저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The effect of in vitro osteogenic induction
on in vivo tissue regeneration of the stem cells
from dental pulp and periodontal ligament
Yoon Sun Cha
The Graduate School
Yonsei University
Department of Dentistry
[UCI]I804:11046-000000517057
[UCI]I804:11046-000000517057
[UCI]I804:11046-000000517057
[UCI]I804:11046-000000517057
The effect of in vitro osteogenic induction
on in vivo tissue regeneration of the stem cells
from dental pulp and periodontal ligament
Directed by Professor Je Seon Song
A Dissertation Thesis
Submitted to the Department of Dentistry
and the Graduate School of Yonsei University
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Dentistry
Yoon Sun Cha
June 2018
Acknowledgements
먼저, 저의 지도교수님이신 송제선 교수님께 깊은 감사의 마음을 표합니다. 교수님께서 따뜻한 격려와 조언으로 이끌어주셔서 이 논문을 시작할 수 있었고, 또 잘 마무리할 수 있었습니다. 또한 연구에 가장 큰 힘이 되어주신 전미정교수님께도 감사드립니다. 소아치과의사로서의 꿈을 키우게 해주신 최병재 교수님, 학문적으로, 학문 외적으로 많은 조언과 가르침을 주셨던 이제호 교수님, 늘 따뜻한 마음으로 지켜봐주신 최형준 교수님, 부족한 저를 믿어주시고 용기를 주셨던 김성오 교수님께도 감사의 인사를 전합니다. 연구와 논문을 마무리하기까지 함께 고민하며 도와주셨던 김승혜교수님, 언제나 밝은 미소로 응원해주셔서 정말 감사했습니다. 소아치과의사로서의 길을 열어주시고 한결 같은 믿음으로 키워주신 김지훈교수님께도 감사의 인사를 전합니다. 환자를 먼저 생각할 줄 아는, 깨어있는 소아치과의사가 되겠습니다. 항상 보고싶고 고마운 김수현선생님, 든든한 의국원이었던 배두환선생님, 그리고 함께 동고동락했던 의국 동기 박소영, 오지현, 김효진선생님에게도 감사드립니다. 한없는 사랑으로 키워주신 부모님. 믿음으로 사랑으로 채워주시는 어머님, 아버님. 이 세상에 하나뿐인 내 인생의 동반자 운. 그리고 소중한 예린, 현우, 뱃속에서 건강히 잘 있어준 막내. 저의 길을 응원해주고 제 삶이 빛날 수 있게 해준 우리 가족에게 감사를 전합니다. 2018 년 6 월 차 윤 선 드림Table of Contents
Abstract ... iii
I. Introduction ... 1
II. Materials and Methods ... 3
1. Subjects and predifferentiation ... 3
2. Transplantation ... 5
3. Histology and Immunohistochemistry(IHC) ... 6
4. Quantity of alkaline phosphate activity(ALP) in transplants ... 8
5. Transplant gene expression analysis by qRT-PCR ... 9
6. Statistical analysis ... 11
III. Results ... 12
1. Histologic Characteristics, IHC, and ALP activity in DPSC transplants ... 12
2. Gene expression profiles in DPSC transplants : qRT-PCR findings ... 15
3. Histologic Characteristics, IHC, and ALP activity in PDLSC transplants ... 17
4. Gene expression profiles in PDLSC transplants : qRT-PCR findings ... 20
IV. Discussion ... 23
V. Conclusion ... 28
References ... 29
List of Tables and Figures
Table 1. Primer sequences and sizes used for qRT-PCR ... 10 Figure 1. Histological characteristics of dental pulp stem cell transplants. ... 13 Figure 2. Relative gene expressions in dental pulp stem cell transplants. ... 16 Figure 3. Histological characteristics of periodontal ligament stem cell transplants. .... 18 Figure 4. Relative gene expressions in periodontal ligament stem cell transplants. ... 21
Abstract
The effect of in vitro osteogenic induction
on in vivo tissue regeneration of the stem cells
from dental pulp and periodontal ligament
Yoon Sun Cha
Department of Dentistry The Graduate school, Yonsei University
(Directed by professor Je Seon Song, D.D.S.,M.S.,Ph.D.)
The aim of this study was to determine the effects of in vitro osteogenic differentiation on the in vivo tissue regeneration of dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs).
DPSCs and PDLSCs were predifferentiated for 0, 4, or 8 days with an osteogenic medium, and then transplanted into subcutaneous pockets in immunocompromised mice. The transplants were harvested 9 weeks after transplantation, and the characteristics of the newly formed tissues in vivo were analyzed by histological staining; examining alkaline phosphate (ALP) activity; immunohistochemical staining for osteocalcin, dental sialoprotein, and type XII collagen; and
quantitative real-time polymerase chain reaction to analyze the expression patterns of the following genes; RUNX2, OC, DMP1, DSPP, POSTN, CP23, and Col XII.
In DPSC transplants, the amount of new tissues was similar in all groups, whereas in predifferentiated transplants the OC and DSPP expression were higher than undifferentiated transplants. Predifferentiated PDLSC transplants generated more hard tissue and expressed higher alkaline phosphatase activity than undifferentiated transplants. In particular, 8-day predifferentiated PDLSC transplants formed the tissue closer to cementum/PDL complex in vivo as confirmed by the higher expression levels of POSTN, CP23, and Col XII.
Although there was no significant increase in tissue-forming ability among DPSCs after predifferentiation, predifferentiated DPSCs generated hard tissue closure to dentin. Also, predifferentiated PDLSCs appeared to be able to generate higher-quality and greater amounts of tissue for dental regeneration than undifferentiated PDLSCs.
The effect of in vitro osteogenic induction
on in vivo tissue regeneration of the stem cells
from dental pulp and periodontal ligament
Yoon Sun Cha
Department of Dentistry The Graduate school, Yonsei University
(Directed by professor Je Seon Song, D.D.S.,M.S.,Ph.D.)
I. Introduction
Stem-cell-based regenerative dentistry has emerged as a promising alternative for the current treatment options, which have unavoidable limitations and are clinically unpredictable. Mesenchymal stem cells (MSCs) are undifferentiated cells that are capable of self-renewal and multilineage differentiation (Gronthos, et al., 2000; Seo, et al., 2004), and can be used for targeted tissue regeneration.
In recent decades, human teeth and periodontal tissue have been found to be a niche of postnatal stem cells. All dental tissue-derived MSCs have the same origin as the neural crest, which may have additional merit in the field of dental tissue engineering (Gronthos,
et al., 2000; Seo, et al., 2004). It has been shown that dental tissue-derived MSCs not only have superior odontogenic capability, but also higher replicative potential and better growth characteristics compared with bone marrow mesenchymal stem cells (Tamaki, et al., 2013; Yu, et al., 2007). Among the several kinds of dental tissue-derived MSCs, those from dental pulp (Gronthos, et al., 2000) and the periodontal ligament (PDL) (Seo, et al., 2004) are particularly useful for autologous stem cell therapies.
The most critical component for targeted tissue regeneration appears to be the isolation of appropriate cell populations and the establishment of optimal conditions for growth and differentiation. Several studies have been conducted with a view to establishing the ideal growth/culture conditions for stem cells (Castano-Izquierdo, et al., 2007; Wang, et al., 2011). In particular, changing the period of induction can be helpful to achieve variously differentiated cells, which can, in turn, be expected to affect the resultant tissue regeneration. However, although there have been several such studies using bone marrow MSCs (Castano-Izquierdo, et al., 2007; Peters, et al., 2009; Ye, et al., 2012), as yet there have been no similar investigations involving dental pulp stem cells (DPSCs) or periodontal ligament stem cells (PDLSCs).
Thus, the aim of this study was to examine the regeneration effects from predifferentiated DPSCs and PDLSCs with different periods of time. The effects of altering the period of in vitro osteogenic differentiation were investigated by observing the characteristics and gene expression patterns of the resulting regenerated tissue in vivo.
II. Materials and Methods
1. Subjects and predifferentiation
Human permanent teeth (n = 16) were extracted for dental treatment from 16 patients (aged 6–44 years; 7 males and 9 females) under approved guidelines set by the Institutional Review Board of the Dental Hospital, Yonsei University (2-2012-0046), Seoul, Korea. The pulp and PDL tissues were subjected to primary culture using the outgrowth method as described in a previous study (J. S. Song, et al., 2012). The explants were cultured in growth medium (alpha minimum essential medium [Invitrogen, Carlsbad, CA, USA] supplemented with 10% fetal bovine serum [Invitrogen], 100 U/ml penicillin, 100 μg/ml streptomycin [Invitrogen], and 2 mmol/L L-glutamine [Invitrogen]) at 37°C in 5% CO2. Cells were blended at passage 1 respectively, and used at passage 3-5. The cells
were assigned to 1 of 3 groups according to the planned predifferentiation period as follows: 0 (control), 4, and 8 days. Predifferentiation was performed as described elsewhere (Kim, et al., 2015). In brief, cells were seeded at a density of 3.0×103 /cm2 then
exposed for 4 or 8 days to osteogenic medium (growth medium supplemented with 100 nmol/L dexamethasone [Sigma-Aldrich, St. Louis, MO, USA], 50 µmol/L L-ascorbic
acid 2-phosphate [Sigma-Aldrich], and 2 mmol/L β-glycerophosphate [Sigma-Aldrich]). Cells in the 4-day group were cultured in growth medium for 4 days and then exposed to the osteogenic medium for the subsequent 4 days, whereas those in the 8-day group were
cultured in the osteogenic medium for 8 days. Cells in the 0-day group were not exposed to osteogenic stimuli. The culture medium was refreshed every 3 days, and all groups had reached almost full confluency after 8 days. These cells were used for transplantation.
2. Transplantation
The transplantation procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Yonsei University (#2012-0293). Predifferentiated 3.0x106 cells were mixed with 40 mg of macroporous biphasic calcium
phosphate (MBCP; Biomatlante, Vigneux de Bretagne, France) and subcutaneously transplanted into the dorsal surface of 5-week-old male, immunocompromised mice (n=45, BALB/c-nu, SLC, Shizuoka, Japan), as described in the previous study (Kuznetsov, et al., 1997). Four pockets were made per mouse, and different types of transplants were individually inserted into the pocket randomly as follows: MBCP particles with Day 0, 4, and 8 groups from DPSCs or PDLSCs and MBCP particles only without cell loading. For Day 0, 4, and 8 groups in DPSC and PDLSC transplantation, 28 pockets were used, and for MBCP particles only group, 12 pockets were used. All transplants were retrieved at 9 weeks post-transplantation; of these 28, 16 from each group were subjected to quantitative real-time poltmerase chain reaction (qRT-PCR), and the remaining 12 transplants from each group were divided equally into two fragments for histological, and for alkaline phosphatase (ALP) analysis.
3. Histology and Immunohistochemistry (IHC)
The harvested transplants were fixed with 10% buffered formalin (Sigma-Aldrich) overnight, decalcified with 10% EDTA (pH 7.4; Fisher Scientific, Houston, TX, USA) for 4 weeks, embedded in paraffin, sectioned, and then mounted onto glass slides. The sections were stained with hematoxylin-eosin or Masson trichrome (MT), and then observed with an optical microscope (Olympus BX40, Olympus, Tokyo, Japan). The areas of newly formed hard tissue and of MBCP were measured on hematoxylin-eosin-stained sections using ImageJ software (ImageJ 1.47t, National Institutes of Health, Bethesda, MD, USA).
The hard tissue-forming potential of transplant, calculated by dividing the total area of newly formed hard tissue by the total area of MBCP on the section, was compared as described previously (Mah, et al., 2014). The DPSC transplant sections were immunostained for dentin sialoprotein (DSP) and osteocalcin (OC), while the PDLSC transplant sections were immunostained for collagen type XII (Col XII) and OC. Protease K (Dako, Carpinteria, CA, USA) was used to retrieve the antigen for the OC staining, while no such treatment was performed for DSP and Col XII staining. For DSP staining, sections were incubated with a 1:3000 dilution of the antihuman DSP antibody (sc-33586; Santa Cruz Biotechnology, Santa Cruz, CA, USA). For OC staining, sections were incubated with a 1:2500 dilution of the antihuman OC antibody (#AB10911; Millipore, Temecula, CA, USA). Finally, for Col XII staining, sections were incubated with a
1:8000 dilution of the antihuman Col XII antibody (sc-68862; Santa Cruz Biotechnology). All sections were then incubated with an horseradish peroxide-labeled polymer conjugated with a secondary rabbit antibody using an EnVision+ system kit (Dako). The color was developed using 3,3’-diaminobenzidine substrate (Dako) and counterstained with Gill’s hematoxylin solution (Merck, Darmstadt, Germany). Negative control sections were subjected to the same immunostaining protocol as the other immunostained sections without the primary antibody step and confirmation that the immunostaining observed in the immunostained sections originated from the transplanted cells was obtained by subjecting MBCP transplant sections to immunostaining with the aforementioned primary antibodies as described elsewhere (Kim, et al., 2015; J. S. Song, et al., 2012).
4. Quantity of ALP in the transplants
The level of ALP in the retrieved transplants was measured using the SensoLyte p-nitrophenylphosphate Alkaline Phosphatase Assay Kit (AnaSpec, Fremont, CA, USA) as described previously (Kim, et al., 2015; Mah, et al., 2014). The transplants were rinsed and soaked overnight in PBS (pH 7.4; Invitrogen) and then lysed with Triton X-100 (provided in the kit), according to the manufacturer’s instructions. Briefly, the supernatant of the tissue lysate was harvested, and p-nitrophenylphosphate was added to it, and then ALP was quantified by measuring the absorbance at 405 nm using a Benchmark Plus Microplate Spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). The quantity of ALP was normalized against the total protein quantity in the same lysate supernatant, which was measured using a Thermo Scientific Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA).
5. Transplant gene-expression analysis by qRT-PCR
The relative gene expressions in the transplants were confirmed by qRT-PCR. The expressions of the genes encoding dentin sialophosphoprotein (DSPP), dentin matrix acidic phosphoprotein 1 (DMP1), runt-related protein 2 (RUNX2), and OC were measured in DPSC transplants, and those of RUNX2, OC, and the genes encoding Col XII, cementum protein 23 (CP23), and periostin (POSTN) were measured in PDLSC transplants. In brief, the total RNA was extracted from the transplants by using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The integrity and concentration of the extracted RNA were measured using a spectrophotometer (NanoDrop ND-2000, ThermoScientific, Waltham, MA, USA). Complementay DNA was synthesized from RNA (500 ng) using a Maxime RT PreMix kit (oligod(T)15primer;IntronBiotechnology,Seoul,Korea)accordingtothe manufacturer’s instructions. A qRT-PCR assay was performed with SYBR Premix EX Taq (Takara Bio, Otsu, Japan) and an ABI 7300 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA), also according to the manufacturer’s instructions. The sequences and sizes of the primers are given in Table 1. The expression level of each gene was normalized to that of the gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the relative expression levels of genes was calculated using the 2–ΔΔCt method (Livak and
Schmittgen, 2001). The expression level of each gene in the transplants was calculated relative to its expression level in the unloaded (cell-free) MBCP (vehicle) transplants.
Table 1. Primer sequences and sizes used for qRT-PCR. The annealing procedures were
performed at 60ºC for all primers.
Gene Primer sequence (5’–3’) Size (bp) Reference
DSPP F: GGGATGTTGGCGATGCA
R: CCAGCTACTTGAGGTCCATCTTC 70 (Wei, et al., 2007)
DMP1 F: GATCAGCATCCTGCTCATGTT
R: AGCCAAATGACCCTTCCATTC 109 (Atkins, et al., 2009)
RUNX2 F: CACTGGCGCTGCAACAAGA
R: CATTCCGGAGCTCAGCAGAATAA 127 (Qian, et al., 2010)
OC F: CAAAGGTGCAGCCTTTGTGTC
R: TCACAGTCCGGATTGAGCTCA 150 (Garlet, et al., 2007)
CP23 F: AACACATCGGCTGAGAACCTCAC
R: GGATACCCACCTCTGCCTTGAC 142 (Fujii, et al., 2008)
POSTN F: CACAACCTGGAGACTGGAC
R: TGTCTGCTGGATAGAGGAG 322
(Tomokiyo, et al., 2008)
Col XII F: CGGACAGAGCCTTACGTGCC
R: CTGCCCGGGTCCGTGG 180 (Fujii, et al., 2008)
GAPDH F: TCCTGCACCACCAACTGCTT
R: TGGCAGTGATGGCATGGAC 100 (Fujii, et al., 2008)
Abbreviations: qRT-PCR, quantitative RT-PCR; F, forward primer; R, reverse primer;
DSPP, gene encoding dentin sialophosphoprotein; DMP1, gene encoding dentin matrix
acidic phosphoprotein 1; RUNX2, gene encoding runt-related protein 2; OC, gene encoding osteocalcin; CP23, gene encoding cementum protein 23; POSTN, gene encoding periostin; Col XII, gene encoding collagen type XII; GAPDH, gene encoding glyceraldehyde-3-phosphate dehydrogenase.
6. Statistical analysis
The data analysis was performed at least 3 times independently for each transplant. Statistical analysis was performed using SPSS software (version 19.0; SPSS, Chicago, IL, USA). Multiple comparison testing was conducted using the Kruskal-Wallis test (p<0.05), followed by the Mann-Whitney U test (with Bonferroni correction; p<0.017).
III. Results
1. Histological characteristics, IHC, and ALP activity in DPSC
transplants
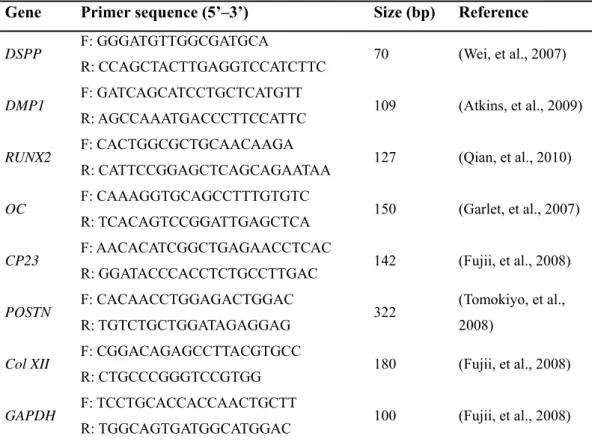
DPSCs formed amorphous hard tissues along the periphery of the MBCP carrier (Figure 1A). The blue-stained portion in Masson’s trichrome-stained samples was largest in the 8-day group followed by the 4-day group and then the control group (Figure 1Ag– i). OC was expressed mainly in the cells lining the margin of the hard tissue in all groups but was particularly remarkable in the 4-day group (Figure 1Aj–l). On the other hand, DSPP was expressed in both the cells and the ECM adjacent to the hard tissue and was most strongly stained in the 8-day group (Figure 1Am–o). The hard tissue-forming ability and ALP activity did not differ significantly between the groups (Figure 1B and C,
Figure 1. Histological characteristics of dental pulp stem cell (DPSC) transplants. (A)
Representative images of sections cut from regenerated tissue formed by DPSCs predifferentiated for 0 days (control; a, d, g), 4 days (b, e, h), and 8 days (c, f, i), stained with hematoxylin-eosin (HE; a–f) and Masson’s trichrome (MT; g–i). Higher-magnification images of the boxed areas in a, b, and c are shown in d/g, e/h, and f/i, respectively. Scale bars: 100 μm for a–c and 20 μm for d–i. Representative images of transplants of DPSCs differentiated for 0 days (a, d), 4 days (b, e), and 8 days (c, f) immunostained with antihuman osteocalcin (OC; a–c) and antihuman dentin sialoprotein (DSP; d–f) antibodies. The red arrows indicate examples of positively immunostained cells. Scale bars: 20 μm. (B) Histogram of the percentage of newly formed tissue relative
to macroporous biphasic calcium phosphate (MBCP) for each of the DPSC groups. Data are mean and standard deviation values. There were no statistically significant differences in the relative amounts of newly formed tissue produced by the DPSCs predifferentiated for 0, 4, and 8 days (Kruskal-Wallis test, p<0.05; each group contained the same number of subjects). (C) Alkaline phosphatase (ALP) activity of DPSC transplants. The Y-axis indicates the ratio of ALP (ng) to total protein (μg) in the transplants; data are mean and standard deviation values. The ALP data did not differ significantly between the three groups (Kruskal-Wallis test, p>0.05; each group contained the same number of subjects).
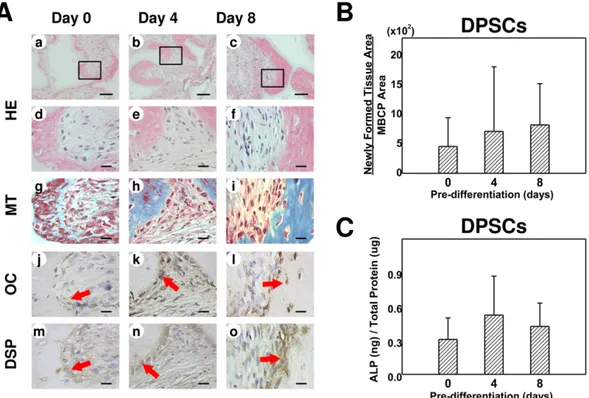
2. Gene expression profiles in DPSC transplants: qRT-PCR findings
The expression patterns of several genes were analyzed using qRT-PCR (Figure 2). The expression level of OC and DSPP was significantly higher in the predifferentiated groups (both 4-day and 8-day) than in the control group (p<0.017). However, the expression levels of RUNX2 and DMP1 did not differ significantly between the groups (p>0.05).
Figure 2. Relative expression levels of the genes encoding runt-related transcription
factor 2 (RUNX2), OC (OC), dentin matrix acidic phosphoprotein 1 (DMP1), and dentin sialophosphoprotein (DSPP) in DPSC transplants. Data are mean and standard deviation values. The expressions of OC and DSPP differed significantly between the three groups (*Kruskal-Wallis test followed by post hoc analysis with the Mann-Whitney U test; Bonferroni correction, p<0.017; each group contained the same number of subjects). The expressions of DMP1 and RUNX2 did not differ significantly between the differently predifferentiated DPSC groups (Kruskal-Wallis test; p>0.05).
3. Histological characteristics, IHC, and ALP activity in PDLSC
transplants
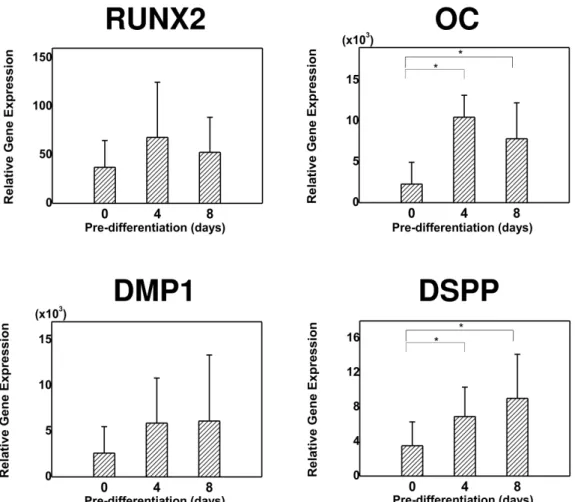
After 9 weeks of transplantation, PDLSCs also produced hard tissues at the periphery of the MBCP carrier (Fig. 3A). The newly formed hard tissues exhibited a fibrous matrix with an irregular wave pattern, and contained embedded cementocytelike cells, somewhat resembling cementum. Dense collagen bundles were observed adjacent to this cementumlike tissue, proceeding toward the interstitial tissue; these were particularly prominent in the 8-day group (Fig. 3Af). IHC revealed that OC was expressed in the cell lining and the cells entrapped in the cementumlike tissues (Fig. 3Aj–l). However, Col XII was detected in the interstitial area, most apparently in the 8-day group (Fig. 3Am–o). The hard tissue-forming ability and ALP activity was significantly higher in the 4- and 8-day groups compared with the control group (Fig. 3B and C, p<0.017).
Figure 3. Histological characteristics of periodontal ligament stem cell (PDLSC)
transplants. (A) Representative images of sections cut from regenerated tissue formed by PDLSCs predifferentiated for 0 days (a, d, g), 4 days (b, e, h), and 8 days (c, f, i) stained with HE (a–f) and MT (g–i). Higher-magnification images of the boxed areas in a, b, and
c are shown in d/g, e/h, and f/i, respectively. The red arrow in f indicates deposition of
collagen fibers resembling Sharpey’s fibers. Scale bars: 100 μm for a–c and 20 μm for d–
i. (C) Representative images of transplants of PDLSC differentiated for 0 days (a, d),
4 days (b, e), and 8 days (c, f) immunostained with antihuman OC (a–c) and antihuman collagen XII (Col XII; d–f) antibodies. The red arrows indicate examples of positively immunostained cells. Scale bars: 20 μm. (B) Histogram of the percentage of newly
formed tissue relative to MBCP for each of the PDLSC groups. Data are mean and standard deviation values. *Statistically significant differences (Kruskal-Wallis test followed by post hoc analysis with the Mann-Whitney U test; Bonferroni correction,
p<0.017; each group contained the same number of subjects). (C) ALP activity of
PDLSC transplants. The Y-axis indicates the ratio of ALP (ng) to total protein (μg) in the transplants. Data are mean and standard deviation values. *Statistically significant differences (Kruskal-Wallis test followed by post hoc analysis with the Mann-Whitney U test; Bonferroni correction, p<0.017; each group contained the same number of subjects).
4. Gene expression profiles in PDLSC transplants: qRT-PCR findings
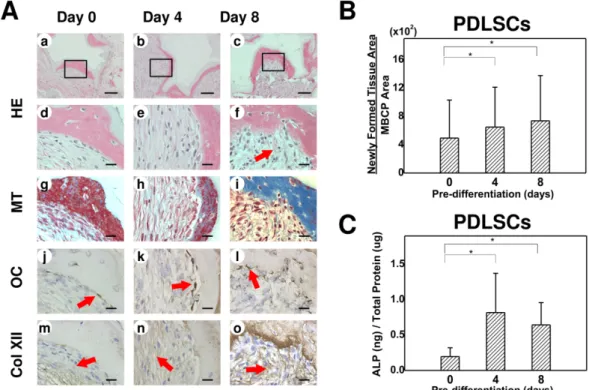
qRT-PCR was used to analyze the gene expression patterns of POSTN, CP23, Col XII,
OC, and RUNX2 in the tissue formed by the transplanted PDLSCs (Fig. 4). The
expressions of OC and RUNX2 differed slightly between the three differently predifferentiated groups, but the differences between them were not statistically significant (p>0.05). The expression levels of genes related to the cementum/PDL complex (POSTN, CP23, and Col XII) were highest in the 8-day group, and were significantly higher than in the control group (p<0.017)
Figure 4. Relative expression levels of RUNX2, OC, and the genes encoding periostin
(POSTN), cementum protein 23 (CP23), and Col XII (Col XII) in variously predifferentiated PDLSC transplants. Data are mean and standard deviation values. The expressions of POSTN, CP23, and Col XII differed significantly between the PDLSCs predifferentiated for 0, 4, and 8 days (*Kruskal-Wallis test followed by post hoc analysis with the Mann-Whitney U test; Bonferroni correction, p<0.017; each group contained the same number of subjects). The expressions of RUNX2 and OC did not differ significantly between the three groups (Kruskal-Wallis test, p>0.05).
IV. Discussion
The optimal use of dental stem cells requires a solid understanding of the process of cell differentiation toward the specific lineage and tissue regeneration. In this study, DPSCs and PDLSCs that had been predifferentiated in vitro for 8 days were as effective at generating new tissues when transplanted in vivo as those that had been predifferentiated for 4 days and transplanted. Furthermore, the tissue regenerated from predifferentiated PDLSCs appeared to be closer to cementum/PDL-like tissue than that regenerated from undifferentiated cells.
The hard tissue-forming ability of DPSC and PDLSC transplants was maintained when predifferentiated for 8 days in the present study. There were no statistically significant differences between groups of DPSC transplants, and predifferentiated PDLSC transplants (both 4-day and 8-day) exhibited statistically significant increased amounts of hard tissue formation relative to undifferentiated PDLSC transplants. These findings differ somewhat from those of previous studies performed with BMMSCs. It was shown that predifferentiated BMMSCs had greater bone-forming potential than undifferentiated BMMSCs (Peters, et al., 2009; Ye, et al., 2012), but when the predifferentiation period was increased, the amount of hard tissue formed from BMMSCs was decreased (Castano-Izquierdo, et al., 2007; Sikavitsas, et al., 2003). In contrast, there was no such reduction in the amount of hard tissue formed from DPSCs and PDLSCs predifferentiated for 4 and 8 days in the present study. The negative correlation between differentiation and
proliferation (Lian and Stein, 1992) provides insights for stem cell engineering, showing the importance of an appropriate period of predifferentiation that is sufficient to trigger differentiation toward a specific lineage, but not long enough to suppress proliferation. Because dental stem cells have a greater proliferation ability compared with BMMSCs (Tamaki, et al., 2013; Yu, et al., 2007), it can be assumed that their tissue-regenerating capability would be maintained with longer periods of predifferentiation. Although it is possible that even longer periods of predifferentiation could result in a reduction in proliferation rates or tissue regeneration, this was not verified in the present study. Further study is required to establish the optimal predifferentiation protocols for dental stem cells in order to maximize their potency in the field of tissue engineering.
The tissue generated from predifferentiated DPSCs was an amorphous hard matrix without any of the organized tubules typically seen in dentin (Gronthos, et al., 2000). The present study used an ectopic in vivo model, with cells loaded onto MBCP as a vehicle being transplanted into the dorsal surface of immunosuppressed mice. MBCP-only transplants (ie., without any stem cells) generated no hard tissue after 9 weeks posttransplantation (data not shown). MBCP is known to have osteoconductive ability, since the mineral chemistry resembles that of human bone (Min, et al., 2015). It has been shown in several other studies that the effectiveness of the subcutaneous MBCP model is limited with respect to dentinogenesis (Kim, et al., 2015; Zhang, et al., 2008). Thus, the unnatural environmental conditions of MBCP could be an important limitation of this study in the context of dental tissue regeneration. It would be advantageous to use dentin
or teeth that could mimic the original environmental conditions to facilitate dentinogenesis from DPSCs because the chemical composition, 3-dimensional structure, and chemotactic effects can differentially promote tissue regeneration (Batouli, et al., 2003; Ogata, et al., 1997).
Predifferentiated DPSC transplants exhibited higher OC and DSPP expressions than the undifferentiated transplants. DSPP can also be detected in bone but at a level 1/400 of that found in dentin (Qin, et al., 2002); thus, DSPP is commonly considered to be a representative marker of odontogenic differentiation because of its important role in dentinogenesis (Batouli, et al., 2003). A study of tooth development observed that DSPP transcription was only weakly present in osteoblasts but strongly in odontoblasts (Chen, et al., 2009). Therefore, the tissue generated from predifferentiated DPSCs would have characteristics closer to dentin than that from undifferentiated DPSCs. Meanwhile, stem cells from human exfoliated deciduous teeth (SHEDs) have been shown to exhibit a higher proliferation rate and bone regeneration capacity than DPSCs but cannot regenerate the complete dentin pulp complex and when predifferentiated for a longer period, the expression of DSPP in these cells was reduced (Kim, et al., 2015). Together these findings show that SHEDs are suitable for bone regeneration but not for dentin, while DPSCs can be used for dental tissue regeneration.
Predifferentiated PDLSCs were able to regenerate a greater amount of hard tissue than their undifferentiated counterparts. They exhibited a greater expression CP23, which is a tissue-specific cementoblast gene (Alvarez-Perez, et al., 2006). These findings are in
agreement with those of previous studies that have investigated cementum and PDL regeneration by differentiated human PDLSCs (Flores, et al., 2008a; Flores, et al., 2008b). Flores et al (Flores, et al., 2008b) produced cell sheets composed of human PDLSCs, and after 3 weeks of predifferentiation these cell could regenerate cementum and Sharpey’s fibers in athymic rats, whereas undifferentiated cell sheets were able to produce only disorganized dense fibrous tissue. Hence, the process of predifferentiation is an effective way of establishing complete regeneration of the periodontium.
PDLSCs predifferentiated for 8 days produced the largest amount of regenerated tissue, which contained the perpendicularly oriented collagen bundles seen in cementumlike tissue and formed connective tissue that resembled Sharpey’s fibers. Also, the 8-day group exhibited the strongest expression of Col XII, which is considered a specific molecular marker for mature PDL (Karimbux and Nishimura, 1995). From the point of view of the root development process (Paulsen, 2010), which involves the simultaneous development of cementum and PDL, it could be postulated that cytokines released from cementoblasts could attract undifferentiated stem cells near them and induce them to differentiate into fibroblasts via a “paracrine effect”. There is an increasing body of evidence that the paracrine effects of periodontal tissues may facilitate complete periodontal regeneration. It was observed that the chemoattractants or differentiation-inducing factors released from cementum are different from those released from bone (Ogata, et al., 1997; A. Song, et al., 2012) and promote PDL cell proliferation, migration, and, ultimately, regeneration of functional PDL structure (Hoz, et al., 2012; Metzger, et
al., 1998). All of these findings suggest that the cytokines released from cementoblasts and PDL fibroblasts affect each other mutually in a paracrine manner, consequently promoting complete periodontal regeneration.
The paracrine effects of stem cells and released cytokines have been investigated in the context of tissue regeneration and appear to promote or control the proliferation and differentiation pathways of surrounding cells (Inukai, et al., 2013; Wang, et al., 2011). The gene expression pattern and protein synthesis of BMMSCs have been shown to change through the process of differentiation in several studies (Liu, et al., 2008; Pavlin, et al., 2001). Therefore, it seems likely that cytokines released from differentiated cells differ from those released from undifferentiated stem cells. Because predifferentiated PDLSCs exhibited superior regeneration of cementum/PDL-like tissue, it could be postulated that the paracrine effects of predifferentiated PDLSCs would affect the regeneration of periodontium differently from undifferentiated cells. Further study is needed to elucidate the changes in cytokine release from stem cells at different levels of differentiation.
To the best of our knowledge, the present study is the first to evaluate and compare the efficacy of dental tissue regeneration by differently predifferentiated dental stem cells. Predifferentiated DPSCs and PDLSCs exhibited an increased capability for dental-tissue regeneration, with 4 and 8 days of predifferentiation being sufficient to maintain the hard tissue-forming ability and to achieve differentiation potency. Furthermore, 8 days of predifferentiation appears to promote the commitment of PDLSCs to a specific lineage of differentiation.
V. Conclusion
The effect of predifferentiation for adequate periods in vitro on dental tissue regeneration in vivo was examined in this study. Although no significant increase in hard tissue-forming ability was conferred by predifferentiation of the DPSCs, predifferentiated DPSCs generated hard tissue closer to dentin. Besides, predifferentiated PDLSCs appeared to generate higher-quality and more tissue for dental regeneration than their undifferentiated counterparts.
References
Alvarez-Perez MA, Narayanan S, Zeichner-David M, Rodriguez Carmona B, Arzate H: Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 38(3): 409-419, 2006.
Atkins GJ, Welldon KJ, Halbout P, Findlay DM: Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int 20(4): 653-664, 2009.
Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al.: Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82(12): 976-981, 2003.
Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI: Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 82(1): 129-138, 2007.
Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L, et al.: Runx2, osx, and dspp in tooth development. J Dent Res 88(10): 904-909, 2009.
Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, Ishikawa I: Cementum-periodontal ligament complex regeneration using the cell sheet technique.
Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I: Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol 35(12): 1066-1072, 2008b.
Fujii S, Maeda H, Wada N, Tomokiyo A, Saito M, Akamine A: Investigating a clonal human periodontal ligament progenitor/stem cell line in vitro and in vivo. J Cell
Physiol 215(3): 743-749, 2008.
Garlet TP, Coelho U, Silva JS, Garlet GP: Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci 115(5): 355-362, 2007.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97(25): 13625-13630, 2000.
Hoz L, Romo E, Zeichner-David M, Sanz M, Nunez J, Gaitan L, et al.: Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol Int 36(2): 129-136, 2012. Inukai T, Katagiri W, Yoshimi R, Osugi M, Kawai T, Hibi H, et al.: Novel application of
stem cell-derived factors for periodontal regeneration. Biochem Biophys Res
Commun 430(2): 763-768, 2013.
Karimbux NY, Nishimura I: Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J Dent
Kim S, Song JS, Jeon M, Shin DM, Kim SO, Lee JH: Ectopic Hard Tissue Formation by Odonto/Osteogenically In Vitro Differentiated Human Deciduous Teeth Pulp Stem Cells. Calcif Tissue Int 97(1): 80-89, 2015.
Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al.: Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12(9): 1335-1347, 1997.
Lian JB, Stein GS: Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol
Med 3(3): 269-305, 1992.
Liu F, Akiyama Y, Tai S, Maruyama K, Kawaguchi Y, Muramatsu K, et al.: Changes in the expression of CD106, osteogenic genes, and transcription factors involved in the osteogenic differentiation of human bone marrow mesenchymal stem cells. J
Bone Miner Metab 26(4): 312-320, 2008.
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-408, 2001.
Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, et al.: The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch Oral Biol 59(5): 539-549, 2014.
Metzger Z, Weinstock B, Dotan M, Narayanan AS, Pitaru S: Differential chemotactic effect of cementum attachment protein on periodontal cells. J Periodontal Res 33(2): 126-129, 1998.
Min B, Song JS, Kim SO, Kim KM, Park WS, Lee JH: Osteoconduction capacity of human deciduous and permanent teeth ash in a rat calvarial bone defect model.
Cell Tissue Bank 16(3): 361-369, 2015.
Ogata Y, Niisato N, Moriwaki K, Yokota Y, Furuyama S, Sugiya H: Cementum, root dentin and bone extracts stimulate chemotactic behavior in cells from periodontal tissue. Comp Biochem Physiol B Biochem Mol Biol 116(3): 359-365, 1997. Paulsen DF: Chapter 15. Digestive Tract. In: Histology & Cell Biology:
Examination & Board Review, 5e. The McGraw-Hill Companies, New York, NY. 2010.
Pavlin D, Zadro R, Gluhak-Heinrich J: Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect Tissue Res 42(2): 135-148, 2001.
Peters A, Toben D, Lienau J, Schell H, Bail HJ, Matziolis G, et al.: Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing.
Tissue Eng Part A 15(10): 2947-2954, 2009.
Qian H, Zhao Y, Peng Y, Han C, Li S, Huo N, et al.: Activation of cannabinoid receptor CB2 regulates osteogenic and osteoclastogenic gene expression in human periodontal ligament cells. J Periodontal Res 45(4): 504-511, 2010.
Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, et al.: The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81(6): 392-394, 2002.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al.: Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429): 149-155, 2004.
Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG: Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J Biomed
Mater Res A 67(3): 944-951, 2003.
Song A, Cai J, Pan K, Yang P: Pre-existing root cementum may promote cementoblast differentiation of human periodontal ligament cells. Cell Prolif 45(3): 249-258, 2012.
Song JS, Kim SO, Kim SH, Choi HJ, Son HK, Jung HS, et al.: In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng Part A 18(19-20): 2040-2051, 2012. Tamaki Y, Nakahara T, Ishikawa H, Sato S: In vitro analysis of mesenchymal stem cells
derived from human teeth and bone marrow. Odontology 101(2): 121-132, 2013. Tomokiyo A, Maeda H, Fujii S, Wada N, Shima K, Akamine A: Development of a
multipotent clonal human periodontal ligament cell line. Differentiation 76(4): 337-347, 2008.
Wang YX, Ma ZF, Huo N, Tang L, Han C, Duan YZ, et al.: Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. J Tissue Eng Regen Med 5(5): 354-362, 2011.
Wei X, Ling J, Wu L, Liu L, Xiao Y: Expression of mineralization markers in dental pulp cells. J Endod 33(6): 703-708, 2007.
Ye X, Yin X, Yang D, Tan J, Liu G: Ectopic bone regeneration by human bone marrow mononucleated cells, undifferentiated and osteogenically differentiated bone marrow mesenchymal stem cells in beta-tricalcium phosphate scaffolds. Tissue
Eng Part C Methods 18(7): 545-556, 2012.
Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, et al.: Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell 99(8): 465-474, 2007. Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA: Hard tissue
formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A 14(2): 285-294, 2008.
국문요약
치수 및 치주인대 줄기세포의 사전분화가
조직재생에 미치는 영향
연세대학교 대학원 치의학과 차 윤 선 지도교수: 송제선 줄기세포를 통한 조직재생을 위해서는 목표한 조직으로 재생되도록 적절한 세포를 분리해 세포 분화 및 성장에 필요한 환경을 형성하는 것이 필요하다. 특히 사전분화 과정은 기간이 길수록 줄기세포의 자가복제능은 줄어들지만, 다양한 세포로 분화될 수 있는 줄기세포가 특정 세포로 분화되도록 유도하기 때문에, 조직 재생에 직접적인 영향을 미치는 중요한 과정이다. 이미 골수유래 줄기세포의 다양한 사전분화 기간에 따른 조직재생 효과에 대해서는 여러 연구가 진행되었으나 사람의 치수 및 치주인대 줄기세포를 이용한 연구는 아직 없었다. 따라서 본 연구는 영구치 치수 및 치주인대 줄기세포를 각기 다른 기간 동안 사전분화시킨 뒤 조직재생에 미치는 영향에 대해 알 아보고자 하였다. 사람의 영구치에서 획득한 치수 줄기세포와 치주인대 줄기세포를 각각 0, 4, 8일간 사전분화시킨 뒤, 면역억제된 쥐에 이식하여 9주간 조직재생을 유도하였다. 이후 이식체를 획득하여 재생된 조직에 대해 조직학적 분석, 알칼리성 인산가수분해효소 활성 (ALP activity), 역전사효소 중합효소 연쇄반응분석, 면역화학염색법을 시행하여 비교하 였다. 치수줄기세포의 경우, 사전분화시키지 않은 군과 비교해 사전분화시킨 군에서 새로 형성된 조직의 양은 차이가 없었으나, 더 높은 OC, DSPP 발현을 나타내 상아질에 더 가까운 조직이 재생되었음을 보여주었다. 치주인대줄기세포의 경우, 사전분화시키지 않은 군보다 사전분화시킨 군에서 더 많 은 양의 조직이 형성되었고, 높은 ALP activity를 나타냈다. 특히, 8일간 사전분화시킨 군에서는 POSTN, CP23, Col XII의 높은 발현을 보여, 백악질/치주인대 복합체에 더 가 까운 조직이 형성되었음을 나타내었다.
본 연구를 통해 사전분화시킨 치수 및 치주인대줄기세포로 재생된 조직의 특성을 이해할 수 있었고, 이는 향후 치수 및 치주인대 줄기세포의 사전분화과정을 이용한 치아조직의 재생에 대한 연구에 응용될 수 있을 것으로 기대한다.