저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in Medicine
Differentiation induction for postoperative
temporal bone using human ear mastoid bone
marrow and adipose-derived stem cells
Ajou University Graduate School
Major in Medicine
Department of Medical Sciences
Seong Gu Park
Differentiation induction for postoperative
temporal bone using human ear mastoid bone
marrow and adipose-derived stem cells
Yun-Hoon Choung, Advisor
I submit this thesis as the
Doctoral thesis in Medicine.
February 2019
Ajou University Graduate School
Major in Medicine
Department of Medical Sciences
Seong Gu Park
i
- ABSTRACT -
Differentiation induction for postoperative temporal bone using
human ear mastoid bone marrow and adipose-derived stem cells
Injured bones have a limited capacity for self-repair in most cases, including otolaryngology. In the case of patients with chronic otitis media or people who need a cochlear implant surgery, the mastoid bone must be drilled out. However, it results in temporal bone defects which can cause various complications such as dizziness, accumulation of keratin debris, otorrhea, or recurrent lesions. To compensate for these disadvantages of open cavity mastoidectomy, reconstruction of the posterior wall of the external auditory canal and mastoid obliteration have been attempted. Autologous tissues, alloplastic and biosynthetic materials have all been used in mastoid obliteration. Autologous tissues are much safer and more cost-effective than alloplastic or biosynthetic materials. However, they also have limitations and are not applicable in every situation because of insufficient amounts of autologous tissues, donor site defects, and reabsorption after surgery. Mastoid bone marrow and adipose tissue can be easily obtained during the surgery without any additional invasive procedures, and also be great source of stem cell. Mastoid bone is normally characterized with air cells for gas exchange and also remains empty space after mastoidectomy. Therefore, mastoid bone requires different bone regeneration strategy from compact bones including cranial bones. Naturally, the scaffold
ii
may have to be different depending on the kinds of target bones. Artificial scaffolds must also be biodegradable, allowing extracellular matrix to occupy the empty space when the biomaterial is degraded. The aim of this study was to investigate the effectiveness of mastoid bone marrow and adipose tissue-derived stem cells, which are acquired through mastoidectomy, and to evaluate their effects on bone formation in combination with scaffold to regenerate temporal bone defects.
The human mastoid bone marrow and adipose tissues showed stem cell phenotypes, and these characteristics were maintained up to passage 5. Mastoid bulla and cranial bone defects were induced in Sprague-Dawley rats and the rats were divided into five groups: (1) control, (2) human ear adipose-derived stromal cells (hEASCs), (3) hEASCs + osteogenic differentiation medium (ODM), (4) hEASCs + polycaprolactone (PCL) scaffolds, and (5) hEASCs + PCL scaffolds + ODM. Osteogenesis was evaluated by micro-computed tomography and histology. Compared with the control group, the groups transplanted with hEASCs and PCL scaffolds had significantly higher bone formation along the periphery of the mastoid bulla area. Moreover, ODM synergistically enhanced bone formation in mastoid bulla defects. Our results suggest that combining hEASCs with PCL scaffolds represents a promising method for anatomical and functional reconstruction of postoperative temporal bone defects following mastoidectomy
Key word: Osteogenesis, Postoperative temporal bone, Mastoidectomy, Mastoid bone marrow, Adipose tissue
iii
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· v
LIST OF TABLES ··· vii
Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 5
1. Cell isolation and culture ··· 5
2. Fluorescent activated cell sorting analysis ··· 8
3. Multi-lineage differentiation of cells ··· 8
4. Cytochemistry ··· 9
5. Immunofluorescence ··· 9
6. Western blot ··· 10
7. Histological analysis ··· 10
8. Animal experimental design ··· 11
9. PCL 3D scaffold ··· 12
10. Surgery ··· 14
11. Micro-CT ··· 14
iv
Ⅲ. RESULTS ··· 16
1. Clinical profiles of the patients ··· 16
2. Characterization of mastoid bone marrow-derived cells ··· 18
3. Maintenance of stem cell phenotype in mastoid bone marrow-derived cells · 20 4. Differentiation of mastoid-derived cells into the chondrogenic, osteogenic, adipogenic, neurogenic and epithelial Lineages ··· 22
5. Characterization of hEASCs ··· 26
6. Bone formation in rat mastoid bulla and cranial bone defects ··· 28
7. ODM synergistically enhances cell/scaffold-based bone formation ··· 31
Ⅳ. DISCUSSION ··· 34
Ⅴ. CONCLUSION ··· 39
REFERENCES ··· 40
v
LIST OF FIGURES
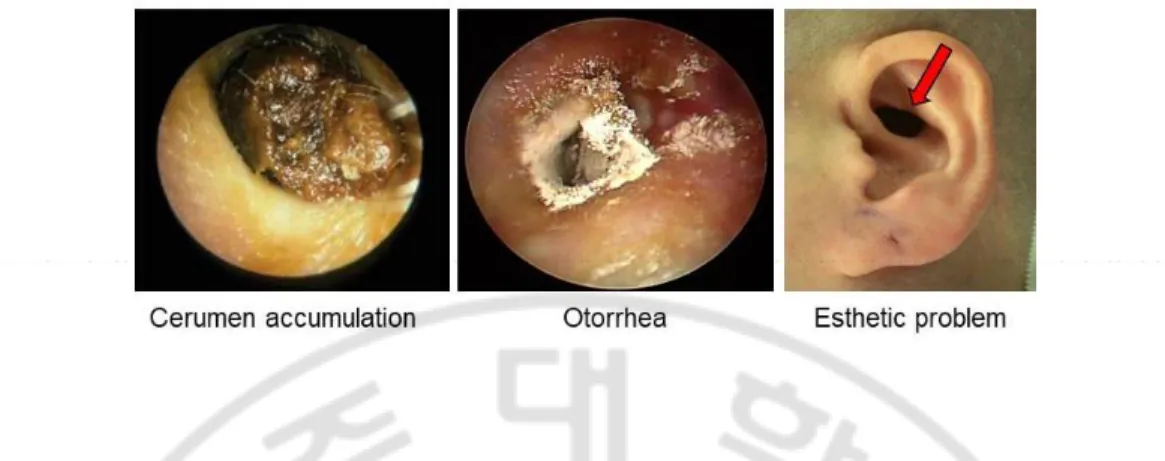
Fig. 1. Various complications after mastoidectomy such as accumulation of cerumen and keratin debris, otorrhea, or esthetic problems ··· 4
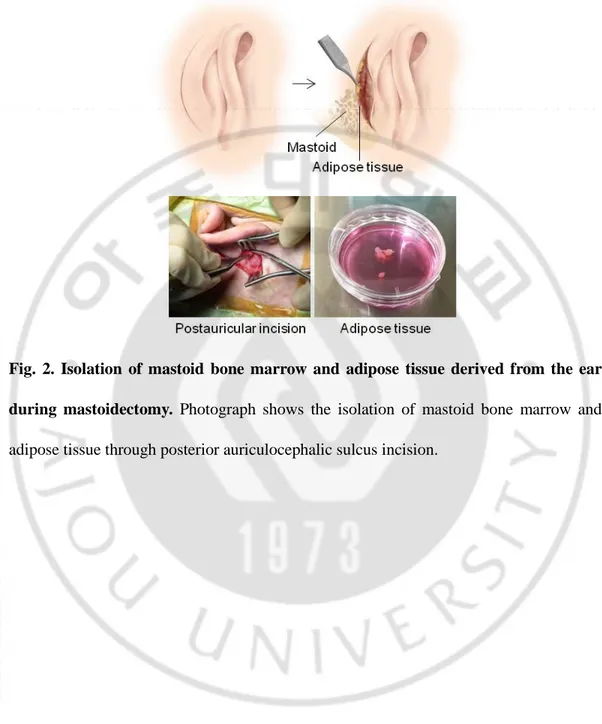
Fig. 2. Isolation of mastoid bone marrow and adipose tissue derived from the ear during mastoidectomy ··· 7
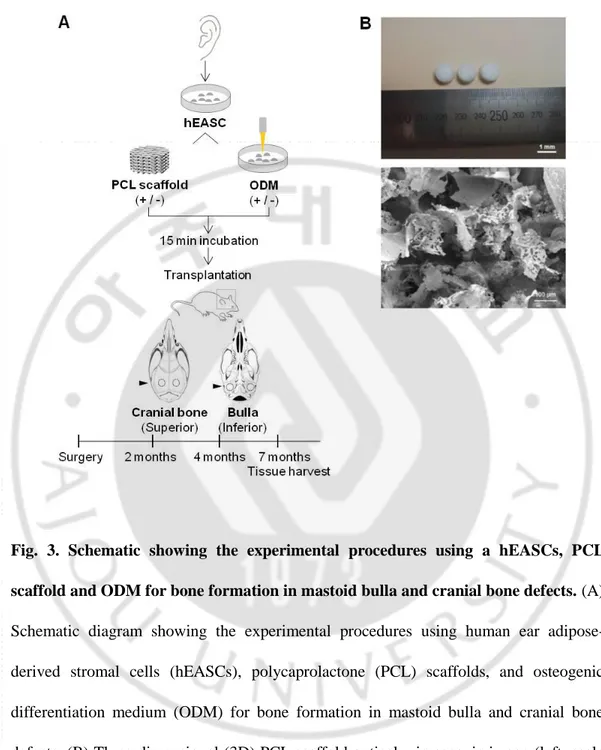
Fig. 3. Schematic showing the experimental procedures using a hEASCs, PCL scaffold and ODM for bone formation in mastoid bulla and cranial bone defects ··· 13
Fig. 4. Characteristics of hMBMCs ··· 19
Fig. 5. Maintenance analysis of the hMBMC stemness at passages 2, 4, 6, 8, 10, and 12 ··· 21 Fig. 6. Multiple differentiation potential of hMBMCs ··· 24
Fig. 7. Characteristics and maintenance analysis of the stemness surface markers and differentiation capacities of hEASCs ··· 27
vi
Fig. 9. Evaluation of in vivo osteogenic effects of hEASCs, PCL scaffold and ODM · 30
Fig. 10. Micro-CT and 3D reconstruction images of tympanic bulla bone formation · 32
vii
LIST OF TABLES
1
I. INTRODUCTION
Mastoidectomy is a surgical procedure performed to remove infected mastoid bone and air cells resulting from ear infections such as otitis media and mastoiditis. This surgical procedure is considered essential for the treatment of chronic otitis media, although it creates temporal bone defects that can cause various complications such as dizziness, accumulation of keratin debris, otorrhea, or recurrent lesions (Fig. 1) (Brzoska M et al., 2005). To compensate for these disadvantages of open cavity mastoidectomy, reconstruction of the posterior wall of the external auditory canal and mastoid obliteration have been attempted (Lee HB et al., 2013). Autologous tissues (such as interior-based flaps or bone pate, cartilage, and adipose tissue), alloplastic materials, and biosynthetic materials have all been used in mastoid obliteration (Lee HB et al., 2013; Matsuno T et al., 2006). Autologous tissues are much safer and more cost-effective than alloplastic or biosynthetic materials. However, they also have limitations and are not applicable in every situation because of insufficient amounts of autologous tissues, donor site defects, and reabsorption after surgery.
The mastoid is the part of the temporal bone that is located behind the ear. In the case of patients with chronic otitis media or people who need cochlear implant surgery, the mastoid bone and adipose tissue must be removed from the ear. During the mastoidectomy process, the bone marrow and adipose tissues were obtained. Unlike several other biocompatible materials, mastoid bone marrow and adipose tissue were a source of mesenchymal stem cells (MSCs), which possess the capacity to differentiate
2
into multiple lineages. Local application of adult stem cells is a promising therapeutic approach for tissue regeneration and repair (Chang J et al., 2013), especially for mastoid bone defects. Moreover, sourcing mastoid bone marrow and adipose tissue for mastoid reconstruction is a minimally invasive procedure, as these behind the ear must be removed for mastoidectomy. Therefore, mastoid bone marrow and adipose tissue could serve as a valuable source of MSCs without additional operations.
Three-dimensional (3D) scaffolds serve as structural and physical supports for cell attachment, growth, regeneration, functional maintenance, and subsequent new tissue development (Hosseinkhani M et al., 2014). Since most bone defects are irregular in shape, the essential characteristics of scaffolds include the feasibility of volume control for irregular bone geometry, adequate mechanical strength, and a porous structure that can transport oxygen and nutrients to assist in new bone formation. Mastoid bone is normally characterized with air cells for gas exchange and also remains empty space after mastoidectomy. On the other hand, cranial bone has two layers of compact bone with a layer of sponge bone between them. Therefore, mastoid bone requires different bone regeneration strategy from compact bones including cranial bones. Naturally, the scaffold may have to be different depending on the kinds of target bones.Artificial scaffolds must also be biodegradable, allowing extracellular matrix to occupy the empty space when the biomaterial is degraded (Jones JR et al., 2009). Polycaprolactone (PCL) is one of the most widely used U.S. Food and Drug Administration (FDA)-approved synthetic polymers, as it is highly biocompatible and biodegradable and non-toxic (Jensen J et al., 2015).
3
Recently, researchers in the field of tissue engineering have adopted the use of soluble growth factors to promote lineage-specific differentiation of MSCs. A wide range of growth factors, such as bone morphogenetic protein (BMP), transforming growth factor beta, insulin-like growth factor, platelet-derived growth factor, and basic fibroblast growth factor have been applied in bone tissue engineering (Nauth A et al., 2010). BMP-2 and platelet-rich plasma (PRP) containing a variety of growth factors are reported to enhance the reconstruction of temporal dorsal bulla bone (Jang CH et al., 2016; Kim SE et al., 2013) However, which growth factors promote mastoid bone formation most efficiently is not yet known. To the best of our knowledge, studies regarding tissue engineering techniques for bone formation in temporal bone defects and mastoid bulla are severely lacking.
The purpose of this study was to investigate the biological properties of human ear mastoid bone marrow cells (hMBMCs) and adipose-derived stromal cells (hEASCs) and to evaluate their effects on bone formation in combination with PCL scaffolds and osteogenic differentiation medium (ODM) to regenerate temporal bone defects.
4
Fig. 1. Various complications after mastoidectomy such as accumulation of cerumen and keratin debris, otorrhea, or esthetic problems
5
II. MATERIALS AND METHODS
1. Cell isolation and culture a. Mastoid bone marrow
Mastoid bone marrow was isolated from patients undergoing mastoidectomy. The study was approved by the Institutional Review Board of the Ajou University School of Medicine (AJIRB-MED-SMP-11-003). Ten patients who underwent mastoidectomies at the Department of Otolaryngology, Ajou University Hospital, Suwon, Republic of Korea, were enrolled in the study. During mastoidectomy, around 1 cc of bone marrow tissues was obtained with a curette from areas without any infection of inflammation and immediately transferred into microtubes filled with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco-BRL, Grand Island, NY, USA) in the operating room, and was delivered promptly to our laboratory.
Bone marrow was washed with phosphate buffer solution (PBS) containing penicillin and streptomycin, digested with 100 U/mL collagenase type II (GIBCO BRL, Burlington, Ontario, Canada) with low-glucose DMEM, and incubated for 2 h at 37°C. The stromal fraction was collected by centrifugation at 1200 rpm for 5 min and then passed through a 100-μm cell strainer (BD Falcon, Erembodegem, Belgium) to remove large cell clumps and particles. For cell culture and expansion of hMBMC, the cells were incubated in low-glucose DMEM supplemented with 10% fetal bovine serum (Gibco, Milan, Italy) and 1% penicillin (GenDEPOT, Barker, TX, USA) at 37°C under a 5% CO2 atmosphere. After 24 h, non-adherent cells were removed and the medium was changed.
6
When the cells were 50% confluent, they were harvested by trypsinization and subcultured in a fresh medium.
b. Adipose tissue
Adipose tissue was isolated from patients undergoing mastoidectomy. This study was approved by the institutional review board of the Ajou University School of Medicine (AJIRB-MED-SMP-11–002). Twenty patients who underwent mastoidectomies at the Department of Otolaryngology, Ajou University Hospital, Suwon, Republic of Korea, were enrolled in the study. Subcutaneous adipose tissue was removed after postauricular incision and immediately transferred to microtubes filled with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco-BRL, Grand Island, NY) in the operating room and promptly delivered to our laboratory (Fig. 2). Harvested human adipose tissue was washed with phosphate-buffered saline (PBS) containing penicillin and streptomycin and then digested with 100 U/mL collagenase type I (Gibco BRL, Burlington, ON, Canada) for 2 h at 37°C. Cells were collected by centrifugation for 5 min at 3000 rpm, and the supernatant was discarded. The pellet was resuspended and then passed through a 100-μm cell strainer (BD Falcon, Erembodegem, Belgium) to remove large cell clumps and particles. For culture and expansion of hEASCs, the cells were incubated in low-glucose DMEM supplemented with 10% fetal bovine serum (Gibco, Milan, Italy) and 1% penicillin and streptomycin (GenDEPOT, Barker, TX) at 37°C under a 5% CO2 atmosphere. After 24 h, non-adherent cells were removed, and the medium was changed. Upon reaching 50% confluency, the cells were harvested by trypsinization and subcultured in fresh medium.
7
Fig. 2. Isolation of mastoid bone marrow and adipose tissue derived from the ear during mastoidectomy. Photograph shows the isolation of mastoid bone marrow and adipose tissue through posterior auriculocephalic sulcus incision.
8
2. Fluorescent activated cell sorting analysis
The cells were characterized based on their expression of stem cell-specific markers. The cells (1 x 106/mL) were incubated with fluorescein isothiocyanate (FITC)-conjugated CD45, CD29, CD90, and CD105 (BioLegend, San Diego, CA) for 1 h at 4°C. Cells were analyzed by flow cytometry using BD FACSAriaTM III (BD Biosciences, San Jose, CA). Cells that were not incubated with primary antibodies were used as the negative staining control. To evaluate the maintenance of stemness, the MSCs were analyzed by FACS during each passage.
3. Multi-lineage differentiation of cells
Early-passage cells (passage < 3) were plated at an appropriate density on a culture dish, depending on the differentiation lineage. Five procedures were tested: (1) chondrogenesis [5 x 104 cells/cm2; Chondrogenesis Differentiation Kit, GIBCO, San Diego, CA, USA], (2) osteogenesis [1 x 105 cells/cm2; DMEM supplemented with 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA) and 50 μg/mL ascorbate-2 phosphate (Sigma-Aldrich)], (3) adipogenesis [5 x 105 /cm2; Chondrogenesis Differentiation Kit, GIBCO)], (4) neurogenesis [5 x 103/cm2; neurogenic differentiation medium (Promocell, Heidelberg, Germany)], and (5) epithelial genesis [5 × 104/cm2; DMEM media supplemented with 5 μM all trans-retinoic acid (ATRA, Sigma-Aldrich)]. All the differentiation media were replaced every 3–4 days for 14–22 days except the media for neurogenesis which were changed every day for 8 days.
9
4. Cytochemistry
Chondrocytes were indicated with Alcian blue staining (pH 2.5; Sigma-Aldrich). The cells were fixed with 4% paraformaldehyde for 20 min and then incubated with 3% acetic acid at pH 2.5 for 3 min and incubated in Alcian blue solution at pH 2.5 for 30 min. Osteocytes were observed by mineralization of the extracellular matrix and calcium deposits. The cells were fixed with 80% cold ethanol for 1 h at 4°C. After the cells were rinsed with distilled water (DW), they were incubated with 2% Alizarin Red S solution (Sigma-Aldrich) at pH 5.5 for 10 min. Adipocytes were confirmed by production of lipid droplets using Oil Red O (Sigma-Aldrich). The cells were fixed with 4% paraformaldehyde for 20 min, overlaid with Oil Red O solution for 6 min, and then washed with 85% propylene glycol and
DW. The samples were stored in PBS at 4°C.
5. Immunofluorescence
Cells were grown on a glass coverslip (Marienfeld, Lauda-Koenigshofen, Germany). The cells were fixed in 4% paraformaldehyde for 20 min and permeabilized using 0.2% Triton-X 100 in PBS for 10 min. After the cells were washed, they were blocked with 1% bovine serum albumin (GenDEPOT, Barker, TX, USA) in PBS and incubated with rabbit anti-Nestin (Abcam, Cambridge, MA, USA), rabbit antineuronal-specific nuclear protein (NeuN; Millipore, Billerica, MA, USA). Secondary anti-rabbit IgG-FITC or Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were applied for 1 h at room temperature. The nuclei were stained with 1 μg/mL
4’,6’-10
diamidino-2-phenylindole (Invitrogen, Molecular Probes, Carlsbad, CA, USA) in PBS for 2 min at room temperature. Immunofluorescent cells were imaged using an AxioVision LE 4.5 microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA).
6. Western blot
Proteins were loaded in each lane of a sodium dodecyl sulfate polyacrylamide gel electrophoresis. Primary antibodies against cytokeratin 18 (Abcam, Pic, Cambridge, UK), and β-actin (Cell Signaling Technology, Danvers, MA, USA) were used. The immunoblots
were washed and incubated with secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoblot bands were visualized by enhanced chemiluminescence (GenDEPOT, TX, USA).
7. Histological analysis
The mastoid bulla and cranial bone specimens were fixed in 4% paraformaldehyde for 24 h and then decalcified in a decalcifying solution (Calci-Clear Rapid; National Diagnostics, Atlanta, GA) for 5 days. The tissues were dehydrated,
embedded in paraffin, and sectioned at 10 μm, and the sections were stained with
hematoxylin (Youngdong Pharmaceutical Co., Seoul, Korea) and eosin (Muto Pure Chemical Co., Tokyo, Japan) (H & E) and immunohistochemically stained for osteopontin (OPN; Abcam, Cambridge, MA), a marker of mature osteoblasts. For H & E staining, the slides were deparaffinized through two xylene incubations and rehydrated
11
through graded washes of ethanol in water. Then, the samples were washed in tap water and stained with hematoxylin and eosin for 3 and 5 min, respectively. For visualization, the slides were mounted with mounting medium (Thermo Fisher Scientific, Miami, FL). To identify OPN expression, indirect immunohistochemistry was performed using antibodies against OPN. Nonspecific binding was blocked with 1% BSA and 0.2% Triton
X-100 in PBS. Sections were incubated overnight at 4°C with the OPN antibody.
Following a wash in PBS, sections were incubated with a biotinylated secondary antibody (Vector Laboratories Inc., Burlingame, CA) for 1 h and visualized using a 3, 30 diaminobenzidine substrate kit (Vector Laboratories Inc., Burlingame, CA). Nuclei were counterstained with hematoxylin. Bright-field microscopy images were obtained using Picture Frame software (Olympus Optical, BX51, Tokyo, Japan)
8. Animal experimental design
All animal procedures were approved by the Institutional Animal Care and Use
Committee of Ajou University School of Medicine. Thirty-nine male Sprague-Dawley
rats (age 8 weeks) were randomly divided into five groups: (1) control group, (2) hEASCs (S group), (3) hEASCs +PCL scaffolds (SP group), (4) hEASCs + ODM (SM group), and (5) hEASCs + PCL scaffolds + ODM (SPM group). The control group was not treated with any reagents or materials. Bone regeneration was monitored by micro-computed tomography (micro-CT) at 2, 4, and 7 months after transplantation. At 7
months post-transplantation, the animals were sacrificed, and cranial bone and tympanic
12
9. PCL 3D scaffolds
To fabricate porous 3D PCL scaffolds, PCL (MW = 80,000, Sigma-Aldrich, Steinheim, Germany) was first dissolved in chloroform (Sigma-Aldrich, Steinheim, Germany). The 5% (w/v) PCL solution was mixed with salt particles (sodium chloride, Amresco, Solon, OH, USA) as a porogen in the range of approximately 100−200 μm. The mixed solution was poured into a Teflon mold to form the 3D structure. The scaffolds were leached in a distilled water bath for 48 h to eliminate salt particles to form porous 3D structures (Fig. 3B). Finally, the scaffolds were freeze-dried for 72 h. Scaffolds were sterilized by soaking in 70% ethyl alcohol, then washed in PBS. The fabricated PCL scaffolds with 100−200 µm pore sizes were individually placed into 96-well culture plates and incubated for 15 min with hEASCs (5 × 106 cells/20 μL).
13
Fig. 3. Schematic showing the experimental procedures using a hEASCs, PCL scaffold and ODM for bone formation in mastoid bulla and cranial bone defects. (A) Schematic diagram showing the experimental procedures using human ear adipose-derived stromal cells (hEASCs), polycaprolactone (PCL) scaffolds, and osteogenic differentiation medium (ODM) for bone formation in mastoid bulla and cranial bone defects. (B) Three-dimensional (3D) PCL scaffold optical microscopic image (left, scale bar: 1 mm) and SEM image (right, scale bar: 100 μm).
14
10. Surgery
The Sprague-Dawley rats were anesthetized by intraperitoneal injection with Zoletil 50 (0.1 cc/100 g; Virbac Laboratoire) and Rompun 2% (0.02 cc/100 g; Bayer Korea). For the mastoid bulla defects, the anterior midline neck skin was incised using a scalpel to expose both sides of the bulla (anterior approach), and 3 × 3-mm holes were made in the bulla using a drill. Then all mucosae inside the bulla were cauterized with 10% trichloroacetic acid. hEASCs (5 × 106) were transplanted directly into the mastoid bulla in the control group (without ODM) and SM group (with ODM), and hEASC-seeded 3D PCL scaffolds were placed in the bulla cavity in the SP group (without ODM) and SPM group (with ODM). The incisions were closed layer-by-layer. For cranial bone defects, 5 × 5-mm holes were made on the left and right sides of the cranial bone using a trephine bur. The hEASCs, PCL scaffolds (5 × 5 mm), and ODM were transplanted into the defect following the same design as in the bulla defect study.
11. Micro-CT
Radiological examination was performed using Skyscan 1076 (Skyscan, Konitch, Belgium) micro-CT, at a resolution of 36.44 pixels and exposure time of 300 ms, with an energy source of 35 kV and current of 170 mA. Each bulla and cranial bone, consisting of 622 slices on average, were scanned. The CT images were processed using MIMICS 16.0 3D imaging software (Materialise’s Interactive Medical Image Control System, Leuven, Belgium). Newly formed bone was identified by assigning a threshold for total bone mineral content. For quantitative comparisons among groups, we
15
calculated bone volume (BV, mm3) and BV/total volume (BV/TV, %) from the micro-CT images.
12. Statistical analysis
Statistical comparisons between two groups were calculated by the Mann-Whitney U test and comparisons among three groups by the Kruskal-Wallis test using SPSS software version 12.0 (SPSS, Inc., Chicago, IL, USA). P value of P < 0.05 was considered significant.
16
III. RESULTS
1. Clinical profiles of the patients
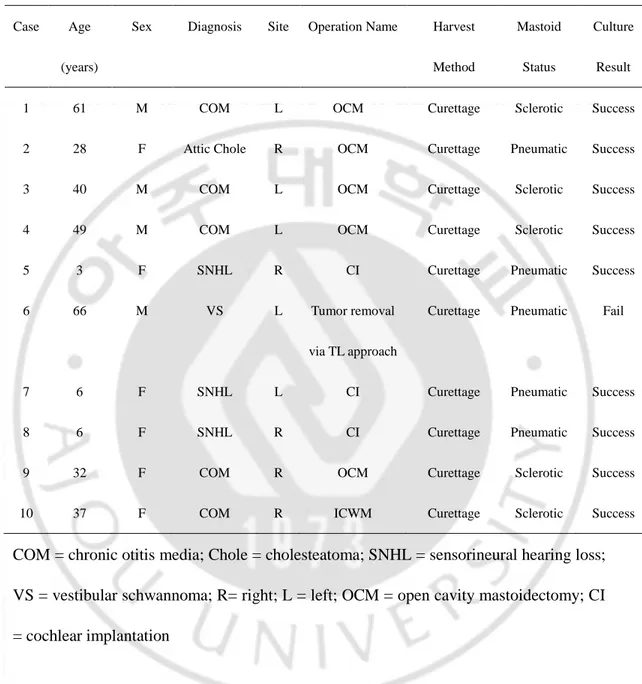
The characteristics of the patients are summarized in Table 1. The average age of the patients was 32.8 ± 22.6 years (range; 3 - 66 years). Their diagnoses were chronic otitis media (n = 5), cholesteatoma (n = 1), sensorineural hearing loss (n = 3) requiring cochlear implantation, or vestibular schwannoma needing surgery via the translabyrinthine approach. The degree of mastoid pneumatization was divided into 3 types (pneumatic, sclerotic, and diploic) according to the different amounts of pneumatized air cells. Five (50%) patients showed the pneumatized type, while the others (50%) revealed the sclerotic variant. Culture was unsuccessful only for 1 patient (10%) with a pneumatized mastoid bone.
17
Table 1. Clinical characteristics of patients.
Case Age
(years)
Sex Diagnosis Site Operation Name Harvest
Method
Mastoid
Status
Culture
Result
1 61 M COM L OCM Curettage Sclerotic Success
2 28 F Attic Chole R OCM Curettage Pneumatic Success
3 40 M COM L OCM Curettage Sclerotic Success
4 49 M COM L OCM Curettage Sclerotic Success
5 3 F SNHL R CI Curettage Pneumatic Success
6 66 M VS L Tumor removal
via TL approach
Curettage Pneumatic Fail
7 6 F SNHL L CI Curettage Pneumatic Success
8 6 F SNHL R CI Curettage Pneumatic Success
9 32 F COM R OCM Curettage Sclerotic Success
10 37 F COM R ICWM Curettage Sclerotic Success
COM = chronic otitis media; Chole = cholesteatoma; SNHL = sensorineural hearing loss; VS = vestibular schwannoma; R= right; L = left; OCM = open cavity mastoidectomy; CI = cochlear implantation
18
2. Characterization of mastoid bone marrow-derived cells
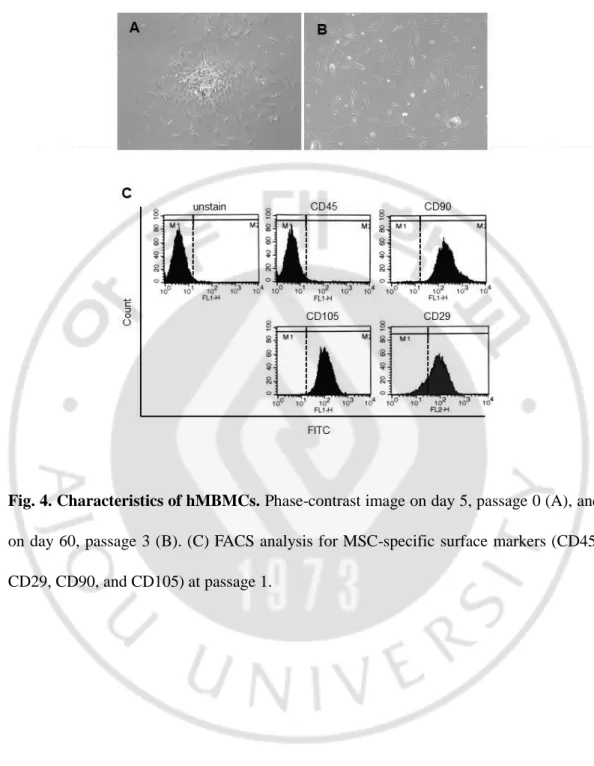
During passage 0, hMBMCs showed characteristic neurosphere-like clusters of cells that changed into a much more stretched form resembling fibroblasts during subsequent passaging over 60 days (passage 3) (Fig. 4). The characteristics of the isolated cells were first evaluated by flow cytometry using CD markers to identify potential stem cells. CD105 (endoglin) is a member of the transforming growth factor beta family, which has been linked with multi-lineage differentiation capacity (Alev C et al., 2010), while CD29 (integrin beta 1) is responsible for mediating cell-to-cell adhesion and is involved in cell growth, differentiation, migration, and death (Langan RC et al., 2012). CD90 (Thy-1) is expressed in colony forming cells with high proliferative potential and is also used to characterize stem cell populations. In contrast, CD45 is expressed in hematopoietic stem cells (Kucia M et al., 2005).In our study, as shown in Fig 4B, populations of cells yielded positive results for CD90, CD105, and CD29 (99.52%, 99.88%, and 85.49%, respectively) and negative results for CD45 (0.97%) at passage 1. These data indicated that adherent cells derived from mastoid bones display an MSC phenotype.
19
Fig. 4. Characteristics of hMBMCs. Phase-contrast image on day 5, passage 0 (A), and on day 60, passage 3 (B). (C) FACS analysis for MSC-specific surface markers (CD45, CD29, CD90, and CD105) at passage 1.
20
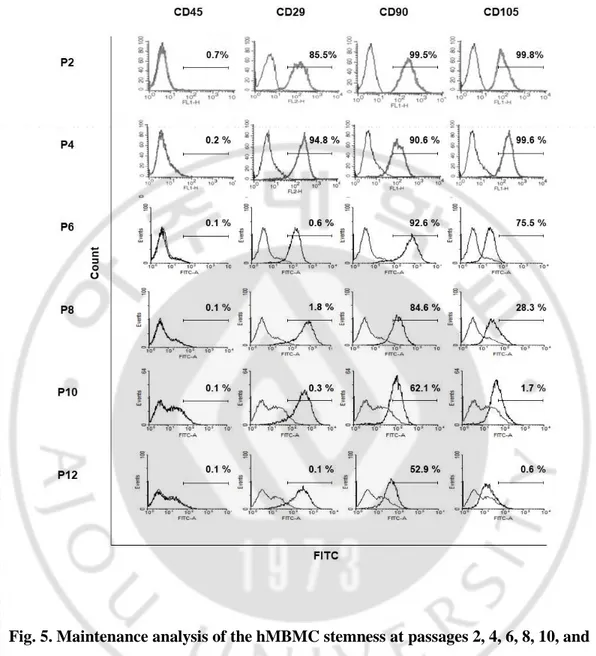
3. Maintenance of stem cell phenotype in mastoid bone marrow-derived cells Expansion and maintenance of stem cell phenotype in vitro are required for clinical autologous cell transplantation. However, the efficiency or changes in hMBMCs during prolonged culture have not yet been studied well. The multipotent ability of MSCs gradually decreases depending on the passage number (Kucia M et al., 2005). To evaluate the stemness of the hMBMCs, we cultured hMBMCs during passage 12 and assessed the levels of MSC immune-phenotypes by using flow cytometry. Interestingly, hMBMCs stably maintained their stem cell phenotype up to passage 4 (Fig. 5). However, after passage 4, we observed a gradual decrease in the expression of CD90, CD105, and CD29 (Fig. 5).
21
Fig. 5. Maintenance analysis of the hMBMC stemness at passages 2, 4, 6, 8, 10, and 12. To FITC:fluorescein isothiocyanate.
22
4. Differentiation of mastoid-derived cells into the chondrogenic, osteogenic, adipogenic, neurogenic and epithelial Lineages
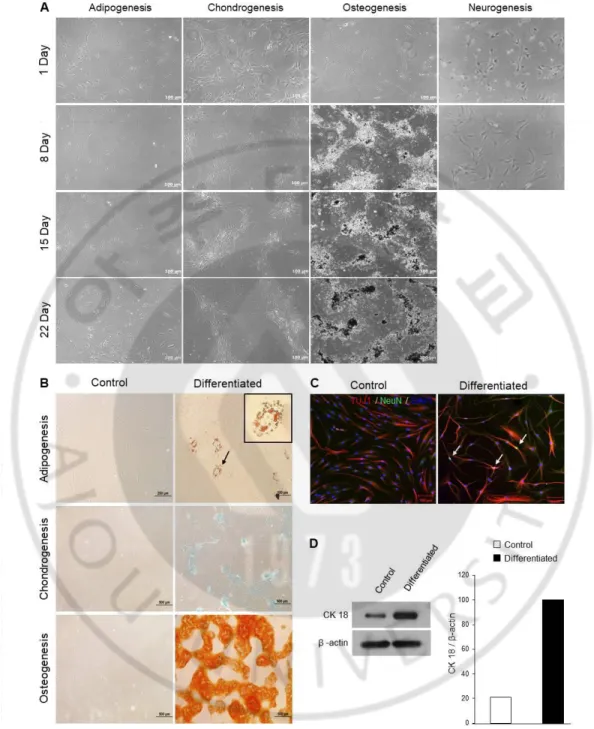
To induce hMBMCs differentiation, we used a culture medium containing specific supplements. On incubation with an adipogenic induction medium for 22 days, the morphologic features of the hMBMCs were changed from typical fibroblast-shaped cells to polygonal cells containing droplets in the cytoplasm (Fig. 6A). To observe the lipid-droplet accumulation more clearly, hMBMCs were stained with Oil Red O. Oil Red O-positive lipid droplets were found in all the cells. In contrast, Oil Red O-positive cells were not detected in the control hMBMCs (Fig. 6B). Under the chondrogenic condition, hMBMCs acquired a cuboidal morphology (Fig. 6A). Alcian blue (pH 2.5) was used to demonstrate the presence of acidic mucopolysaccharides and proteoglycans, which are useful markers of mature chondrocytes (Mitchell JB et al., 2006). With commercial chondrogenic differentiation media, high efficiency was noted for bluish staining (acidic mucosubstances), but no staining was observed in the control hMBMCs (Fig. 6B). Next, we observed robust growth and cluster formations after osteogenic induction. Alizarin Red S staining showed large amounts of calcium deposits in hMBMCs, but not in the control cells (Fig. 6B). In the case of neuronal differentiation, we found that hMBMCs stopped proliferation and entered the differentiated state after neuronal induction as opposed to the results seen for the control hMBMCs. Moreover, the cells exhibited a contracted cytoplasm and spherical cell bodies. Neuronal differentiation was analyzed using an immature neuronal marker, TUJ1 (beta-tubulin 3), and a mature neuronal marker, NeuN. As shown in Fig. 6C, the TUJ1 and NeuN markers were expressed in a small
23
percentage of cells. Finally, we tried to induce epithelial cell differentiation in the presence of retinoic acid. Retinoic acid has been reported to induce differentiation toward the epithelial lineage (Palva T et al., 1979). After 22 days, in the presence of a retinoic acid-containing medium, the hMBMCs exhibited a change in morphological features. Western blot analysis was performed to quantify the epithelial cell marker, cytokeratin 18. As shown in Fig 6D, the cytokeratin 18 level significantly increased in the retinoic acid-containing medium.
24
Fig. 6. Multiple differentiation potential of hMBMCs. (A) Phase-contrast images obtained on days 1, 8, 15, and 22 after differentiation induction. (B) Specific phenotype images with Oil red O (for the adipogenic lineage, upper), Alcian blue (for the
25
chondrogenic lineage, middle) and Alizarin Red S (for the osteogenic lineage, lower) staining. (C) Immunocytochemical co-staining with TUJ1 (red) and NeuN (green, arrows) after 8 days of neuronal induction. The picture on the left side shows the undifferentiated hMBMCs. (D) Western blot analysis of cytokeratin 18 after epithelial cell induction. β-actin was used as a loading control.
26
5. Characterization of hEASCs
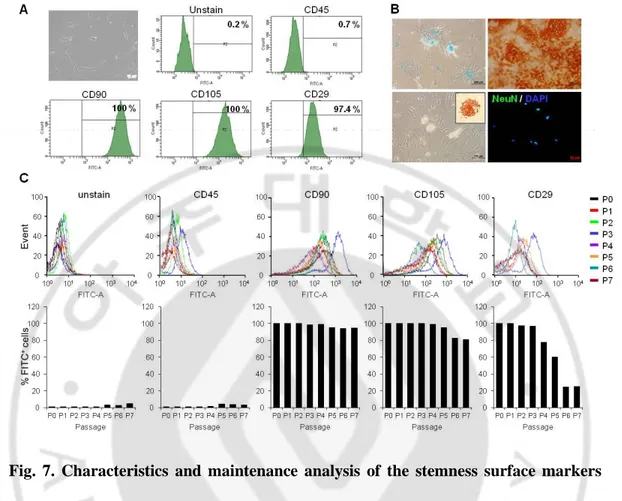
The hEASCs isolated from patients who underwent mastoidectomy were characterized by flow cytometry for several cell surface markers, including CD45 for hematopoietic cells and CD90, CD105, and CD29 for MSCs. We found that hEASCs expressed CD90, CD105, and CD29 (100%, 100%, and 97.4%, respectively) and were negative for CD45 (0.7%) at passage 0 (Fig. 7A). In vitro differentiation assays indicated that the hEASCs induced chondrogenic, osteogenic, adipogenic, and neurogenic differentiation as shown by alcian blue staining, alizarin red S staining, oil red O staining, and NeuN immunofluorescence, respectively (Fig. 7B). hEASCs isolated from behind the ear thus show potential as a source of cells for bone reconstruction. Studies have shown that the potential and maintenance of stem cells may change depending on their origin over a progression of cell passages. However, not all researchers have used the same isolation and passage conditions for adult stem cells. Therefore, we evaluated the stemness of hEASCs. The hEASCs stably maintained their stem cell phenotype up to passage 4. However, after passage 5, we observed a gradual decrease in the expression of CD105 and CD29 (Fig. 7C). Because serial passaging of stem cells influences their phenotype, stem cells must be cultured with care.
27
Fig. 7. Characteristics and maintenance analysis of the stemness surface markers and differentiation capacities of hEASCs. (A) Representative fluorescence-activated cell sorting (FACS) analysis showed positive for mesenchymal stem cell (MSC) specific surface markers (CD45, CD90, CD105 and CD29) at passage 1. (B) Multipotent differentiation potential of hEASCs. Specific treatments induced hEASCs to undergo chondrogenic (Alcian blue staining), osteogenic (Alizarin red S staining), adipogenic (oil red O staining), and neurogenic (immunocytochemical staining with NeuN, FITC) differentiation. An increased numbers of differentiated cells were observed in the hEASC population. (C) Maintenance analysis of hEASC stemness at passages 0‒7. The hEASCs consistently maintained their stem cell phenotype up to passage 4. After passage 5, gradual decreases of CD105 and CD29 were observed. FITC: fluorescein isothiocyanate.
28
6. Bone formation in rat mastoid bulla and cranial bone defects
On CT images taken 2 and 4 months after surgery, the SP (hEASCs + PCL) and SPM groups (hEASCs + PCL + ODM) showed significant new bone formation (Fig. 8A). In contrast, the groups without PCL scaffolds (control, S, and SM groups) did not show any regenerated bone. Histological staining and OPN immunohistochemistry also showed new bone formation at the periphery of the bulla defects in the groups transplanted with PCL scaffolds (Fig. 8B, C). In addition, the cranial bone defects of the SM (hEASCs + ODM) and SP groups (hEASCs + PCL) showed new bone formation, but there were no observable effects of ODM (Fig. 9). These results indicate that the PCL scaffold was essential for new bone formation in the mastoid bulla defects, but not in the cranial bone defects.
29
Fig. 8. In vivo osteogenic effects of hEASCs, PCL scaffold and ODM. Coronal plane computed tomography (CT) images (A), H&E staining (B), and immunohistochemical analysis of osteopontin (OPN; C) of the bulla cavity at 2 and 4 months after mastoid bulla obliteration in the animal study. The hEASCs + PCL and hEASC + PCL + ODM groups showed significant bone formation compared with other experimental groups. The yellow and black arrows indicate rat mastoid bulla defects. Scale bars: 2 mm.
30
Fig. 9. Evaluation of in vivo osteogenic effects of hEASCs, PCL scaffold and ODM. Coronal plane computed tomography (CT) images (A), H&E staining (1.25X (B), 20X (C) of the cranial bone at 2 and 4 months after bone defects in the animal study. Scale bars: 2 mm.
31
7. ODM synergistically enhances cell/scaffold-based bone formation
For quantitative evaluation of bone mineral density and the effects of ODM on the SP group (hEASCs + PCL), we performed micro-CT and 3D-image conversion using the MIMICS 16.0 software on new bone defects in vivo, including the control, SP (hEASCs + PCL), and SPM (hEASCs + PCL + ODM) groups. As shown in the 3D images (Fig. 10C), bone formation in the SP and SPM groups occurred along the periphery of the bulla defects and grew into the scaffolds (Fig. 10A, C). In the control group, there was no evidence of new bone formation at the defect. After 7 months, the BV was 25.8 ± 1.0 mm3 in the control, 34.2 ± 4.7 mm3 in the SP group, and 43.3 ± 2.5 mm3 in the SPM group. The BV/TV was 18.0 ± 1.8% in the control, 23.2 ± 2.6% in the SP group, and 27.8 ± 4.2% in the SPM group (Fig. 10D). There were significant differences among the three groups (P < 0.05). This suggests that the hEASCs and PCL scaffold have potential for osteogenesis, and ODM may greatly enhance osteogenic induction.
The cranial bone also showed increased BV in the SP and SPM groups compared with the control group. However, ODM did not induce any significant differences (Fig. 11).
32
Fig. 10. Micro-CT and 3D reconstruction images of tympanic bulla bone formation. (A) Micro-CT analysis of tympanic bulla in the control, hEASCs + PCL, and hEASCs + PCL + ODM groups at 5 and 7 months. (B) Representative micro-CT 3D reconstructed images of the bulla cavity bone. Quantitative morphometric analysis of bone volume (C) and bone volume/total volume ratio (D) in the bone defect as assessed by micro-CT. Area within the yellow box in the 3D image (above) is viewed at a higher magnification (below). The yellow and black arrows indicate rat mastoid bulla defects.
33
Fig. 11. Micro-CT and 3D reconstruction images of cranial bone formation. (A) Micro-CT analysis of cranial bone in the control, hEASCs + PCL, and hEASCs + PCL + ODM groups at 5 and 7 months. (B) Representative micro-CT 3D reconstructed images of cranial bone. Quantitative morphometric analysis of bone volume (C) and bone volume/total volume ratio (D) in the bone defect as assessed by micro-CT. The yellow and black arrows indicate rat cranial bone defects.
34
IV. DISCUSSION
After open cavity mastoidectomy for treatment of chronic otitis media, several complications including recurrent otorrhea, poor hearing aid fit, and the presence of keratin debris may persist. These complications have been reported to occur in approximately 10% of cases, even under the most expert surgical treatment (Palva T et al., 1979). To minimize complications, various autologous tissues and alloplastic materials are frequently used to fill in bone defects. Of these, the musculoperiosteal flap is used most commonly, because it can be manipulated easily and is resistant to infection (Lee HB et al., 2013). However, it is often resorbed, resulting in a large cavity. Other autologous tissues such as cartilage or bone pate are also insufficient to compensate for bone defects (Estrem SA et al., 1999).
In recent years, there has been an increasing interest in stem cell-based tissue engineering (Kagami H et al., 2014), particularly the use of adult MSCs for regenerative medicine, because of the simplicity of isolation and ex vivo expansion of these cells (Nasaf A et al., 2007). Sources of MSCs include bone marrow, adipose tissue, umbilical cord, and amniotic fluid. hMBMCs and hEASCs in particular are easily harvested from the posterior auriculocephalic sulcus during mastoidectomy, without any additional invasive procedures. Therefore, we chose ear-derived mastoid bone marrow and adipose stromal cells as a new source of cells for mastoid bone reconstruction. We first evaluated the regenerative potential of hMBMCs and hEASCs in vitro and subsequently mastoid bone reconstruction in vivo.
35
In this study, MSCs could be obtained from most of the patients (9/10, 90% success rate). Culture was unsuccessful only for 1 patient with a pneumatic mastoid bone. We think that MSCs could be obtained from most mastoid bones even with the sclerotic type as seen in this study. According to their diagnosis, 6 patients had chronic infection or inflammation and it can be another concern. However, we harvested mastoid bone marrow aseptically from areas without any infection, and succeeded culture from all those patients. For successful harvesting MSCs, we recommend the use of curettage rather than an aspiration technique and the harvested area should be the mastoid tip without infection.
The hMBMCs and hEASCs showed stem cell phenotypes by expressing CD29, CD90, and CD105, but not CD45. These characteristics were maintained up to at least passage 5, but the expression of CD29 and CD105 began to decrease by passage 6. This finding is consistent with a previous study showing that senescence-like morphology and the number of senescence-associated β-galactosidase-positive cells increased at passage 6 in bone marrow-derived stromal cells (Zheng Y et al., 2016). The authors suggested that p53 plays a critical role in upregulating autophagy during senescence in bone marrow-derived stromal cells (Zheng Y et al., 2016). This relationship between passage number and stem cell potential has been established. Cells at passage 20 showed an expanded cytoplasm and significantly decreased differentiation capacity (Wan Safwaki WK et al., 2011). Although the underlying mechanisms remain unclear, previous reports have suggested that mitochondria, autophagy, genomic stability, and growth factor-mediated changes may affect MSC senescence as the cultivation time and passage number increase
36
(Stab BR et al., 2016; Kundrotas G et al., 2016). In addition, changes in stem cell phenotype may differ depending on the MSC origin, which could significantly impact their clinical potential (Meng X et al., 2017). Therefore, stemness must be considered before using MSCs for tissue engineering.
Regarding MSC delivery, a commonly used approach in cell-based therapy is to inject stem cells suspended in saline buffer or culture medium directly into the injured tissues. However, this procedure has not shown satisfactory outcomes; thus, an appropriate supportive 3D scaffold should be applied in cell-based tissue engineering strategies. Scaffolds provide physical and mechanical support, spatial structure, and an adequate biochemical environment for cell behavior (Sivak WN et al., 2014). Another major problem in bone tissue engineering is insufficient oxygen and nutrient supplies, which ultimately cause cell death (Rai B et al., 2010). In previous studies, the porous structure of 3D scaffolds facilitated sufficient oxygen diffusion and nutrient delivery.
We selected PCL as a scaffold for mastoid bulla defect regeneration using hEASCs. PCL is a synthetic, non-toxic, absorbable polymer that can be widely applied in the field of tissue engineering. Porous PCL scaffolds have favorable mechanical properties and are resistant to rapid hydrolysis (Park SY et al., 2016). Jang et al. reported that PCL scaffolds used with human umbilical cord serum(Jang CH et al., 2014) or beta-tricalcium phosphate(Jang CH et al., 2013) led to improved bone formation compared with controls. Our micro-CT imaging and histological results also revealed a significant difference in bone formation between the groups with and without PCL scaffolds. Interestingly, the positive effect of the scaffolds was much greater for mastoid bulla bone
37
formation than for cranial bone formation. This difference may be due to the different environments of these structures. The mastoid bulla does not have any supporting inner structures for cell adhesion in the cavity, and the fluid contents in the bulla can be removed through the Eustachian tube, whereas in the cranial bone defect area, transplanted cells can be supported by the periosteum and supplied with nutrients by adjacent tissues. Therefore, we suggest that a scaffold should be used for stimulating bulla bone formation. Whether PCL scaffolds can exert osteogenic effects on the mastoid bulla bone without the addition of stem cells remains to be elucidated. However, there are some supporting reports that scaffold alone without cell seeding may not have much influence on bone formation. The polyesters (MPEG-(PLLA-co-PGA-co-PCL) scaffold alone showed almost no view of bone-like ingrowths in the bone defects. On the other hand, a significant bone ingrowth was observed when the human dental pulp stem cells were seeded on scaffolds (Kwon DY et al., 2015). And even though bone formation occured with collagen/chitosan/HA scaffold (CCHS) alone, significant difference in bone formation was observed between the CCHS group and the bone marrow-MSC combined with CCHS group (Sun H et al., 2014).
Bone defects of small, non-critical size undergo spontaneous regeneration by recruiting osteoblast-like cells or stem cells from adjacent tissues. These cells are induced to differentiate into osteoblasts by various hormones and growth factors, forming new bone (Marsell R et al., 2011). However, bone defects of a critical size cannot naturally heal without assistance from cells, scaffolds, or growth factors. In this study, we used a commercial ODM to evaluate the effects of osteoinductive growth factors. We observed a
38
greater volume and density of regenerated bone in the ODM group than in the non-ODM group. Previous studies showed that Escherichia coli-derived rhBMP-2, BMP-2, and PRP enhanced osteogenesis in mastoid obliteration (Jang CH et al., 2016; Kim SE et al., 2013). We suggest that ODM is a good option for supplying the nutrients and growth factors necessary for bone formation.
A recent study by Skoloudik et al. (2016) revealed that delivery of stem cell-based biomaterials composed of human bone marrow-derived MSCs (hMSCs), hydroxyapatite, and tissue glue was successful in regenerating temporal bulla bone (Skoloudik et al., 2016). They found that guinea pigs implanted with hMSCs showed a significantly higher ratio of new bone formation than that of non-implanted control animals. Additionally, they evaluated inflammatory processes and angiogenesis but did not find significant differences. However, stem cells can directly or indirectly secrete various immuno- or angiogenic modulatory factors, growth factors, and cytokines that affect their regenerative ability (Gnecchi M et al., 2012).
39
V. CONCLUSION
In this study, we isolated and identified a new type of MSCs from mastoid bone marrow and adipose tissue obtained during mastoidectomies. Both hMBMCs and hEASCs possessed the general features of MSCs, including morphology, stemness, and multiple differentiation including adipogenesis, chondrogenesis, osteogenesis, neurogenesis, and epithelial differentiation under specific stimulation. Also, we have shown for the first time that hEASCs combined with porous PCL scaffolds significantly facilitated mastoid bulla bone formation. Thus, this combination of hEASCs with PCL scaffolds represents a promising approach for the anatomical and functional reconstruction of postoperative temporal bone defects following mastoidectomy.
40
REFERENCES
1. Brzoska M, Geiger H, Gauer S, Baer P: Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 330:142-150, 2005 2. Lee HB, Lim HJ, Cho M, Yang SM, Park K, Park HY, Choung YH: Clinical
Significance of beta-Tricalcium Phosphate and Polyphosphate for Mastoid Cavity Obliteration during Middle Ear Surgery: Human and Animal Study. Clin Exp
Otorhinolaryngol 6:127-134, 2013
3. Matsuno T, Nakamura T, Kuremoto K, Notazawa S, Nakahara T, Hashimoto Y, Satoh T, Shimizu Y: Development of beta-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent Mater J 25:138-144, 2006
4. 4 Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, Soo C, Al Hezaimi K, Zou W, Chen X, Mooney DJ, Wang CY: NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci
U S A 110:9469-9474, 2013
5. Hosseinkhani M, Mehrabani D, Karimfar MH, Bakhtiyari S, Manafi A, Shirazi R: Tissue engineered scaffolds in regenerative medicine. World J Plast Surg 3:3-7, 2014
6. Jones JR, Atwood RC, Poologasundarampillai G, Yue S, Lee PD: Quantifying the 3D macrostructure of tissue scaffolds. J Mater Sci Mater Med 20:463-471, 2009 7. Jensen J, Kraft DC, Lysdahl H, Foldager CB, Chen M, Kristiansen AA, Rolfing JH,
41
and beta-TCP facilitates migration and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Eng Part A 21:729-739, 2015
8. Nauth A, Giannoudis PV, Einhorn TA, Hankenson KD, Friedlaender GE, Li R, Schemitsch EH: Growth factors: beyond bone morphogenetic proteins. J Orthop
Trauma 24:543-546, 2010
9. Jang CH, Choi CH, Cho YB: Effect of BMP2-Platelet-rich Plasma-Biphasic Calcium Phosphate Scaffold on Accelerated Osteogenesis in Mastoid Obliteration.
In Vivo 30:835-839, 2016
10. Kim SE, Yun YP, Song HR, Choi KH, Kim BH, Lee EK, Song JJ: Bone formation of middle ear cavity using biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 using animal model.
Int J Pediatr Otorhinolaryngol 77:1430-1433, 2013
11. Alev C, McIntyre BA, Ota K,Sheng G: Dynamic expression of Endoglin, a TGF-beta co-receptor, during pre-circulation vascular development in chick, Int J Dev
Biol, 54:4, 737, 2010
12. Langan RC, Mullinax JE, Ray S, Ray S, Raiji MT, Schaub N: A Pilot Study Assessing the Potential Role of non-CD133 Colorectal Cancer Stem Cells as Biomarkers. J Cancer, 3:231, 2012
13. Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ: Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells, Leukemia, 19:7, 1118, 2005
42
Storms RW, Goh B, Kilroy G, Wu X, Gimble JM: Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24:376-385, 2006
15. Palva T: Mastoid obliteration. Acta Otolaryngol Suppl ;360:152-154, 1979
16. Estrem SA, Highfill G: Hydroxyapatite canal wall reconstruction/mastoid obliteration. Otolaryngol Head Neck Surg 120:345-349, 1999
17. Kagami H, Agata H, Inoue M, Asahina I, Tojo A, Yamashita N, Imai K: The use of bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering: basic science to clinical translation.
Tissue Eng Part B Rev 20:229-232, 2014
18. Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, Fouillard L: Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr 13:217-226, 2007
19. Zheng Y, Lei Y, Hu C, Hu C: p53 regulates autophagic activity in senescent rat mesenchymal stromal cells. Exp Gerontol 75:64-71, 2016
20. Wan Safwani WK, Makpol S, Sathapan S, Chua KH: The changes of stemness biomarkers expression in human adipose-derived stem cells during long-term manipulation. Biotechnol Appl Biochem 58:261-270, 2011
21. Stab BR, 2nd, Martinez L, Grismaldo A, Lerma A, Gutierrez ML, Barrera LA, Sutachan JJ, Albarracin SL: Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci 8:299,
43
2016
22. Kundrotas G, Gasperskaja E, Slapsyte G, Gudleviciene Z, Krasko J, Stumbryte A, Liudkeviciene R: Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 7:10788-10802. 2016
23. Meng X, Xue M, Xu P, Hu F, Sun B, Xiao Z: MicroRNA profiling analysis revealed different cellular senescence mechanisms in human mesenchymal stem cells derived from different origin. Genomics 2017
24. Sivak WN, Bliley JM, Marra KG: Polymeric biomaterials for nerve regeneration: fabrication and implantation of a biodegradable nerve guide. Methods Mol Biol 1162:139-148, 2014
25. Rai B, Lin JL, Lim ZX, Guldberg RE, Hutmacher DW, Cool SM: Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials 31:7960-7970, 2010
26. Park SY, Choi JW, Park JK, Song EH, Park SA, Kim YS, Shin YS, Kim CH: Tissue-engineered artificial oesophagus patch using three-dimensionally printed polycaprolactone with mesenchymal stem cells: a preliminary report. Interact
Cardiovasc Thorac Surg 22:712-717, 2016
27. Jang CH, Cho YB, Choi CH, Jang YS, Jung WK, Lee H, Kim GH: Effect of umbilical cord serum coated 3D PCL/alginate scaffold for mastoid obliteration. Int J
44
28. Jang CH, Cho YB, Yeo MG, Kim GH: Mastoid obliteration using three-dimensional composite scaffolds consisting of polycaprolactone/beta-tricalcium phosphate/collagen nanofibers: an in vitro and in vivo study. Macromol Biosci 13:660-668, 2013
29. Kwon DY, Kwon JS, Park SH, Park JH, Jang SH, Yin XY, Yun J, Kim JH, Min BH, Lee JH, Kim WD, Kim MS: A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci Rep 5:12721, 2015 30. Sun H, Wu Y, Fu D, Liu Y, Huang C: SIRT6 regulates osteogenic differentiation of
rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-κB signaling pathway. Stem Cells 32:1943-1955, 2014
31. Marsell R, Einhorn TA: The biology of fracture healing. Injury 42:551-555, 2011 32. Skoloudik L, Chrobok V, Kalfert D, Koci Z, Sykova E, Chumak T, Popelar J, Syka J,
Laco J, Dedkova J, Dayanithi G, Filip S: Human Multipotent Mesenchymal Stromal Cells in the Treatment of Postoperative Temporal Bone Defect: An Animal Model.
Cell Transplant 25:1405-1414, 2016
33. Gnecchi M, Danieli P, Cervio E: Mesenchymal stem cell therapy for heart disease.
45 - 국문요약 -
인간 귀 유양골수 및 지방조직 유래 줄기세포를 이용한
유양골 분화 유도
아주대학교 대학원 의학과 박 승 구 (지도교수: 정 연 훈) 유양돌기 절제술(mastoidectomy)은 중이염 및 유양돌기염 환자 수술에서 병변 함기세포 제거와 재발을 막기 위해 시행된다. 이 술식은 병변이 제거되고 정상기능을 유지하며 재발률이 낮은 장점이 있으나, 공동(open cavity)으로 인해 치유기간이 길어 만성적 이루, 현훈, 각소(keratin debris) 축적, 보청기 착용 등의 문제점이 따른다. 이러한 유양돌기 절제술에 따른 공동 문제를 줄이기 위해 삭개된 유양동에 자가골분, 연골, 지방, 합성골 등을 이용하여 유양동을 폐쇄하는 방법이 적용되어 왔다. 자가조직은 합성골에 비해 안전성과 비용 측면에서 효과적임에도 불구하고, 충분한 양을 얻지 못하거나 흡수 정도를 예측하기 어렵다는 단점이 있다. 줄기세포(stem cell)는 여러 조직으로 분화할 가능성을 지닌 미분화 세포로, 유양골수(mastoid bone marrow)와 지방조직(adipose tissue)은 성체 중간엽 줄기세포를 포함하며, 유양동 삭개술시, 2 차적인 침습적 시술46 없이 얻기 용이한 세포 재료가 된다. 유양골은 두개골과 달리, 공기세포 (air cell)가 존재하며 수술 후 공동은 빈 공간으로 남기 때문에 유양골조직과 유사한 구조의 3 차원적 지지체 (scaffold)가 요구된다. 폴리카프로락톤 (Polycaprolactone; PCL) 은 합성고분자 재료로, 천연고분자의 스캐폴드에 비해 가격이 낮으며 분자구조와 분자량 조절이 가능해 물리, 기계적 특성 및 생분해성을 조절할 수 있는 장점을 지닌 FDA 승인된 지지체로 조직공학에서 널리 활용되고 있다. 본 연구는 유양돌기삭개술 시 획득되는 인간 유래 유양골수와 지방조직 내 줄기세포로서의 유용성을 확인하고, 유양동 결손 동물모델에 3 차원 다공성 지지체와 세포, 성장인자를 조합시켜 공동에 이식해 골 재생효과를 알아보는 것을 목표로 하였다. 유양골수와 지방조직 유래 세포에서 중간엽 줄기세포의 표면항원인 CD90, CD105, CD29 를 90% 이상 발현하며, 조혈모세포의 표면항원인 CD45 는 발현하지 않았다. 이러한 발현 양상은 5 계대(passage)까지 유지되었으며 이후 점차 감소되었다. 골, 연골, 지방, 신경, 상피세포로의 분화 유도 후 각 세포염색을 통해 관찰한 결과, 다분화능 (multi-potency) 또한 확인되었다. 동물실험은 생후 8 주령 Sprague-Dawley rat으로, 총 24 마리의 유양골과 두개골 결손모델에 (1) 대조군, (2) 귀 지방유래 세포군, (3) 귀 지방유래 세포 + 골 분화촉진 성장인자군, (4) 귀 지방유래 세포 + PCL 지지체군, (5) 귀 지방유래 세포 + PCL 지지체 + 골 분화촉진 성장인자군으로 분류하고, CT (computed tomography)와 Hematoxylin & Eosin (H&E), 골 분화 지표인 osteopontin 면역염색을 통해 골형성을
47 분석하였다. CT를 시행한 결과, 귀 지방유래 세포 + PCL 지지체군에서 상당한 골 분화가 관찰되었으며, H&E와 면역염색 결과에서도 유양골 결손 기저부로부터 골형성이 시작되는 것을 확인하였다. 이에 골 분화촉진 성장인자군이 첨가되었을 때, 유양골 형성의 상승효과가 관찰되었다. 그러나 이러한 효과가 두개골 결손모델에서는 관찰되지 않았다. 본 연구에서는 귀 지방유래 세포와 3 차원 다공성 지지체를 이식하여 유양골 형성의 유의미한 효과를 보았다. 향후 유양돌기 절제술시 발생하는 부작용에 자가조직과 지지체를 이용한 본 기술이 유용하게 적용될 것으로 기대된다. 핵심어: 골형성, 유양돌기절제술, 유양돌기, 지방조직, 줄기세포