IC50 of the each test drug was first determined by evaluating its individual dose-dependent cytotoxicity, and then each value was further evaluated using a fixed concentration of another test drug. The interaction between 5-FC and TMZ was then quantified by determining the CI as described above. The IC50 values for all the assays were plotted, and a hypothetical additive line was drawn between two IC50 values from either drug alone. Above and below the additive line are the hyper- and hypo-additive regions, indicating antagonistic and synergistic interactions, respectively (Zhao L et al. 2004).
11
In vivo therapeutic effects
Wild-type C57BL/6 mice, athymic nude BALB/c mice (6 weeks age) were anesthetized with intra-peritoneal injection by 75 mg/kg ketamine and postured in a stereotaxic device (Stoelting, Wood Dale, IL, USA) for right cerebral hemisphere. GL261/GFP cells were harvested by trypsinization, washed three times with PBS, and finally resuspended in PBS with a density of 1x104 cell/µl and inoculate 3x104 cells/mouse (3 µl).Frozen MSC/CD cells were thawed in 37°C water-bath and absolutely removed DMSO by 5% HSA. And then, mixture of MSC/CD and GL261/GFP were resuspended in PBS with a density of 3x104 cells in 3µl. These cells were inoculated into the striatum (anteroposterior, +0.05 cm; mediolateral, -0.18 cm; dorsoventral, -0.3 cm) using a Hamilton micro-syringe. After surgery, the skin was closed with a clip. The animals were orally administered with 5-FC (1,000 mg/kg/day twice a day)for 7 consecutive days and TMZ was intraperitoneally injected with 5 mg/kg/day for 5 days. The body weights were measured twice a week.
Histological analysis
Mouse brains were removed and fixed for 24 h in 4 % paraformaldehyde in PBS. The next day, these samples were processed in paraffin embedding assay. For immunohistochemistry studies, primary antibodies used were: microglia-specific anti-Iba1 (1:2000; Wako cat.# 019-19741); anti-CD3 (1:100 abcam cat.# ab5690); anti-CD8 (1:100, eBioscience 14-0808); anti-CD4 (1:100, eBioscience 14-9766). After the first incubation, these section were incubated with secondary antibody anti-rat (1:250,#) and anti-rabbit (1:250,#), followed by development using 3,3′-diaminobenzidine (DAB) horseradish peroxidase (HRP) substrate (Vector Laboratories).
12
MRI analysis of tumor growth
Glioma-bearing mice were imaged in a 15.2T MR scanner. Thirty slices of brain at 0.3 mm slice thickness were consecutively taken per each mice. Images were analysis using MicroDicom (Escott EJRubinstein D 2003).
Tumor Area (mm3) = Number of pixels of tumor area
Number of pixels per unit area x Depth
Statistics analysis
Results from at least three independent in vitro experiments were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc test. For animal studies, the results were analyzed using Student’s t-test for single comparisons or ANOVA, followed by Tukey’s HSD post-hoc test for multiple comparisons. Values are expressed as the mean S.E
13
RESULTS
5-FU Sensitivity of glioma cells was preserved after reporter gene labeling
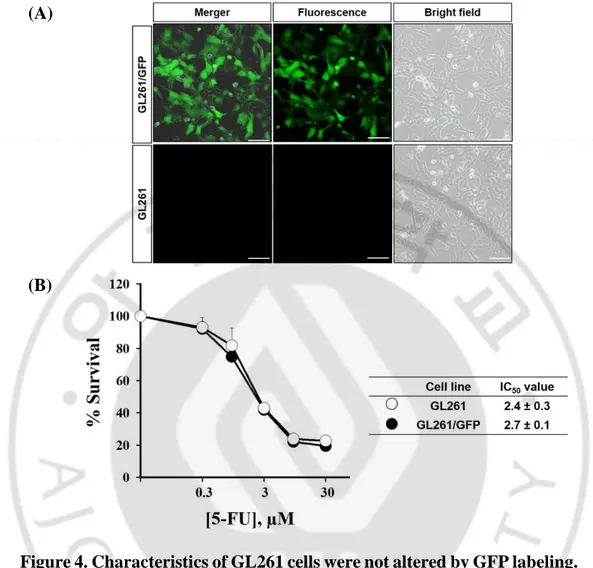
Morphology of GL261/GFP glioma cells were similar to those found in naïve GL261. Subsequently, the 5-FU sensitivity of GL261/GFP and naïve GL261 also were assessed whether GFP expression might altered cell characteristics. MTT assays measuring the mitochondrial activity of surviving cells showed IC50 values of GL261/GFP and naïve GL261 were 2.4 µM and 2.7 µM, respectively. The date indicated that negligible changes were made by transduction of reporter genes in primitive morphology and 5-FU sensitivity.
14
(A)
(B)
Figure 4. Characteristics of GL261 cells were not altered by GFP labeling. (A) The morphology of naïve GL261 and GL261/GFP. (B) The
sensitivity to 5-FU of naïve GL261 (black circle) and GL261/GFP (white circle). Cells were plated 1x104 cells/well (24 well plate) and then incubated with different concentration of 5-FU for 5 days. MTT assays measuring the mitochondrial activity of surviving GL261 and GL261/GFP cells showed IC50 values of 2.4 0.3 µM and 2.7 0.1 µM, respectively. The data indicated that negligible changes were made by transduction of reporter genes in morphology and 5-FU sensitivity. (*p <0.05, use T-test).
15
In vitro Suicide effects of MSC/CD
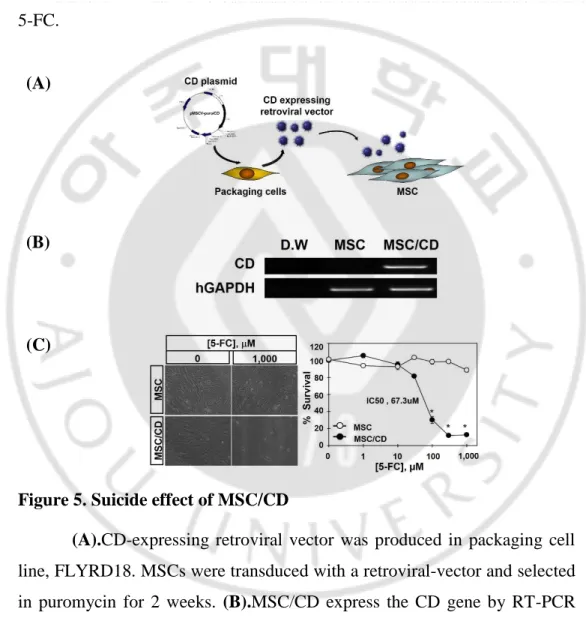
To test the effect of the suicide CD gene on the MSC/CD, they were cultured with 5-FC for 7 days. While the survival rate of the MSC remained consistent, the MSC/CD underwent cell death with an IC50 value of 67.3μM 5-FC.
(A)
(B)
(C)
Figure 5. Suicide effect of MSC/CD
(A).CD-expressing retroviral vector was produced in packaging cell
line, FLYRD18. MSCs were transduced with a retroviral-vector and selected in puromycin for 2 weeks. (B).MSC/CD express the CD gene by RT-PCR analysis. (C).MSC/CD was similar to proliferation and morphology of native MSCs. However MSC/CD was showed suicide effect in the culture medium containing 5-FC. The IC50 value of MSC/CD was 67.3 M.
16
In vitro Bystander effects of MSC/CD against GL261/GFP cells
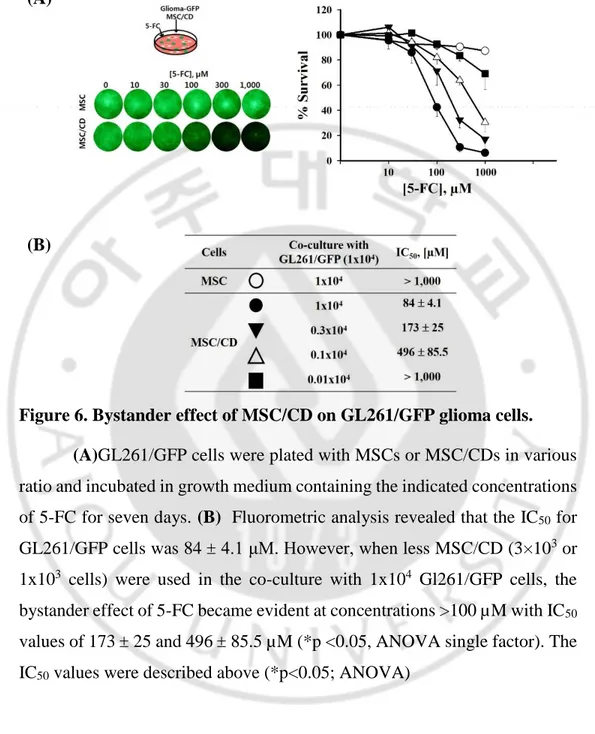
To confirm the bystander effect of MSC/CD with 5-FC against glioma cell lines,GL261/GFP cells were plated with MSCs or MSC/CDs in 1:1 ratio and incubated in growth medium containing the indicated concentrations of 5-FC for seven days. Fluorometric analysis revealed that the IC50 for GL261/GFP cells was 84 μM. However, when less MSC/CD (3x103 or 1x103 cells) were used in the co-culture with 1x104 Gl261/GFP cells, the bystander effect of 5-FC with IC50 values of 173 and 433.2 µM, respectively. The parental MSCs did not exert any cytotoxic effects on glioma cells even in the presence of this highest 5-FC concentration. Fluorometric assays indicated that the bystander effect of MSC/CD and 5-FC was dependent not only the concentration of 5-FC, but also on the ratio of therapeutic MSCs to glioma cells.
17
(A)
(B)
Figure 6. Bystander effect of MSC/CD on GL261/GFP glioma cells. (A)GL261/GFP cells were plated with MSCs or MSC/CDs in various
ratio and incubated in growth medium containing the indicated concentrations of 5-FC for seven days. (B) Fluorometric analysis revealed that the IC50 for GL261/GFP cells was 84 4.1 μM. However, when less MSC/CD (3×103 or 1x103 cells) were used in the co-culture with 1x104 Gl261/GFP cells, the bystander effect of 5-FC became evident at concentrations >100 µM with IC50 values of 173 25 and 496 85.5 µM (*p <0.05, ANOVA single factor). The IC50 values were described above (*p<0.05; ANOVA)
18
Combination effect of TMZ and 5FU in GL261/GFP cells in vitro
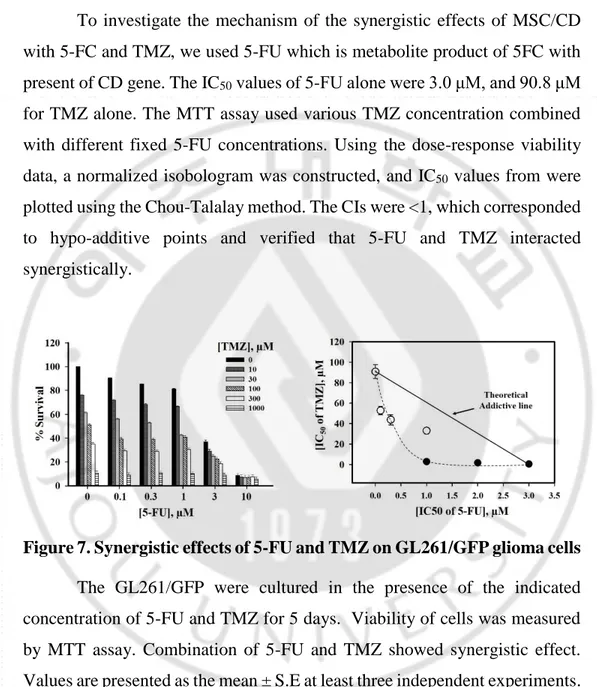
To investigate the mechanism of the synergistic effects of MSC/CD with 5-FC and TMZ, we used 5-FU which is metabolite product of 5FC with present of CD gene. The IC50 values of 5-FU alone were 3.0 μM, and 90.8 μM for TMZ alone. The MTT assay used various TMZ concentration combined with different fixed 5-FU concentrations. Using the dose-response viability data, a normalized isobologram was constructed, and IC50 values from were plotted using the Chou-Talalay method. The CIs were <1, which corresponded to hypo-additive points and verified that 5-FU and TMZ interacted synergistically.
Figure 7. Synergistic effects of 5-FU and TMZ on GL261/GFP glioma cells
The GL261/GFP were cultured in the presence of the indicated concentration of 5-FU and TMZ for 5 days. Viability of cells was measured by MTT assay. Combination of 5-FU and TMZ showed synergistic effect. Values are presented as the mean ± S.E at least three independent experiments.
19
Combination effect of MSC/CD+5-FC and TMZ in GL261/GFP cells
Figure 8. Synergistic interaction of MSC/CD with 5-FC and TMZ on GL261 glioma cells
To evaluate the combinatorial interaction of MSC/CD with 5-FC and TMZ, MSC/CD and GL261/GFP were co-cultured with 5-FC and TMZ. The IC50 values of 5-FC and TMZ alone were 71.3 and 20 μM, respectively. Importantly, combined treatment lowered the drug’s individual IC50 values interactively. To evaluate the combinatorial interactions, the IC50 values shown in plotted using the Chou-Talalay method and the combination indices were < 1, which corresponding to hypo-additive points and indicated 5-FC and TMZ acted synergistically.
20
21
Figure 9. Kinetics of tumor growth:
(A) 3x104 GL261/GFP cells resuspended in 3 µl PBS were intracranially inoculated into striatum as described in method part. Mice were sacrificed at indicated day (1d, 7d, 10d, 19d, n=3). (B) Representative MR images showed tumor growth with 15.2 Tesla. Tumor volume were measured using MicroDcom softwave (4d, 11d, 18d) (n=3).
22
In vivo therapeutic effect
TMZ were cytotoxic to both cancer and normal cells when administered systemically. To avert TMZ’s cytotoxicity on the MSC/CD and retain the synergy, we sequentially treated the mouse glioma model with MSC/CD followed by 5-FC first and then TMZ. Next day, 5-FC and TMZ were administrated by oral administration with 1000 mg/kg twice in a day, during 7 days and intraperitoneal injection with 5 mg/kg, one time in a day, during 5 days, respectively. A similar TMZ dose finding study as for the. Concentration of TMZ (5 mg/kg) in combination with a fixed dose of 5-FC (1000 mg/kg) equal with GBM patients which is the dose used during first-line chemotherapy in clinical trials were administered. All mice were sacrificed on day 20 after glioma cell inoculation. Mice with large tumor burden, regardless of treatment group, showed weight loss. Importantly, the tumor areas decreased more dramatically with the combination treatments. Treatment of single MSC/CD-5-FC regimen and TMZ or combination resulted in statistically reduced tumor areas compared with the control group. These results demonstrate a synergistic efficacy when combining MSC/CD with TMZ treatment in syngeneic orthotopic glioma model. The Kaplan-Meier graph indicated that the median survival of the control group alone was 19 days, similarly, MSC/CD treated tardy tumor state group (D10), while that of the MSC/CD at tumor initial state groups (D0 and D1, respectively) showed a slight increase of 7 days.
23
(A)
(B)
(C) (D)
Group M.S. O.S. Mean
PBS 18 26 20.3
MSC/CD 26 29 27.1
TMZ 31 35 31.6
MSC/CD + TMZ 43 49 42.5
Figure 10. Synergistic efficacy of MSC/CD with TMZ in syngeneic glioma model
(A) Schematic diagram to evaluate antitumor effect of MSC/CD + TMZ in
syngeneic mice GL261/GFP glioma cells and MSC/CD cells were transplanted with 3x104 cells and administrated 5-FC and TMZ at indicated
24
time points with 1000 mg/kg/day P.O. of 5-FC and 5 mg/kg/day of TMZ in C57BL/6 mice, respectively. (B) At 19d, glioma-bearing mice were taken MRI to measure tumor mass. The tumor volume (mm3) showed synergistical antitumor effect of MSC/CD and TMZ therapy in syngeneic glioma mouse model (n=5) (C).The Kaplan-Meier graph indicated that the survival of mice (n=8).
25
Contribution of immune-component factors in preclinical model
(A)
(B)
Figure 11. Inflammatory response of immune system in preclinical model (A) Immunohistochemistry analysis in tumor including CD3+pan T cells, CD4+Th cells, CD8+Tc cells, Iba1+microglia/macrophage. (B) Quantification of CD3+pan T cells, CD4+Th cells, CD8+Tc cells (n=3) and
26
percentage of CD3+/CD4+Th cells, CD3/CD8+Tc cells, CD11b+macrophage, B220+B cells in blood by FACS analysis (n=5)
Population of CD3+pan-T cells, CD4+Th cells significantly increased in MSC/CD, TMZ single treated mice and combination of MSC/CD and TMZ treated mice compared with PBS group, furthermore, CD8+Tc cells population in therapy treated mice were higher than non-treat mice. On these other hands, the number of CD3+/CD4+Th, CD3+/CD8+Tc and CD11b+macrophage were increased in the blood in treated groups compared to PBS groups. However, the number of B220+B cells were not altered between treated groups and PBS groups. These observation suggested that CD3+pan-T cells and CD4+Th cells play important role in prevent glioma growth in MSC/CD + 5-FC and TMZ therapy. Microglia and macrophages population, are also known antigen-presenting cells in brain, infiltrate into tumor site particularly therapy treated mice, however their function within this study were still unclear. These results further support a mechanism of action for the combination of MSC/CD and TMZ that involves activation of an antitumor immune response.
27
Critical function of immune system influent to tumor rejection in preclinical model
(A)
(B)
(C)
Group C57BL/6 Athymic Balb/c M.S. O.S. Mean M.S. O.S. Mean
PBS 23 26 23 23 25 23.4
MSC/CD 28 35 31 27 28 26.4
TMZ 35 37 34.6 30 32 29.8
28
Figure 12. Critical function of immune system synergistically contributed the combination of TMZ and MSC/CD therapy to prolong life-span of glioma-bearing mice
(A) Schematic diagram to examine function of immune system
contributed the combination of TMZ and MSC/CD therapy. (B) Immunocompetent mice (C57BL/6) were inoculated with GL261/GFP glioma cells and treated with MSC/CD and TMZ. (C)Immunocompromised (Athymic Balb/c mice)were inoculated with GL261/GFP glioma cells and treated with MSC/CD and TMZ.The survival rates of animals were shown in Kaplan-Meier graph. Treatment with MSC/CD + TMZ elicited higher therapeutic effects in immune-competent mice compared immunocompromised mice. The results indicate that immune components play an important role in suppressing tumor growth in a preclinical model (n=5).
To evaluate effect of the role of immune components which contribute MSC/CD with TMZ therapy, immunocompetent mice and immunocompromised mice were inoculated GL261/GFP glioma cells and single treated with MSC/CD, TMZ or combination of MSC/CD and TMZ with same scheme and results were shown survival rate of mice by Kaplan-Meier graph.In the immune-compromised mouse model, mice that received MSC/CD, TMZ or combination of MSC/CDand TMZ showed poor short-term survival compared with PBS group. On the other hand, in similar experiments in immune-competent mice, glioma-bearing mice treated therapy led to statistically significant efficacy and long-term survivors.Therefore, immune components play an important role in eradicating tumor in our preclinical model.
29
DISCUSSION
Effective suicide gene therapies rely on strong bystander cytotoxicity and successful delivery of therapeutic genes to tumor sites. In this study, I showed that MSCs can overcome physical barriers to serve as efficient vehicles for delivering suicide gene to brain tumors.
Several recent studies have shown that MSCs can be utilized to deliver therapeutic genes to brain tumors. Genetically modified MSCs that express tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (Kim SM et al. 2008), interleukin-2 (IL-2) (Nakamura K et al. 2004) or interferon (Nakamizo A et al. 2005) have been shown to inhibit tumor growth and prolong the lifespan of tumor-bearing animals. However, these therapies may not be effective for all types of brain tumors, since some tumors can escape from TRAIL-mediated cell death (Malhi HGores GJ 2006) or immune surveillance (Watters JJ et al. 2005). In our study, MSC/CD originated from human displayed robust antitumor effect in fully immunosurveillance model- immunocompetent mice model. The glioma-bearing mice treated with combination MSC/CD (D0) and TMZ (D10) exhibited a more significant improvement in median survival of mice compared with control groups. These results demonstrate a synergistic efficacy when combining MSC/CD with TMZ treatment in syngeneic orthotopic glioma model.In this study, I demonstrated a human-applicable, ex vivo therapeutic modality utilizing bone marrow-derived MSCs as cellular vehicles to deliver therapeutic CD gene into brain tumors. My outcomes underscore the potential advantages of auto-graft MSCs over allograft NSCs for the treatment of established tumors.
30
Of note, Bracci et al highlight that tumor cell death after treatment with select chemotherapeutic agents can reveal tumor antigens and promote epitope spread through ICD. Additionally, several chemotherapeutic agents have specificity for immunosuppressive cells in the tumor microenvironment which, upon clearance, leads to reactivation of immune effector function. (Ghiringhelli FApetoh L 2013; Bracci L et al. 2014). The antineoplastic agent 5-FU has been reported to promote ICD, deplete immunosuppressive cells (specifically MDSCs) from the tumor microenvironment, and activate the adaptive arm of the immune system (Bracci L et al. 2014). Both CD4+Th and CD8+Tc cells were significantly increased in tumors of treated animals. An increase in the ratio of effector T cells to tumor targets is favorable during promotion of an antitumor immune response, especially when Tregs remain unchanged as seen here. In fact, the work described herein showed that while 5-FC was detectable throughout the circulation, 5-FU was only present at detectable levels within the tumor tissue.Hiraoka et al assessed specific lymphocyte subsets that contribute to tumor eradication upon tumor re-challenge in antibody-mediated depletion of CD4+Th, CD8+Tc, and nature killer (NK) cells. The tumors rejection were observed in all animals treated CD8 or NK antibody, however, CD4 or combination of anti-CD4 and anti-CD8 antibody treated groups prevented tumor rejection in previously cured animals (Hiraoka K et al. 2017). These data indicate the importance of CD4+Th cells in antitumor and antiviral immunity, andCD4+ cells may be even more efficient as cytolytic effectors than CD8+Tc cells (Perez-Diez A et al. 2007).In our study, population of CD3+pan-T cells, CD4+Th cells significantly increased in therapy treated groups compared with PBS group, furthermore, CD8+Tc cells population in therapy treated mice were higher than non-treat mice. These observation suggested that CD3+pan-T cells and CD4+Th cells play important role in prevent glioma growth in our therapy.
31
Microglia and macrophages population, are also known antigen-presenting cells in brain, infiltrate into tumor site particularly therapy treated mice, however their function within this study were still unclear.
In the immune-compromised mouse model, mice that received MSC/CD, TMZ or combination of MSC/CDand TMZ showed poor short-term survival compared with PBS group. On the other hand, in similar experiments in immune-competent mice, glioma-bearing mice treated therapy led to statistically significant efficacy and long-term survivors.Therefore, immune components play an important role in eradicating tumor in preclinical model.We previously showed that combination of MSC/CD and TMZ resulted in long-term survival in intracranial tumor-bearing mice. In this current study, we have demonstrated that comparable long-term survival can also be achieved with MSC/CD and TMZ in the immune-competent mouse model. These results further support a dual mechanism of action for the combination of MSC/CD and TMZ that involves both direct tumor chemoablation and the consequent activation of an antitumor immune response.
32
CONCLUSIONS
Dual mechanism of action for the combination of TMZ and MSC/CD + 5-FC that involves both direct tumor chemoablation and the consequent activation of an antitumor immune response.
33
Supplementary data
(A)
(B)
Group M.S. O.S. Mean
TMZ 26 29 25.7
MSC/CD 18 23 19.4
TMZ + MSC/CD 25 30 26
S1. Efficacy of MSC/CD with TMZ in syngeneic glioma model: (A) Schematic diagram to evaluate antitumor effect of MSC/CD + TMZ in
syngeneic mice GL261/GFP glioma cells and MSC/CD cells were transplanted with 3x104 cells and administrated 5-FC and TMZ at indicated time points with 1000 mg/kg/day P.O. of 5-FC and 5 mg/kg/day of TMZ in C57BL/6 mice, respectively. (B) The Kaplan-Meier graph indicated that the survival of mice (n=8).
34
REFERENCES
Aboody K, Najbauer J and Danks M: Stem and progenitor cell-mediated tumor selective gene therapy. Gene therapy 15: 739-752, 2008
Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM and Perides G: Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro-oncology 8: 119-126, 2006
Ahmed AU, Tyler MA, Thaci B, Alexiades NG, Han Y, Ulasov IV and Lesniak MS: A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Molecular pharmaceutics 8: 1559, 2011
Alvarez RD, Gomez-Navarro J, Wang M, Barnes MN, Strong TV, Arani RB, Arafat W, Hughes JV, Siegal GP and Curiel DT: Adenoviral-mediated suicide gene therapy for ovarian cancer. Molecular therapy 2: 524-530, 2000
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E and Saulnier P: Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine 13: 1050-1059, 2007
Bracci L, Schiavoni G, Sistigu A and Belardelli F: Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death & Differentiation 21: 15-25, 2014
Chang DY, Yoo SW, Hong Y, Kim S, Kim SJ, Yoon SH, Cho KG, Paek SH, Lee YD and Kim SS: The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. International journal of cancer 127: 1975-1983, 2010
Damia G and D’Incalci M (1998). Mechanisms of resistance to alkylating agents. Multiple Drug Resistance in Cancer 2, Springer: 165-173.
35
Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, Kim SU, Garcia E, Metz MZ and Najbauer J: Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer research 67: 22-25, 2007
Escott EJ and Rubinstein D: Free DICOM Image Viewing and Processing Software for Your Desktop Computer: What’s Available and What It Can Do for You 1. Radiographics 23: 1341-1357, 2003
Frank RT, Najbauer J and Aboody KS: Concise review: stem cells as an emerging platform for antibody therapy of cancer. Stem Cells 28: 2084-2087, 2010
Ghiringhelli F and Apetoh L: Chemotherapy and immunomodulation: from immunogenic chemotherapies to novel therapeutic strategies. Future Oncology 9: 469-472, 2013
Gutova M, Shackleford GM, Khankaldyyan V, Herrmann KA, Shi X-H, Mittelholtz K, Abramyants Y, Blanchard MS, Kim SU and Annala AJ: Neural stem cell-mediated CE/CPT-11 enzyme/prodrug therapy in transgenic mouse model of intracerebellar medulloblastoma. Gene therapy 20: 143-150, 2013
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W and Mariani L: MGMT gene silencing and benefit from temozolomide in glioblastoma. New England Journal of Medicine 352: 997-1003, 2005
Hiraoka K, Inagaki A, Kato Y, Huang TT, Mitchell LA, Kamijima S, Takahashi M, Matsumoto H, Hacke K and Kruse CA: Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro-oncology 2017
Imitola J, Raddassi K, Park KI, Mueller F-J, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL and Walsh CA: Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proceedings of the National Academy of Sciences 101: 18117-18122, 2004
36
Immonen A, Vapalahti M, Tyynelä K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N and Ylä-Herttuala S: AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Molecular therapy 10: 967-972, 2004
Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, Garcia E, Kim SU, Barish ME and Aboody KS: Neural Stem Cell Targeting of Glioma Is Dependent on Phosphoinositide 3‐ Kinase Signaling. Stem cells 26: 1575-1586, 2008
Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park S-H, Sung Y-C and Jeun S-S: Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer research 68: 9614-9623, 2008
Kuratsu J-i, Yoshizato K, Yoshimura T, Leonard EJ, Takeshima H and Ushio Y: Quantitative study of monocyte chemoattractant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. Journal of the National Cancer Institute 85: 1836-1839, 1993
Logg CR, Robbins JM, Jolly DJ, Gruber HE and Kasahara N: Retroviral replicating vectors in cancer. Methods Enzymol 507: 199-228, 2012
Longley DB, Harkin DP and Johnston PG: 5-fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer 3: 330-338, 2003
Malhi H and Gores GJ: TRAIL resistance results in cancer progression: a TRAIL to perdition? Oncogene 25: 7333-7335, 2006
Nagasawa DT, Chow F, Yew A, Kim W, Cremer N and Yang I: Temozolomide and other potential agents for the treatment of glioblastoma multiforme. Neurosurgery clinics of North America 23: 307-322, 2012
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G and Dembinski J: Human bone marrow–derived
37
mesenchymal stem cells in the treatment of gliomas. Cancer research 65: 3307-3318, 2005
Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y and Hamada H: Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene therapy 11: 1155-1164, 2004
Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F, Manabe D, Thompson TC and Kumon H: Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Molecular Therapy 15: 834-840, 2007
Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T and Group S-CCCS: Phase II study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. British journal of cancer 83: 141, 2000
Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O and Matzinger P: CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 109: 5346-5354, 2007
Portsmouth D, Hlavaty J and Renner M: Suicide genes for cancer therapy. Molecular aspects of medicine 28: 4-41, 2007
Seol HJ, Jin J, Seong D-H, Joo KM, Kang W, Yang H, Kim J, Shin CS, Kim Y and Kim KH: Genetically engineered human neural stem cells with rabbit carboxyl esterase can target brain metastasis from breast cancer. Cancer letters 311: 152-159, 2011
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B and Belanger K: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet oncology 10: 459-466, 2009
38
Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG, Research EOf, Tumor ToCB, Groups R and Group NCIoCCT: Changing paradigms—an update on the multidisciplinary management of malignant glioma. The oncologist 11: 165-180, 2006
Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C and Bogdahn U: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine 352: 987-996, 2005
Watters JJ, Schartner JM and Badie B: Microglia function in brain tumors. Journal of neuroscience research 81: 447-455, 2005
Zhao L, Wientjes MG and Au JL: Evaluation of combination chemotherapy. Clinical Cancer Research 10: 7994-8004, 2004