저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
i
A novel function of tissue inhibitor of
metalloproteinase-1 as a chemoattract
molecule for human neural stem cells
By
Soo Youn Lee
Major in Neuroscience
Department of Biomedical Sciences
ii
A novel function of tissue inhibitor of
metalloproteinase-1 as a chemoattract
molecule for human neural stem cells
By
Soo Youn Lee
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Master of Neuroscience
Supervised by
Myung Ae Lee, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
iii
This certifies that the dissertation
of Soo Youn Lee is approved.
SUPERVISORY COMMITTEE
Hye Sun Kim
Joon Kyu Lee
Myung Ae Lee
The Graduate School, Ajou University
December, 22nd, 2008
iv - ABSTRACT -
A novel function of tissue inhibitor of metalloproteinase-1 as a
chemoattract molecule for neural stem cells
Recently neural stem cells (NSCs) were reported to migrate to damaged brain
area, such as brain tumor or ischemic region. So, we hypothesized that human
glioma or ischemic tissues secreted chomoattract molecules that induce NSC
migration toward that area. In present study, we reported one candidate protein,
TIMP-1, commonly expressed from microarray and proteomic data.
Imuunostaining showed that expression of TIMP-1 is up-regulated in all glioma
tissues compared to normal brain tissues. In addition, TIMP-1 dramatically
induced migration of NSCs, but not NIH3T3 cells in a does-dependent manner,
and showed the maximum activity at a concentration of 100mg/ml. This induction
of NSC-specific migration may be derived from the difference of TIMP-1 receptor
expression because F3 highly expressed all TIMP-1 receptors, but NIH3T3 cells
do not. After then, to investigate if CD63, one of TIMP-1 membrane receptors,
mediate NSC migration by TIMP-1 on the cell surface, we established
CD63-knockdown F3 cell lines using a vector-based small hairpin RNA (shRNA)
strategy. Interestingly, CD63 knockdown blocked almost all NSC migration by
TIMP-1 in vitro migration assays. In addition, TIMP-1-mediated migration
mediated through ERK and Cdk5 signaling pathway, as PD98059 and roscovitin
v
reduced the migration by 26% and 45%, respectively. All these results
demonstrated that TIMP-1 is a powerful chemoattract molecule for NSC, and may
be promising drug for brain tumor therapy.
Key words: Tissue inhibitor of metalloprotein-1 (TIMP-1), neural stem cell migration, Brain tumor, Chemoattract molecule, CD63
vi
TABLE OF CONTENTS
ACKNOWLEDGEMENTS………...ⅰ ABSTRACT ………..……….………..…….... vi TABLE OF CONTENTS ……….………..…... vi LIST OF FIGURES ……….………..……….……... vi LIST OF TABLE….……….………..….. vi LIST OF ABBREVIATION ……….………..…. vi . I Ⅰ NTRODUCTION ……… 1 . M Ⅱ ATERIALS AND METHODS ……….. 51. Cell culture……….………...……….……….5
2. Immunohistochemistry ……….………...………….………. 5
3. Boyden chamber chemotaxis assay……….6
4. RNA preparation and Reverse transcription-polymerase chain reaction (RT-PCR)…6 5. Western blot analysis ……….………...…….. 7
6. Immunoprecipitation………... 8
7. Establish Cd63-knockdown cell lines ………..………..… 8
8. Flow cytometric analysis……….………... 9
9. Reagent and antibody……….………... 10
. R Ⅲ ESULTS ……..……….……… 11
1. Expression Pattern of TIMP-1 in human Brain Tumors ……….…....……. 11
2. TIMP-1 induced Human Neural stem migration……….. 12
vii
4. Different expression pattern of receptors in several cell lines…... 13
5. Interaction of with TIMP-1 Integrin β1 and CD63 in HNSC Cells... 15
6. Efficient Silencing of CD63 expression by RNAi... 15
7. CD63 is a key mediator for NSC migration by TIMP-1………...………..16
8. ERK and CDK5 independent pathways for stimulating TIMP-1 HNSC cell Migration ... 17 . Ⅳ DISCUSSION ... 27 Ⅴ. CONCLUSION ……..………. 29 REFERENCES ..…………..……….. 30 국문요약 ... 39
viii
LIST OF FIGURES
Fig. 1. Schematics of choosing tissue inhibitor of metalloproteinase-1 as a
chemoattractant molecule secreted by brain tumor samples ... 3
Fig. 2. TIMP-1 expression in human glioblastoma tissues ... 19
Fig. 3. TIMP-1 elicited a concentration-dependent migration of hNSCs ...20
Fig. 4. TIMP-1 induces migration in NSC-type specific manner ... 21
Fig. 5. CD63 mediates TIMP-1 binding to the cell surface and TIMP-1 co-localization with integrin β1……….……… 22
Fig. 6. Efficient silencing of CD63 expression in HNSCs ………..………. 24
Fig. 7. CD63 is a mediator of migration of NSC ………...………..……… 25
ix
LIST OF TABLES
x
LIST OF ABBREVIATIONS
TIMP-1, tissue inhibitor of metalloproteinase-1 NSCs, human neural stem cell line
NIH3T3, mouse fibroblasts cell line
HEK293, human embryonic kidney cell line P1, transfection of Prep4 vector cell line
1
.
Ⅰ
INTRODUCTION
Malignant brain tumors, glioblastoma multiform, remain virtually untreatable and inevitably lethal despite extensive surgical excision and adjuvant radiotherapy and chemotherapy. Their treatment resistance is related to their exceptional migratory nature and ability to insinuate themselves seamlessly and extensively into normal neural tissue, often migrating great distances from the main tumor mass. These cells are responsible for the recurrent tumor growth near the borders of the resection cavity. It is this behavior that has also limited their accessibility to otherwise promising gene therapeutic vectors and interventions (Kramm et al, 1995). Intriguingly, one of the cardinal features of neural stem cells (NSCs) is their exceptional migratory ability (Heese et al, 2005; Aboody et al, 2000; Alvarez-Buylla & Temple, 1998; Flax et al, 1998; Gage, 2000; Lynch et al, 1999; McKay, 1997; Weiss et al, 1996; Yandava et al, 1999). Indeed, it is their migratory capacity that has made them so useful in therapeutic paradigms demanding brain wide gene and cell replacement in various animal models of neurodegeneration (Lynch et al, 1999; Yandava et al, 1999). Many experiments showed that NSCs in the adult brain are able to migrate in a directed way toward pathologically altered tissues (Aboody et al., 2000; Benedetti et al., 2000; Ehtesham et al., 2002); Specially NSCs displayed a strong tendency to migrate toward gliomas. (Aboody et al., 2000) Neural stem cells (NSCs) as well as neural progenitor cells have the biological potential to differentiate into different CNS cell subtypes, including neurons, astrocytes, and oligodendrocytes. (Park et al., 2002; Vescovi et al., 1999; Villa et al., 2000). Similar to neural progenitor cells during CNS
2
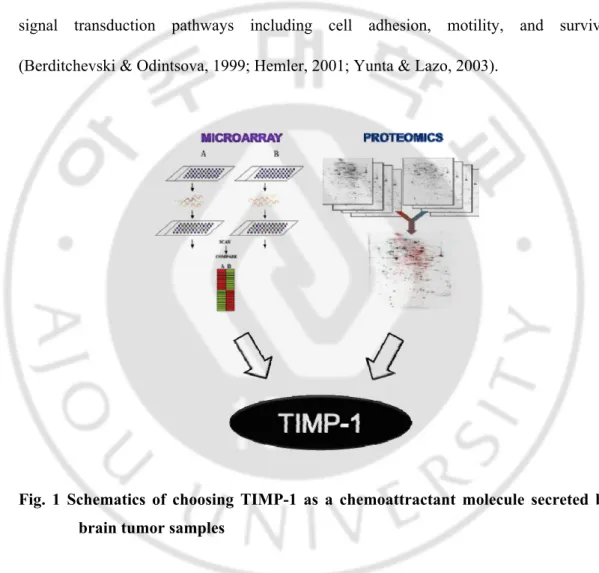
development. Precursor cell migration is a well-known. Many different factors have been identified that guide the motility of these cells, including soluble factors, cell adhesion molecules, and extracellular matrix components. In contrast, very little is known about the specific mechanisms responsible for NSC migration in adult brain. In the present study, we investigated the effects of TIMP-1 on the directional motility of the Human neural stem cell line (F3). The family of tissue inhibitors of metalloproteinases (TIMPs), consisting of four members (TIMP-1 to -4), regulates dynamic processes of extracellular matrix (ECM) turnover and remodeling as well as activities of growth factors and their cell surface receptors, in part through inhibition of matrix metalloproteinases (MMPs). However, mounting evidence suggests that TIMPs can regulate angiogenesis, cell survival, proliferation and apoptosis independent of their MMP inhibitory activity via interactions with cell adhesion molecules or growth factor receptors (Guedez et al, 1998; Airola et al, 1999; Slee et al, 1999; Liu et al, 2003, 2005; Qi et al, 2003). Since then, it has been suggested that TIMP-1 has pleiotropic activities in the regulation of proliferation, cell survival, differentiation and migration independent ofits MMP inhibitory activity. Recent studies show that TIMP-1 interacted with the CD63/integrin β1 complex (a main tetraspanin-interacting integrin) on the cell surface of human breast epithelial MCF10A cells. These complexes are regulation of cell signaling (As shown in Fig.1). CD63 interacted with TIMP-1. (Jung et al, 2006). In this study we demonstrated a CD63 dependent TIMP-1 association with integrin β1 on the cell surface of human neural stem cell and this complex are regulation of NSC migration.CD63 is one of 28 members of the tetraspanin family, a group of hydrophobic proteins containing four transmembrane
α-3
helices, two extracellular loops, and a short cytoplasmic tail (Berditchevski & Odintsova, 1999; Hemler, 2001; Yunta, & Lazo, 2003).Twenty tetraspanin proteins are estimated to be expressed on the cell surface and have been implicated in diverse cellular processes such as cell proliferation, polarization, activation, adhesion, migration, and differentiation(Escola et al, 1998; Hemler, 2001; Jang & Lee, 2003). CD63, CD82 and CD151 interact with cell adhesion molecules such as integrins, regulating intracellular signal transduction pathways including cell adhesion, motility, and survival (Berditchevski & Odintsova, 1999; Hemler, 2001; Yunta & Lazo, 2003).
Fig. 1 Schematics of choosing TIMP-1 as a chemoattractant molecule secreted by brain tumor samples
Our previous report reported one secreted proteins that are up-regulated in human brain tumor from microarray data and proteomics data actually expressed highly in brain
4
tumor samples compared to normal tissues with real-time PCR. We hypothesized that this secreted protein from human glioma may be putative chemioattracts and provide clues to the molecular pathway involved in neural stem cell migration to glioma. In the present study, we investigated for one candidate protein TIMP-1 to induce NSCs migration and the molecular mechanisms involved in such migration of human neural stem cells.
5
. Materials and Methods
Ⅱ
1. Cell culture
Immortalized human neural stem cell lines (NSCs) HB1.F3, F5 and A4 was established as described previously (Kim et al., 2003), and maintained and passaged on uncoated culture dishes in Dulbecco’s modified medium (DMEM, GIBCO-BRL Life Sciences) with 10 % Fetal bovine serum (FBS, Hyclone), 10 μg/ml penicillin-streptomycine (Gibco). HEK293 (human embryonic kidney cell line) and NIH3T3 (mouse fibroblasts cells) were grown in DMEM (sigma-Aldrich) supplemented with 10% FBS (Hyclone), 10 μg/ml penicillin-streptomycine (Gibco). All cells are incubated at 37 ℃ in an incubator of 5 % CO2/95 % air.
2. Immunochemistry
Tissue was fixation in 4 % paraformaldehyde. Frozen tissue sections (20 μM) were cut onto slides coating by gelatin. Sections were air-dried at 4 ℃ for 1h before stain. Tissue was washing PBS two times. Endogenous peroxidase activity was quenched by incubating with 0.3 % hydrogen peroxide for 5 min. Tissue were permeabilized with 0.2 % triton X-100 in PBS and 5 % BSA for 30 min at room temperature blocked with 10 % normal goat serum in PBS for 1 h. Sections were subsequently incubated at 4 ℃ overnight after room temperature 30 min with a 1:50 dilution of mouse anti-human TIMP-1 monoclonal antibody (R&D system). After washing, slides were incubated with mouse anti-mouse IgG followed by VECTASTAIN® Elite ABC Kit (Vector Laboratories,
6
CA), according to the manufacturer's instructions. Tissues were counter-stained with hematoxylin QS (vector CA).
3. Boyden chamber chemotaxis assay
Recombinant human TIMP-1 was the chemoattractants for migration (R&D Systems) in a Boyden chamber chemotaxis assay. TIMP-1 were diluted in 10 % FBS DMEM (Hyclone, GIBCO-BRL Life Sciences) and added to the lower compartments of the Boyden chamber (Neuro Probe Inc., MD, USA). 10 % FBS DMEM were added to lower compartments as negative assay controls, respectively. Lower-chamber wells were overlaid with a porous membrane filter (8 mm) that was polycarbonate (costar 3422). The chamber was assembled experimental cells were added to the upper compartment of the apparatus at a density of 5 X 104 cells/100 μl well and incubated at 37 ℃, 24 h and
TIMP-1 add down chamber and incubation 37 ℃, 12 h to allow for cell migration. After the polycarbonate filter was removed, the cells adhering to its upper surface were wiped off with a filter wiper. The filter was dried, fixed and stained with Hematoxlin (Sigma). The cells of at least four randomly selected fields were counted under the Image Analysis System (Olympus BX51, KY-LINK), and expressed as the average number of migrating cells.
4. RNA preparation and reverse transcription-polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen) from cell lines as described previously (Bonny, 1995). cDNA was synthesized using 2 μg total RNA in the presence
7
of superscriptII and oligo(DT) 12-18 (Invitrogen). The PCR was performed in a 50 μl reaction solution containing 5 μl 10X PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 50
pmol primer, 5 μl 10X diluted cDNA, and 1U of Tag DNA Polymerase (Invitrogen). The PCR conditions were as follow: 30 cycles as 94 ℃ for 45 seconds, annealing temperature (show table I) 45 seconds, 72 ℃ for 45 seconds and a final extension for 10 min as 72 ℃. PCR products were separated in a 1.2 % agarose gel with TAE buffer.
Table. 1 Oligonucleotide primers used for RT-PCR analysis (H: Human M: Murine)
Gene Forward primer Reverse primer Annealing
Temperature Bp H CD63 CCCGAAAAACAACCACACTGC GAT AGGAGGCTGAGGAGACC 55℃ 361 M CD63 CATTGGTGTAGCGGTTCAGGTTG CATTCCCA AGCCCACAGTTATG 55℃ 449 H CD81 GGATCCTTTGTCAACAAGGACCAG AAGCTTCTTCCCGGAGAAGAGGTCATCG 55℃ 279 M CD81 TACCTGGAACTGGGAAACAA GCTACCACAATGGCTGCAAT 55℃ 503 H CD9 TTCGGCCCAGGCTAAGTTAG CGGCAAGCCAGAAGATGAAG 55℃ 101 M CD9 CAGTGCTTGCTATTGGACTATG GCCACAGCAGTCCAACGCCATA 55℃ 377 H Integrin α3 CTGGAAAGGAAACAGCTACATGATT CCTGCATCGTGTACCCAATATAGA 55℃ 110 M Integrin α3 CACCAACATTAGGGCGCTGTC TCCTGGTTGATGTCACCGATGC 55℃ 360 H Integrin α6 GTGACTGCTGCTGCCGAAA GGTACTCCCGAATACTGAGCTACAG 55℃ 110 M Integrin α6 AACATCAGAGACAAGCTGCG CTCAGTTCCTGTAAGCGGA 55℃ 437 H Integrin β1 CTGCAAGAACGGGGTGAATG CACAATGTCTACCAACACGCCC 55℃ 302 M Integrin β1 GAATGTGTTCAGTGCAGAGC ATTGGGATGATGTCGGGACC 60℃ 261
5. Western blot analysis
Total cell lysate were prepared by two cold lysis buffers. RIPA lysis buffer (1 % Sodium deoxycholoate, 0.1 % SDS, 20 mM Tris-HCl, pH 7.5, 1 % Triton X-100, and 50 mM NaCl) is used for analyses of all other proteins except CD63. The other is 1% Brij 96
8
lysis buffer (1% Brij 96, 25 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 2 mM
PMSF 1x protease inhibitor cocktail) for CD63 protein analysis. After incubation for 1 h on ice, samples were centrifuged at 13,000 rpm for 20 min at 4 ℃. The concentration of protein in the extracts was determined using a Dc protein assay kit (Bio-Red) (Quantitative et al., 2000). 10 - 100 μg of cell extract was separated on 8 – 10 % SDS-PAGE gel. After electrophoresis, gel was transferred onto a PVDF membrane (Millipore, USA) for 1 and half hour. The membrane was blocked for 1 hr with 5 % BSA in TBS-T. Mouse monoclonal antibody to human CD63 (Abcam), mouse monoclonal antibody to human TIMP-1 (R&D systems) and rabbit polyclonal antibody to human integrin β1 (Millipore) were diluted in 5 % BSA in TBS-T, and incubated at 4 ℃ overnight. The membrane was washed three times of 15 min in TBS-T. After blocked with 5 % skim milk in TBS-T for 1 h, it was incubated for 1 hr with peroxidase-conjugated secondary antibody (1:2000, Zymed). Immunoblot were detected using ECL (Amersham) kit and visualized after exposure to a film.
6. Immunoprecipitation
Cell lysates were obtained by lyzing the cell monolayer with 1 % Brij 96 lysis buffer. Nuclear fraction was discarded after centrifugation at 5000 rpm for 10 min, and supernatants were precleared by incubation with agarose beads conjugated with secondary antibody 4 h at 4 °C. The precleared lysates were incubated with 1 μg/ml anti-rTIMP-1 or anti-CD63 antibody at 4 °C overnight. Agarose beads with immune complexes were collected with centrifugation at 5,000 rpm for 10 min, and followed by 3
9
washes with the immunoprecipitation buffer. Immune complexes were then eluted from the beads with 2x SDS sample buffer and subjected to SDS-PAGE.
7. Establishment of CD63 knockdown cell lines
Knockdown of CD63 expression was performed using the short hairpin-activated gene silencing system (Lee, Y. J. et al., 2004). The plasmids pREP4 containing CD63 shRNA mRNA sequences were expressed under the pREP4 (Invitrogen), which allows for long term suppression of gene expression. The sequence of shRNA used for knockdown of CD63 expression was previously reported to specifically suppress CD63 gene expression in human breast epithelial MCF10A cells(Jung et al., 2006).
CD63 shRNA target: 5’-GGTCTAGAAAA AAGGACTCGGTCCTTCGAC A C G G A A G A G A C T C A A G C T T C A A T C C C T T C C A T G T C G A A G A A C C GAGTCCGGTGTTTCGTCCTTTCCACAA-3’. Three day after transfection with these pREP4 constructs using polyethyleneimine, Human neural stem cell cells were grown in the presence of 200 μg/ml hygromycin B for 9 days for selection. Any dying cells were removed, and the cells selected were reseeded on the plates, further grown in DMEM containing 30 μg/ml hygromycin B for the indicated times.
8. Flow cytometric analysis
Cells were incubated with 2 μg/ml anti-CD63 monoclonal antibody (abcam, ab8219) in FACS buffer(1X PBS , 2 %FBS, 0.1% sodium azide) for 1h, washed with cold PBS, and then incubated with saturating concentrations of FITC-conjugated goat anti-mouse
10
IgG (KPL) for 30 min at RT. After being washed with FACS buffer. Cell surface immunofluorescence was analyzed by flow cytometry performed on a FACS can (Becton Dickinson, San Diego, CA). Data analysis by winMDI version 2.9.
9. Reagents and antibodies
Anti-TIMP-1 antibody (clone 63515) and recombinant human TIMP-1 were purchased from R&D Systems. Anti-TIMP-1 ab-2 from Sigma. Anti-CD63 antibody is from Abcam, integrin β1 from Millipore. HRP-conjugated mouse IgG and anti-rabbit IgG from Zymed. Transfection reagent was Lipofectamine from Invitrogen. Hygromycin B from Invitrogen. Fluorescein-labeled affinity purified antibody to mouse IgG (KPL 01-18-06).
11
.
Ⅲ
RESULTS
Expression pattern of TIMP-1 in human brain tumors
As noted above, Tissue inhibitor of metalloproteinase-1(TIMP-1) can function as an anti-apoptotic and survival factor, and its levels increase in response to inflammation. However recently report show that such processes may contribute to exacerbation of the malignant process. Higher levels of TIMP-1 correlate with poor clinical outcome for patients (Kossakowskaet al., 2000). And our previous data show that TIMP-1 is over expression in human brain tumors than normal brain tissue of microarray and proteomics. So we essentiality need that to definite expression pattern of timp-1 in human brain tumor. Relationship between TIMP-1 expression and clinic pathological features Tissue inhibitor of metalloproteinase-1 expression in human brain tumors was investigated by immunohistochemical analysis. Positive staining for TIMP-1 was restricted to tumor cells. When comparing the expression patterns of the brain tumors (Fig. 1E-H) and normal brain specimens (Fig 1.A-D). TIMP-1 showed a correlation with brain tumor over the range of Brain Tumors tissue. For TIMP-1, expression was never detectable by IHC in normal brain tissue but expressed dramatically for brain tumor tissues. Intensity of staining varied among different patients and in different areas of the tumor. Immunoreactive products had a fine granular appearance and localized in the cytoplasm and displayed a heterogeneous of tumor cells. We detected excessive expression area (Indication arrows in Fig1). Previous reported that TIMP-1 has effects on ECM breakdown, angiogenesis, cell morphology, and cell survival/death, it is the net outcome
12
of the balance of these functions that would determine the pathophysiology of glioma cells (Gardner & Ghorpade, 2003). So we supposed that this area is looks like blood vessel and we suggest that TIMP-1 is over expression in blood vessel. Therefore, TIMP-1 secreted when brain tumor metastasis. We hypothesized that Timp-1 induced the migration of neural stem cell. We arrived at conclusion, TIMP-1 was the only expression protein in the human Brain Tumors. Furthermore our data are consistent with a potential chemoattract molecule function for TIMP-1.
TIMP-1 induced Human Neural stem migration.
previous reported that neural stem cell migration toward glioma in vitro(Heese et al, 2005). These findings suggest that soluble factors secreted by Brain tumor are potent inducers of NSC migration, but that the responsible factors are produced to a varying extent by the different cell lines. However, the factors responsible for NSC migration in the adult CNS are unknown. Our previous data show that Brain tumor secreted TIMP-1.
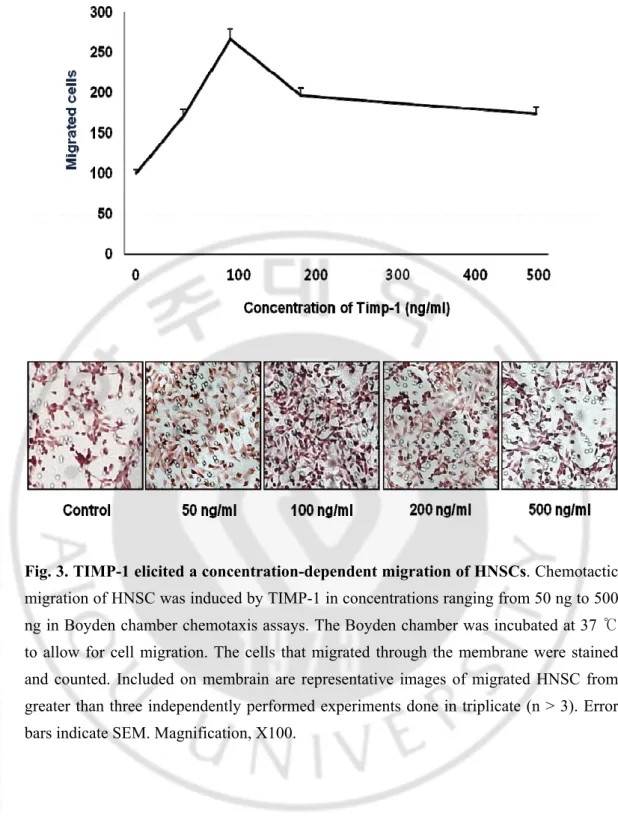
TIMP-1 is up regulated in regions of Brain tumor injury and inflammation and attracts migratory cells. So we investigated the neural stem cell migration after stimulation with the TIMP-1. Boyden chamber assays was performed for measuring the Human Neural stem cell movement. Boyden chamber assays were used to assay TIMP-1 in concentrations ranging from 50 ng/ml to 500 ng/ml. TIMP-1 induced the typical bell- shape curve for the human neural stem cell chemoattraction. The effects were concentration dependent, and strongest motogenic responses were consistently observed at the highest protein concentration. (100 ng/ml). Stimulatory effects ranged from
0.7-13
fold stimulation (50ng/ml) to 1.6 fold stimulation (100ng/ml). (Fig. 2) TIMP- 1 specific factor responsible for NSC chemoattraction migration.
Migration capacity of F3 and NIH3T3 Cells in Vitro
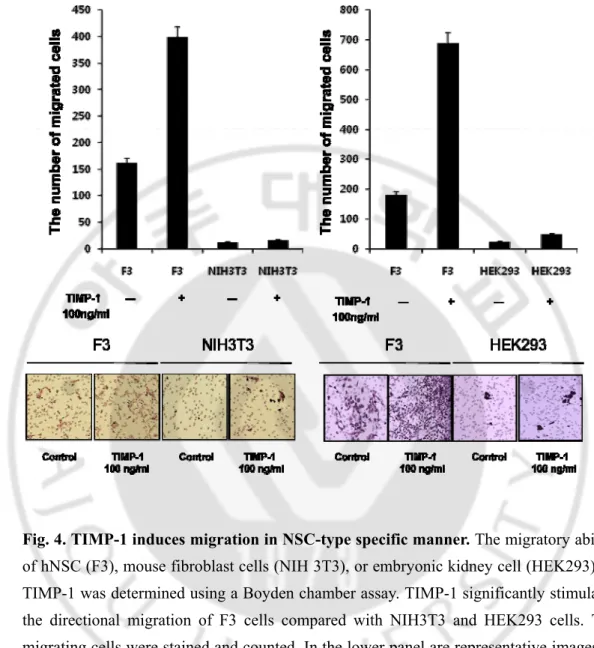
We observed that TIMP-1 induced human neural stem cells. Recent studies have shown that showing the remarkable migratory capacity of NSCs to the infiltrating tumor cell in the normal brain. In contrast, the NIH 3T3 fibroblast cells did not migrate and remained localized at the area of the injection site (Kim et al, 2006). We next compared the in vitro migratory capacities of the two cell lines Human neural stem cell (F3) and Mouse fibroblast cell (NIH3T3) in TIMP-1 by using the boyden chamber assay as described in materials and methods. Our previous data showed the maximum activity at a concentration of 100 ng/ml (Fig. 2), accordingly we treated TIMP-1 100ng/ml. F3 Stimulatory effects 1.3-fold stimulation but NIH3T3 effects -8.7 fold stimulation (100ng/ml). Human neural stem cell (F3) migrated with the typical bell- shape curve for the TIMP-1 chemoattraction. But mouse fibroblast cell (NIH3T3) does not migrate. (Fig. 3) Importantly, the migration capacity of human neural stem cells was significantly higher than that of the mouse fibroblast cells (NIH3T3) on TIMP-1. (Fig. 3).
Expression of TIMP-1 receptors in various cells
Pevious migration data show that only HNSC migrated by TIMP-1. We guess reason of this situation. So we hypothesize that they have different receptor expression pattern. Recent studies from our laboratory found that TIMP-1 expression correlated with the
14
levels of active integrin β1 on the cell surface in a CD63-dependent manner. And TIMP-1 affects CD63’s regulation of the integrin signaling pathway in a cell-type specific manner (Chirco et al, 2006). Our lab had microarray data in HNSC. So we compare expression patterns of tetraspanins (CD9, CD81, CD63) and integrins (β1, α3, α6), in microarray data. Almost cell line is expression tetraspanins (CD9, CD81, and CD63) and integrins (β1, α3, α6). Especially F3 is most expression of CD63 and β1. Next we compared expression tetraspanins (CD9, CD81, and CD63) and integrins (β1, α3, α6) by PT-PCR analysis in HNSCs, HEK293 and NIH3T3. We performed RT-PCR analysis using primers specific for tetraspanins (CD9, CD81, and CD63) and integrins (β1, α3, α6) cDNA to compare the expression in HNSCs, HEK293 and NIH3T3. The bands of these integrins and TM4 were clearly detected both in HNSCs, HEK293 and NIH3T3 by RT-PCR. As a control, the presence of mRNA for GAPDH and β–actin was examined. All integrin and TM4 mRNA revel expression is NIH3T3 better than HNSCs and HEK293 expect CD81. Especially CD63 is extremely expression in NIH3T3 rather than HNSC and HEK293. We investigated that of expression pattern of CD63 and integrin β1 in protein level. We performed western blot analysis using mouse monoclonal Antibody CD63 (abcam) and rabbit polyclonal anti-body integrin β1 (Millipore). HNSC (F3), NIH3T3 and Human embryonic kidney cell (HEK293) were examined for surface expression of CD63 and integrin β1 by western-blot (Fig.4 C, D). The CD63 protein with 30–70% of its size (30– 60 kDa) being heavily glycosylated (Stippet al, 2003) exhibited a diffuse distribution on SDS–PAGE under a nonreducing condition Surprising F3 and HEK293 expressed CD63 and integrin β1 but NIH3T3 was not detected. And CD63 and integrin β1 was extremely
15
expression in F3 rather than HEK293. This results show that HNSC (F3) has most powerful migration ability. Due to F3 have a lot of receptors.
Interaction of with TIMP-1 and integrin β1 and CD63 in HNSC Cells
Previous reports show that CD63 interacts with the C-terminal, but not with the N-terminal domain of TIMP-1, suggesting TIMP-1 interaction with CD63. Here, we asked whether TIMP-1 interacts with CD63/integrin complex, and if so, whether these interactions are critical for TIMP-1 regulation of cell migration. Since integrin β1 is thought to be one of the main tetraspanin-interacting integrins (Berditchevski et al, 1995, 1996, 1997), we first examined CD63 interactions with integrin. As shown in Fig. 4E and F, anti-CD63 antibodies immunoprecipitated integrin β1 and TIMP-1 from lysates of F3 cells, which suggests that these proteins are forming a complex. We observed that CD63 is interacted with integrin β1. In addition, TIMP-1 is interacted with CD63. These interactions may be very importance for NSC migration.
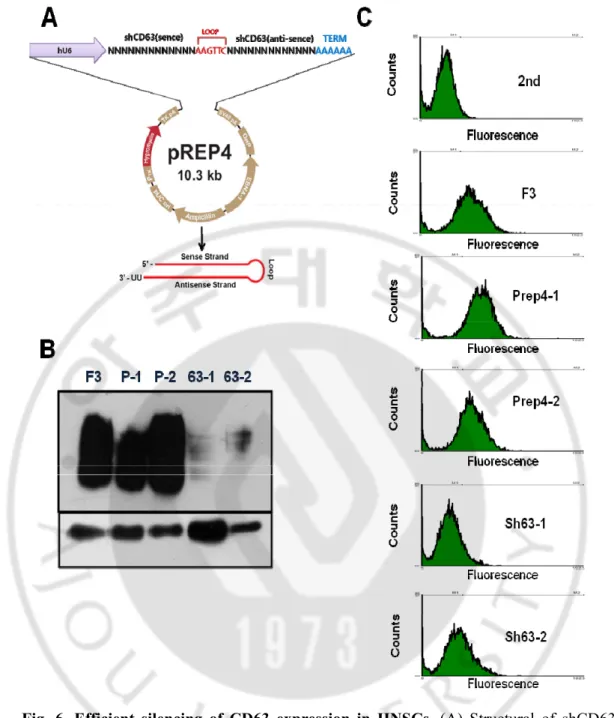
Efficient silencing of CD63 expression by shRNA system
Our data show that TIMP-1 and CD63 and integrin complex are important for HNSC migration. Specifically, CD63 is mediator in TIMP-1/CD63/integrin β1 complex. To evaluate the significance of CD63 for TIMP-1 binding on the cell surface, we established CD63-knockdown F3 cells using a vector-based small hairpin RNA (shRNA) strategy. To examine the role of CD63 in mediating the surface binding of TIMP-1 and integrin β1. We had knocked down CD63 in HNSC cells using a shRNA approach. CD63 shRNA
16
sequence is showed in Method. The shCD63-F3 cells were stably transfected with the Prep4 vector containing CD63 target sequences (Fig6.A).Western-blot analyses confirmed significant down regulation of CD63 expression in cells receiving specific CD63 shRNA target sequences (Fig6.B). Compared with wild-type control cells, cell lines bearing two of the CD63 shRNAs (shCD63-1, and shCD63-2) shRNA displayed almost loss of cell surface CD63. Conceivably, cell surface expression of CD63 could be more sensitive to suppression by RNAi than total CD63 expression. So we evaluated CD63 expression in with wild-type control cell and two of the shCD63-F3 cells populations by flow cytometry (Fig6.C). FACS Results show that cell surface expression, total levels of CD63 in wild-type, shCD63-1, and shCD63-2 cells. shRNA displayed a almost loss of cell surface CD63. As a result our shCD63-F3 cell lines are low expression CD63.
CD63 is a key mediator for NSC migration by TIMP-1
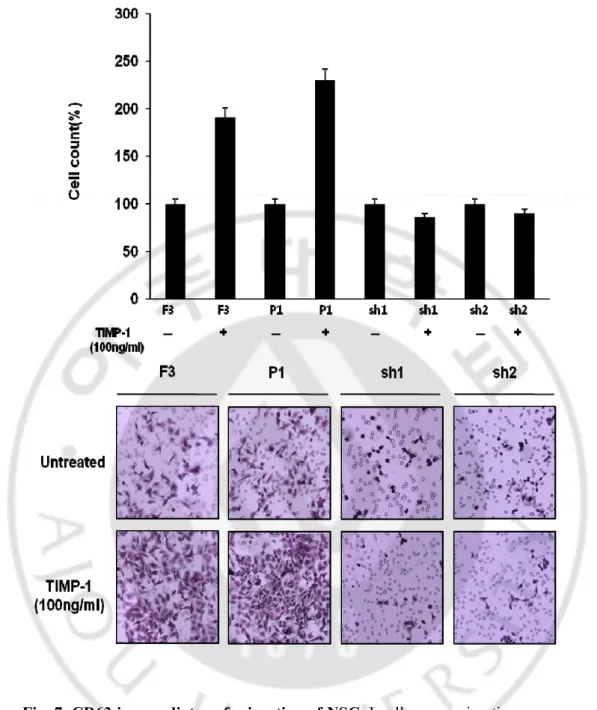
We hypothesis that Cd63 is key mediator in Human Neural stem cell migration. we next compared the in vitro migratory capacities of the 4 kinds of cell lines Human neural stem cell (F3), shCD63-F3-1, and shCD63-F3-2 cells and pREP4 cell line in TIMP-1 by using the boyden chamber assay as described under Experimental Methods. our previous data showed the maximum activity at a concentration of 100 ng/ml (Fig. 2), accordingly we treated TIMP-1 100ng/ml. F3 and pREP4 cell Stimulatory effects 2-fold stimulation but shCD63 cells effects -0.2 fold stimulation (100ng/ml). These results show that CD63 is key mediator in NSC migration by TIMP-1(Fig 4)
17
ERK and CDK5 are independent pathways for stimulating TIMP-1-mediated NSC migration
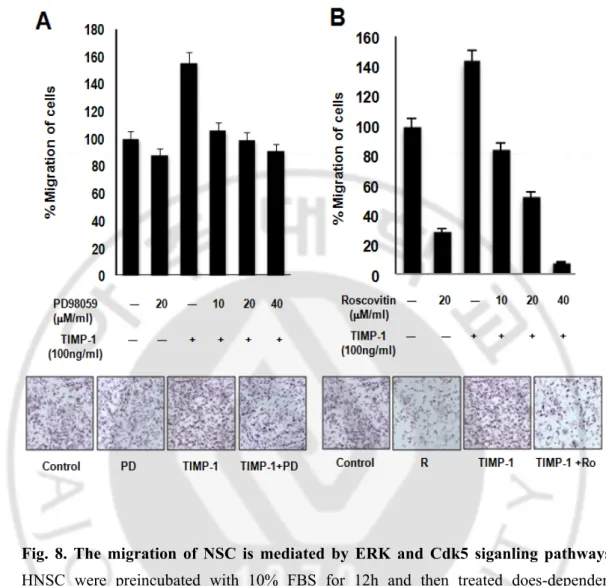
Previous paper reported TIMP-1 inhibits both the intrinsic and extrinsic cell death pathways through activation of the FAK-PI3K pathway. These effects are mediated by activation of the extracellular regulated kinase (ERK). (Stetler-Stevenson, 2008) and Erk has been implicated in the migration of numerous cell types. The in response to cell matrix proteins, such as fibronectin, vitronectin (Liu et al, 2007) and collagen (Anand-Apte et al., 1997; Klemke et al., 1997; Webb et al., 2000). We hypothesis that Erk has been implicated in the migration of Human neural stem cell. Then we using Erk pathway inhibitors PD98059inhibite the migration of Human neural stem cells. When cells were pre-treated TIMP-1 with the MEK inhibitor, PD098059 does-dependent (10 ~40μM/ml), the response to human neural stem cell migration was entirely blocked. When TIMP-1 stimulated cell migration was maximally inhibited by PD098059 40 μM. and simultaneous addition of PD098059 40 μM had a 37.5% effect. Only pre-treated PD98059 20 μM/ml had a 50% inhibition effect. But it doesn’t have dramatically effect, so we find another signal pathway. previous paper reported CDK5 is a member of the cyclin-dependent kinasefamily of proline-directed protein kinases CDK5 is an essential role in regulating the complex migration Several other neuronal functions of CDK5 have also been identified, including cell-cell adhesion (Liu et al, 2007), cell-matrix adhesion (Tang et al, 1998), neurite extension(Cruz & Tsai, 2004), and cytoskeletal regulation (Miyamoto et al, 2007). CDK5 deficiency on cell migration is consistent with a role for CDK5 in regulating cell-to matrix adhesion. We hypothesis that CDK5 has been
18
implicated in the migration of Human neural stem cell. Then we using CDK5 pathway inhibitors Roscovitin inhibit the migration of Human neural stem cells. When cells were pre-treated TIMP-1 with the CDK5 inhibitor, Roscovitin does-dependent (10 ~40 μM/ml), the response to human Neural stem cell migration was entirely blocked. When TIMP-1 stimulated cell migration was maximally inhibited by Roscovitin 40 μM. And` simultaneous addition of Roscovitin 40μM had a 97% decrease effect. Only pre-treated Roscovitin 20 μM/ml had a 80% inhibitaion effect. Thus Erk signaling is an important factor in the regulation of cell migration. But CDK5 signaling is more powerful factor in the regulation of cell migration.
19
Fig. 2. TIMP-1 expression in human glioblastoma tissues. Immunohistochemical
staining was used to detect the expression of TIMP-1 in normal human brain tissues (A-D) and human glioblastoma tissues (E-H). After DAB staining, sections were counter stained with haematoxylin.
20
Fig. 3. TIMP-1 elicited a concentration-dependent migration of HNSCs. Chemotactic
migration of HNSC was induced by TIMP-1 in concentrations ranging from 50 ng to 500 ng in Boyden chamber chemotaxis assays. The Boyden chamber was incubated at 37 ℃ to allow for cell migration. The cells that migrated through the membrane were stained and counted. Included on membrain are representative images of migrated HNSC from greater than three independently performed experiments done in triplicate (n > 3). Error bars indicate SEM. Magnification, X100.
21
Fig. 4. TIMP-1 induces migration in NSC-type specific manner. The migratory ability
of hNSC (F3), mouse fibroblast cells (NIH 3T3), or embryonic kidney cell (HEK293) by TIMP-1 was determined using a Boyden chamber assay. TIMP-1 significantly stimulates the directional migration of F3 cells compared with NIH3T3 and HEK293 cells. The migrating cells were stained and counted. In the lower panel are representative images of migrated cells in each cell line of three independent experiments done in triplicate (n > 3). Error bars indicate SEM. Magnification, X100.
23
Fig. 5. CD63 mediates TIMP-1 binding to the cell surface and TIMP-1 co-localization with integrin β1. (A) Expression profiles of tetraspanin superfamily and
integrin genes from microarray data of human NSC lines (F3,A4,F5). (B) RT-PCR analysis of tetraspanin superfamily genes and integrin genes in hNSC lines, NIH 3T3, HEK293 cells. GAPDH; internal control. (C) Expression of CD63 and integrin β1 proteins. Each cells was analyzed by western blot. β-actin and tubulin were used as internal control. D. Interaction of CD63 with TIMP1 or integrinβ. Lysates of F3 cells were immunoprecipitated with anti-CD63 or anti-TIMP-1antibody, followed by immunoblotting with anti-integrin β1 or anti-CD63 antibody, respectively.
24
Fig. 6. Efficient silencing of CD63 expression in HNSCs. (A) Structural of shCD63
knockdown vector. (B) Expression of CD63 by western blot. NSCs were stably transfected with shCD63 plasmids. P1 and P2; F3 cells transfected with Prep4 vector, 63-1 and 63-2; transfected with shCD63 knockdown plasmids. (C) FACS analysis of CD63 expression in F3 and CD63 knockdown cells. CD63 expression was evaluated by flow cytometry. Isotype background staining was donw with nonimmune mouse IgG1.
25
Fig. 7. CD63 is a mediator of migration of NSC. In all cases, migration was measured
after 12h and is expressed relative to the migration of unstimulated cells. Included on membrane are representative images of migrated F3 from greater than two independently performed experiments done in triplicate (n > 3).Error bars indicate SD. Magnification, X100.
26
Fig. 8. The migration of NSC is mediated by ERK and Cdk5 siganling pathways.
HNSC were preincubated with 10% FBS for 12h and then treated does-dependent PD98059 or Roscovitin. Other cell groups were treated PD98059 or Roscovitin with TIMP-1. In all cases, migration was measured after 12h and is expressed relative to the migration of unstimulated cells. Included on membrane are representative images of migrated F3 from greater than three independently performed experiments done in triplicate (n > 3).Error bars indicate SD. Magnification, X100.
27
. D
Ⅳ
ISCUSSION
our previously report reported one common secreted proteins that are up-regulated in human brain tumor from microarray data and proteomics data actually expressed highly in brain tumor samples compared to normal tissues with real-time PCR. We hypothesized that these secreted genes from human glioma may be putative chemioattracts and provide clues to the molecular pathway involved in neural stem cell migration to glioma. First we definite expression of TIMP-1 in human brain tumor tissue. Expression of TIMP-1 is increased in human glioma compared with normal tissues using and immunohistochemistry. TIMP-1 is expression in only human brain tissue, but not normal brain tissue did not expression. We investigated for one candidate protein tissue inhibitor of metalloproteinase-1(TIMP-1) to induce NSCs migration and the molecular mechanisms involved in such migration of human neural stem cells. TIMP-1 induced dramatic increase the migration of F3 cells, but not of mouse fibroblasts NIH3T3 cells in a dose-dependent manner, and showed the maximum activity at a concentration of 100 ng/ml. So we investigated as to how TIMP-1 regulates migration activity. We hypothesize that receptor expression is different in cell type specific manner. Therefore we investigated expression of receptors. In previous paper show that TIMP-1 regulates the integrin signaling complex via its interaction with CD63, a member of the tetraspanins. TIMP-1 affects CD63’s regulation of integrin signaling in a cell-type specific manner. TIMP-1 interaction with the tetraspanin member CD63 implies potentially diverse effects of TIMP-1 signaling on many cellular processes (Jung et al, 2006).We experiment
28
expression of tetraspanins and integrins by RT-PCR and microarray data mining. Most abundance expression of CD63 and integrin is F3. And NIH3T3 in mRNA revel. We experiment expression of tetraspanins and integrins by western-blot. Surprising expression of CD63 and integrin was detected only from F3 but not NIH3T3.We identification that migration ability is decided by different expression of receptors. Forward receptor’s interaction. Next confirmed interaction of CD63, integrin and TIMP-1 in F3. CD63 is mediator of migration by TIMP-1 in NSCs. So we performed knock-out CD63 in F3. We established shCD63-F3 cell lines using short hairpin-activated gene silencing system (Lee, Y. J et al., 2004). We confirmed that CD63 protein did not detected in shCD63-F3 cell lines by western blot and FACS. We performed boyden chamber assay using shCD63-F3, F3, prep4-F3. These result show that shCd63-F3 cell lines are dramatic decease at migration than F3, pREP4-1. So we identification CD63 is very important for NSC migration.CD63 interacts with signaling molecules. Increasing evidence suggests that CD63 regulates FAK, Src, Gab2, PI3K, Akt, and PI4K signaling pathways as shown by modulation of these pathways upon anti-CD63 antibody binding to CD63 in breast carcinoma cells and immune cells (Berditchevski and Odintsova, 1999; Sugiura and Berditchevski, 1999; Pfistershammer et al, 2004; Kraft et al,)
2005). These results demonstrated that TIMP-1 is powerful chemoattract molecule for NSC, and may be promising drug for brain tumor therapy. In addition, TIMP-1 may provide critical cues in understanding the migration of neural stem cells in physiological and pathopysiological conditions.
29
Ⅴ
. CONCLUSION
This study showed that TIMP-1 is induced NSCs. And we find mechanism of migration effect, cell signaling and different expression of receptor. These results demonstrated that TIMP-1 is powerful chemoattract molecule for NSC, and may be promising drug for brain tumor therapy. In addition, TIMP-1 may provide critical cues in understanding the migration of neural stem cells in physiological and pathopysiological conditions.
30
REFERENCES
1. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY : Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas.
Proc Natl Acad Sci U S A 97(23): 12846-12851, 2000
2. Aljada IS, Ramnath N, Donohue K, Harvey S, Brooks JJ, Wiseman SM, Khoury T, Loewen G, Slocum HK, Anderson TM, Bepler G, Tan D (Upregulation of the tissue inhibitor of metalloproteinase-1 protein is associated with progression of human non-small-cell lung cancer. J Clin Oncol 22(16): 3218-3229, 2004
3. Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development 126(8): 1655-1664, 1999
4. Berditchevski F, Odintsova E Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol 146(2): 477-492,1999
5. Chirco R, Liu XW, Jung KK, and Kim HR Novel functions of TIMPs in cell signaling.
31
6. Cruz JC, Tsai LH A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14(3): 390-394, 2004
7. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell
Biol 9(3): 255-267, 2007
8. Drucker L, Uziel O, Tohami T, Shapiro H, Radnay J, Yarkoni S, Lahav M, Lishner M Thalidomide down-regulates transcript levels of GC-rich promoter genes in multiple myeloma. Mol Pharmacol 64(2): 415-420, 2003
9. Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273(32): 20121-20127, 1998
10. Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat
32 Biotechnol 16(11): 1033-1039, 1998
11. Gage FH Mammalian neural stem cells. Science 287(5457): 1433-1438, 2000
12. Gardner J, Ghorpade A Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res 74(6): 801-806, 2003
13. Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knauper V, Lafleur MA, Forsyth PA, Edwards DR Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer 85(1): 55-63, 2001
14. Hasegawa M, Furuya M, Kasuya Y, Nishiyama M, Sugiura T, Nikaido T, Momota Y, Ichinose M, Kimura S CD151 dynamics in carcinoma-stroma interaction: integrin expression, adhesion strength and proteolytic activity. Lab Invest 87(9): 882-892, 2007
15. Heese O, Disko A, Zirkel D, Westphal M, Lamszus K Neural stem cell migration toward gliomas in vitro. Neuro Oncol 7(4): 476-484, 2005
33
17. Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim YM, Lee H Homophilic interactions of Tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathways. J Biol Chem 281(34): 24279-24292, 2006
18. Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of Tspan-5 and NET-6 during osteoclastogenesis. Allergol Int 56(4): 457-463, 2007
19. Jang HI, Lee H A decrease in the expression of CD63 tetraspanin protein e levates invasive potential of human melanoma cells. Exp Mol Med 35(4): 317-323, 2003
20. Jung KK, Liu XW, Chirco R, Fridman R, Kim HR Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 25(17): 3934-3942, 2006
21. Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Beliveau R Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis 17(7): 555-566, 1999
22. Kim IS, Kim JH, Kim JS, Yun CY, Kim DH, Lee JS The inhibitory effect of Houttuynia cordata extract on stem cell factor-induced HMC-1 cell migration. J
34 Ethnopharmacol 112(1): 90-95, 2007
23. Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, Carroll RS Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res 12(18): 5550-5556, 2006
24. Kramm CM, Sena-Esteves M, Barnett FH, Rainov NG, Schuback DE, Yu JS, Pechan PA, Paulus W, Chiocca EA, Breakefield XO Gene therapy for brain tumors. Brain
Pathol 5(4): 345-381, 1995
25. Lee JH, Engel W, Nayernia K Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev 73(2): 173-179,2006
26. Liu X, Hu Y, Hao C, Rempel SA, Ye K PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 26(34): 4918-4927, 2007
27. Lynch WP, Sharpe AH, Snyder EY Neural stem cells as engraftable packaging lines can mediate gene delivery to microglia: evidence from studying retroviral env-related neurodegeneration. J Virol 73(8): 6841-6851, 2007
35
28. McKay R Stem cells in the central nervous system. Science 276(5309): 66-71, 1997
29. Michael M, Babic B, Khokha R, Tsao M, Ho J, Pintilie M, Leco K, Chamberlain D, Shepherd FA Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J Clin Oncol 17(6): 1802-1808, 1999
30. Milan J, Charalambous C, Elhag R, Chen TC, Li W, Guan S, Hofman FM, Zidovetzki R Multiple signaling pathways are involved in endothelin-1-induced brain endothelial cell migration. Am J Physiol Cell Physiol 291(1): C155-164, 2006
31. Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci 120(Pt 24): 4355-4366, 2007
32. Miyazaki T, Kato H, Nakajima M, Faried A, Takita J, Sohda M, Fukai Y, Yamaguchi S, Masuda N, Manda R, Fukuchi M, Ojima H, Tsukada K, Kuwano H An immunohistochemical study of TIMP-3 expression in oesophageal squamous cell carcinoma. Br J Cancer 91(8): 1556-1560, 2004
33. Nozaki A, Ikeda M, Naganuma A, Nakamura T, Inudoh M, Tanaka K, Kato N Identification of a lactoferrin-derived peptide possessing binding activity to hepatitis
36
C virus E2 envelope protein. J Biol Chem 278(12): 10162-10173, 2003
34. Oka M, Tagoku K, Russell TL, Nakano Y, Hamazaki T, Meyer EM, Yokota T, Terada N CD9 is associated with leukemia inhibitory factor-mediated maintenance of embryonic stem cells. Mol Biol Cell 13(4): 1274-1281, 2002
35. Shanmukhappa K, Kim JK, Kapil S Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection. Virol J 4: 62, 2007
36. Shapiro JL, Wen X, Okamoto CT, Wang HJ, Lyngstadaas SP, Goldberg M, Snead ML, Paine ML Cellular uptake of amelogenin, and its localization to CD63, and Lamp1-positive vesicles. Cell Mol Life Sci 64(2): 244-256, 2007
37. Shehu-Xhilaga M, Kent S, Batten J, Ellis S, Van der Meulen J, O'Bryan M, Cameron PU, Lewin SR, Hedger MP The testis and epididymis are productively infected by SIV and SHIV in juvenile macaques during the post-acute stage of infection.
Retrovirology 4: 7, 2007
38. Stetler-Stevenson WG Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1(27): re6,2008
37
differentiation of rat primary oligodendrocytes. J Cell Biochem 68(1): 128-137, 1998
40. Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D Is there a neural stem cell in the mammalian forebrain? Trends Neurosci 19(9): 387-393, 1996
41. Yandava BD, Billinghurst LL, Snyder EY "Global" cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A 96(12): 7029-7034,1999
42. Yunta M, Lazo PA Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell Signal 15(6): 559-564, 2003
43. Zhang B, Moses MA, Tsang PC Temporal and spatial expression of tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1 and -2) in the bovine corpus luteum. Reprod
Biol Endocrinol 1: 85, 2003
44. Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu J, Phinney DG, LaMarca HL, Florez L, Morris CB, Hoyle GW, Scandurro AB Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol 35(4): 640-652, 2007
38
45. Gardner J, Ghorpade A Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system.
J Neurosci Res 74(6): 801-806, 2003
46. Heese O, Disko A, Zirkel D, Westphal M, Lamszus K Neural stem cell migration toward gliomas in vitro. Neuro Oncol 7(4): 476-484, 2005
47. Jung KK, Liu XW, Chirco R, Fridman R, Kim HR Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 25(17): 3934-3942, 2006
48. Liu X, Hu Y, Hao C, Rempel SA, Ye K PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 26(34): 4918-4927, 2007
49. 박해철 성체 신경줄기세포와 뇌종양 줄기세포 연구동향 및 향후 전망. Trends in Medical Research - “Cancer Stem Cell” Vol.15(No.2), 2008
50. 신주현, 김일두, 이민형1, 이자경 C6 별아교종양세포주에서 IFNγ에 의한 TIMP-1의 발현 조절을 매개하는 SP1 결합 부위 동정. The Korean J Anat 40(2): 77~83, 2008
39 - 국문요약 -
뇌종양 과 발현 물질인 Timp-1에 의한 신경줄기세포의 이동
유도 기작 연구
아주대학교 대학원 의생명과학과 이 수 연 (지도교수: 이 명 애) 우리의 앞선 연구에서 프로테옴과 마이크로어레이를 통해서 사람 신경교종에서 TIMP-1이 발현하는 것을 확인하였다. 이를 기반으로 본 연구에서는 TIMP-1에 의한 신경줄기세포의 이동을 유도하는 과정에 대하여 연구하고자 하였다. Tissue inhibitor of metalloproteinases (TIMPs)는 다양한 기능을 가진 분비단백질로 TIMP-1, TIMP-2, TIMP-3, 그리고 TIMP-4로 이루어진 단백질 그룹이다 (Nagase et al.,1999). 모든 TIMP는 매우 단단한 1:1 비공유성 결합을 형성하는 방법을 통하여 대부분의 기질 단백분해효소(MMP)의 작용을 억제한다고 그 기능이 알려져 왔다. 최근 들어 신경계에서의 TIMP의 역할의 중요성이 알려지면서, 특히 신경세포 가소성이나, 신경계질환과 관련한 TIMP의 역할에 대한 관심이 높아지고 있다(Gardner et al.,2003; Crocker et al., 2004). TIMP-1은 국소허혈, 다발성 경화증, 후 뇌신경계에서 발현이 현저히 증가한다고 보고 되고 있으며,40 특히 뇌종양 특히 신경교종에서 발현이 증가되어 종양을 악성화 한다고 보고 되고 있다. 먼저 사람 신경교종 (glioma)조직에서 TIMP-1의 발현 정도를 면역조직염색을 통하여 확인한 결과 사람 뇌종양조직에서만 발현하고 정상 뇌조직에서는 발현하지 않는 것을 확인하였다. 이렇게 뇌종양조직에서만 발현하는 TIMP-1에 의해 신경줄기세포의 이동이 유도되는 지를 실험하기 위하여 다른 종류의 세포와 비교하여 실험한 결과 TIMP-1에 의한 세포의 이동에서 신경줄기세포만이 현저하게 증가함을 확인할 수 있었다. 위와 같은 결과는 수용체와 세포신호전달과정의 차이에서 기인하는 것인지 확인하기 위하여 수용체의 발현을 조사하였다. 최근, TIMP-1은 여러 세포주에서 tetraspanin TM4 중의 하나인 CD63 과 integrin β1을 통하여 세포막에서 신호를 전달하고 이런 세포신호에 의하여 세포사, 세포이동, 분열 등에 관여 한다고 보고되고 있다. 신경줄기세포에서 CD63과 integrin β1의 발현을 확인한 결과 다른 세포주들에 비해서 두드러지게 발현함은 물론, 신경줄기세포에서는 이들이 서로 complex를 형성하고 있음을 확인하였다. 이런 상호작용이 신경줄기세포의 뇌손상부위로의 이동에서의 역할을 조사하기 위해 shRNA를 이용한 CD63 knockdown 세포주는 정상적인 신경줄기세포주에 비하여 이동능력이 현저히 떨어지는 것을 확인할 수 있었다. 이는 CD63이 TIMP-1에 의한 신경세포 유도현상에서 중요한 수용체로 작용함을 시사하는 것으로 생각된다. 본 연구는 뇌 특성상 수술치료가 어려운 신경교종 치료에 획기적인 치료법에 공헌할 것이라고 기대된다. 핵심어: TIMP-1, 신경세포주, 신경종양, 뇌종양, CD63, integrin β1