1. Introduction
2)Hair fib er has a hierarchical structure and the diameter of human hair varies between 50 – 100 μm[1-3]. The three main structures of hair fiber are cuticle, cortex and medulla. The outermost layer of hair is called cuticle, of which 8 to 11 layers are overlapped with scales of 40 - 60 μm length and 0.3 - 0.5 μm thickness, covering the hair[3-5]. The layers include the epicuticle, exocuticle, endocuticle and inner layer[4,5]. Each cuticle layer is formed by one
† Nae Gyu Kang (e-mail: ngkang@lghnh.com), call: 02)6980-1533
cell[4]. The epicuticle or the most external layer of cuticle is composed of 25% lipids and 75% proteins including 12% of cystine, containing high content of sulfur[4]. Hair surface is originally hydrophobic due to covalently bound main lipid, or 18-methyl eicosanoic acid (18-MEA) to pro-tein matrix, however, it may be oxidized and becomes more hydrophilic when the cuticle layers are afflicted by washing and chemical treatments. The cortex is filled by cortex cells with 50 - 100 μm length and 3 μm diameter, and occupies 75% of the hair area[4]. In addition, those cortical cells consist of macrofibrils of intermediate filaments (IFs) made
Repairing Damaged Hair Using Pentapeptides of
Various Amino Acid Sequences with Crosslinking Reaction
Wonkyung Choi, Seongkil Son, Sang-Hun Song, Nae Gyu Kang†, and Sun-gyoo Park
LG Household & Health Care, E10 LG Science Park, 70 Magokjungang 10-ro, Gangseo-gu, Seoul 07795, Korea (Received June 11, 2020; Revised August 10, 2020; Accepted August 28, 2020)
Abstract: The aim of this study is to investigate the effect of various pentapeptides on hair repair depending on the characteristics of comprising amino acids using crosslinking agents in hair. Total ten peptides were synthesized with two kinds of amino acids respectively, of which were previously categorized according to R group of the amino acids contributing to the characteristic of each peptide: STTSS (Ser-Thr-Thr-Ser-Ser), LIILL (Leu-Ile-Ile-Leu-Leu), CMMCC (Cys-Met-Met-Cys-Cys), DEEDD (Asp-Glu-Glu-Asp-Asp), RKKRR (Arg-Lys-Lys-Arg-Arg), TAMRA- STTSS, TAMRA-LIILL, TAMRA-CMMCC, TAMRA-DEEDD, and TAMRA-RKKRR. Pentapeptide alone, or pentapeptides with crosslinking agents such as polymeric carbodiimide (PCI) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were treated to chemically damaged hair. Hair diameter and break strength (N = 40/case) were measured to calculate tensile strength of hair for computing hair repair ratio, and fluorescence yields (N = 20/case) were collected for hair treated with TAMRA-peptides. The tensile strength of hair treated with pentapeptides alone, or pentapeptides with cross-linking agents is consistent with the fluorescence yield from the microscope images of the cross-sectioned hair in vision and in numerical values. Pentapeptides consisting of hydrophobic amino acids (LIILL), amino acids with sulfur (CMMCC), and basic amino acids (RKKRR) increased the tensile strength in perm-damaged hair. Pentapeptides with no extra carboxyl/amine groups in R group of amino acids resulted in no significant differences in hair strength and fluorescence yield among hairs treated with alone and with crosslinkers. Pentapeptides with extra carboxyl groups or amine groups enabled further strengthening of hair due to increased bonds within the hair after carbodiimide coupling reaction. The hair repairs of pentapeptides with various amino acid sequences were studied using crosslinking. Depending on the physical characteristics of comprising amino acids, the restoration of damaged hair was observed with tensile strength of hair and fluorescence signals upon cross-sectioned hair in parallel to possibly understand the binding tendency of each pentapeptide within the hair.
from α-helical polypeptide chains in the matrix with high-sulfide content[2,3]. Moreover, it is demonstrated that the properties of human hair are dominated by α -keratin[2,6], and cortex largely determines the tensile be-havior of human hair[7,8]. In the center of hair, the medulla may exist, containing high lipid rate concentration and poor cystine[4].
Human hair contain approximately 65 - 95 w% pro-teins[7]. The protein matrix in which macrofibrils are im-bibed, consists of keratin associated proteins (KAPs) with a high cysteine content[9-12]. Various interactions such as van der Waals forces, hydrophobic interaction, hydrogen bonds, disulfide bridges, or isopeptide bonds may be estab-lished between the peptides and hair keratin. The mechan-ical properties of hair fibers rely on such interactions within keratin or between keratin and peptides from comparatively strong covalent bonds as well as disulfide and isopeptide bonds to weaker, non-covalent interactions with respect to stabilization of macromolecular structure of keratin fi-bers[4,9]. In the previous study[12], peptides with higher content in amino acids with higher hydrophobic side chains and cysteines and fewer amino acids with polar side chains showed higher affinity to hair keratins. It was suggested that the observed affinity were mainly driven by hydro-phobic interactions and disulfide bonds between the pep-tides and the extracted keratin. Nonetheless, the ex-perimental studies supporting peptides of those character-istics in the repair of keratin damage in hair fibers were not yet demonstrated.
In order to improve physical and mechanical properties of hair and prevent protein damage during daily washing and chemical treatments, the diffusion of peptides and ami-no acids with low molecular weight into hair cortex were studied[12-14]. Furthermore, fluorescent dyes are often used to study the diffusion of molecules into hairs[15,16]. The penetration of peptides or protein into keratin sub-strates were investigated with peptides linked with fluo-rescent dyes such as fluorescein 5(6)-isothiocyanate (FITC) or fluorescent dye 5(6)-carboxytetramethyl-rhodamine, suc-cinimidylester (5(6)-TAMRA), upon λex = 544 nm and λem = 572 nm, respectively[17,18].
In this study, pentapeptides of molecular weight < 1,000 Da with various amino acid sequences containing side chain group that are hydrophilic, acidic, hydrophobic, thi-ol-friendly, and basic, were synthesized for the diffusion of peptides into hair. The peptides were bound to TAMRA dye for visualization of peptides in hair utilizing fluo-rescence microscope (Evos FL Auto 2 Imaging System). Furthermore, carbodiimide coupling reaction was applied with the peptides on hair to improve the peptide binding affinity[22,24,25]. Carbodiimide chemistry accounts for the formation of amide bonds by the reaction between carbox-ylic acid and amines with the carbodiimide compounds. Those compounds including representative carbodiimide, 1-ethyl-3-dimethylaminopropyl carbodiimide hydrochloride (EDC-HCl) are generally applied in biochemical area for protein conjugation, surface modification, fluorescence tag-ging, or structure formations. Moreover, there are feasible bindings site for penetrated peptides in hair, since high car-boxylic acid content are found in the endocuticle, the cell membrane complex (CMC of the cortex) and the me-dulla[4]. Thus, EDC carbodiimide coupling reaction within peptides and between peptides and hair is employed as the binding technology for hair repair in expectation for en-hancement of hair properties in comparison with using pep-tides only, especially for peppep-tides with extra amine or car-boxyl groups. Utilizing the carbodiimide chemistry, the pentapeptides were treated to chemically damaged hair for hair repair, in order to probe and improve the affinity of peptides to hair. Since sensitivities of the hair on relative humidity and temperature are reported[2,7], all experiments were conducted in the same condition of humidity 50 - 55%, and temperature 23 ℃. Numerical data from tensile tests and fluorescence tests showed the correlation of repair capabilities by peptide in chemically damaged hair, and an appropriate protein structure was proposed to improve hair condition.
2. Materials and Methods
2.1. Materials
peptides, STTSS (Ser-Thr-Thr-Ser-Ser), LIILL (Leu-Ile- Ile-Leu-Leu), CMMCC (Cys-Met-Met-Cys-Cys), DEEDD (Asp-Glu-Glu-Asp-Asp), RKKRR (Lys-Arg-Arg-Lys-Lys), TAMRA-STTSS, TAMRA-LIILL, TAMRA-CMMCC, TAMRA-DEEDD, TAMRA-RKKRR were purchased from Peptron (Daejeon, Korea). Polymeric carbodiimide (PCI) and EDC were used as the cross-linkers between the amine/ carboxyl group of peptide with carboxyl/amine group of hair with an expectation of covalent bonds to be produced to strengthen the weakened hair. PCI was obtained from LG Household and healthcare (Korea). 1-ethyl-3-dimethylaminopropyl carbodiimide hydrochloride (EDC- HCl, 99%) and N-hydroxysuccinimide (NHS, 98%) were purchased from Sigma Aldrich (USA). NHS was used to stabilize the intermediate in the crosslinking reaction[17, 18].
2.2. Peptide Synthesis
The Trt-Cl resins and all Fmoc amino acids were pur-chased from GL Biochem (China). Coupling reagents and cleavage cocktail reagents were purchased from Sigma Aldrich (USA) and other solvents were purchased from Daejung chemical (Korea).
Peptides were synthesized by Fmoc SPPS (solid phase peptide synthesis) using ASP48S (Peptron, Inc., Korea) and purified by the reverse phase HPLC (Shimadzu Prominence HPLC, Japan) using a Vydac Everest C18 column (250 mm × 22 mm, 10 μm, USA). Elution was carried out with a water-acetonitrile linear gradient (10 - 75% (v/v) of aceto-nitrile) containing 0.1% (v/v) trifluoroacetic acid. Molecular weights of the purified peptide were confirmed using LC/MS (Shimadzu LC/MS-2020 series, Japan). Lyophilize were used FDT-12012 (Operon, Korea). 2.3. General Procedure of Peptide Synthesis
Each peptide was synthesized using a standard sol-id-phase peptide synthesis protocol. The Fmoc protecting group was removed by rocking in 20% piperidine in di-methylformamide (DMF) for 10 min (twice) and cou-pling was carried out using Fmoc amino acid (6 eq), hy-droxybenzotriazole (HOBT) (6 eq), 2-(1H-benzotriazole-
1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, hexafluorophosphate benzotriazole tetramethyl uronium (HBTU) (6 eq), N,N-diisopropylethylamine (DIPEA) (12 eq) in DMF for 2 h. For each step, resin washing was using DMF and methanol for 2 times each.
When the desired sequence was completed, crude pep-tide was cleaved from the resin using a mixture of TFA / EDT / Thioanisole / TIS / DW (90 / 2.5 / 2.5 / 2.5 / 2.5 volume) for 2 h. The solution was precipitated with cold ether and made pallet using centrifuge. The solid was collected and air dried.
Crude peptide was dissolved in DW and purified by the reverse phase HPLC using C18 reverse phase column. Elution was carried out with a water-acetonitrile linear gra-dient (10 - 75% (v/v) of acetonitrile) containing 0.1% (v/v) trifluoroacetic acid. The pure portion of peptide was col-lected and lyophilized.
2.4. Preparation of Chemically Damaged Hair
The protocol of perm treatment was established with in-formation given in previous studies[1,2]. For reducing agent, ammonium thioglycolate solution 50% (Daejung Chemicals & Metals Co., Korea) was dissolved in de-ionized water, and the pH of the solution was controlled with sodium hydroxide 98% (Sigma Chemicals Co, USA). The oxidizing agent was prepared with hydrogen peroxide solution 35% (Junsei Chemical Co, Japan) was dissolved in deionized water, and the pH of the solution was con-trolled with 0.5% NaOH solution.
2 g of 1.0 M ammonium thioglycolate solution at pH 9.5 was applied to 1 g of untreated hair previously washed with 15% sodium laureth sulfate (SLES), for 20 min. The hair tress was soaked and rinsed in 4 mL/s running water for 10 min, followed by application of 2 g of 3% hydrogen peroxide solution at pH 7 as neutralizer for ten min. The hair tress was soaked and rinsed in running water for 5 min, washed with 15% SLES, towel dried, and blow dried for 2 min.
2.5. Treatment of Hair with Peptides Only or with Carbodiimide
For tensile test, STTSS, DEEDD, LIILL, CMMCC, and RKKRR were each diluted to 0.5% solution in deionized water, and PCI or EDC was diluted to 1% solution in 0.1 M phosphate buffer solution (PBS) at pH 5. 2 g of each diluted peptide solution was treated to 1 g of permed hair tress for 15 min. For treatment of carbodiimide for binding peptides to hair, 2 g of PCI or EDC solution was treated to 1 g of hair previously treated with each peptide for 15 min. Considering the total amount of solution treated, 2 g of blank PBS buffer solution at pH 5 was treated to the hair after treatment of peptide, for preparation of hair treat-ed with peptides only. After treatment of peptides solution only or with carbodiimide solution, the hair was shampooed for three times with 15% sodium lauryl ether solution (SLES, LG H&H, Korea).
For fluorescence test, TAMRA-STTSS, TAMRA-LIILL, TAMRA-CMMCC, TAMRA-DEEDD, and TAMRA- RKKRR TAMRA dye were diluted to 0.025% solution in 0.1 M PBS buffer solution at pH 5. 2 g of each diluted TAMRA-peptide solution was treated to 1 g of permed hair tress for 15 min in darkness. For preparation of peptide cross-linked hair, EDC solution was treated for 15 min in darkness, while blank PBS buffer solution was treated to hair for the same time for hair treated with TAMRA-pep-tide only. After treatment of TAMRA-pepTAMRA-pep-tide solution only or with carbodiimide solution, the hair was shampooed for three times with 15% SLES solution.
2.6. Tensile Strength Measurements
Hair samples were prepared by crimping both ends of each hair fiber in 30 mm length with metal crimps (Dia-stron, UK). Laser scan micrometer (LSM-501S, LSM-6200, Japan) was used to measure the cross-sectional area of the hair fiber. Tensile tester (MTT175, Dia-stron, UK) was used to break each hair fiber and measure the break strength in gram force (gmf). One side of the fiber placed in fiber holder was pulled straight in 20 mm/min with converted energy from rotation of motor inside. The pulling force is measured and monitored in graphs with
load cell located on the other side of the fiber holder. Break load per cross-sectional area of each fiber was calculated as break stress, and the repair ratio (%) was later considered for comparison of peptides in hair.
(Equation 1.)
2.7. Fluorescence Microscopy Analysis
Treated hair wrapped with paraffin film was cut into 0.7 cm samples for paraffin embedding. The cut hair was paraf-fin embedded with an embedding center (Shandon Inc., UK) and was frozen overnight. Embedded samples were sliced with M1R rotary microtome (Shandon Inc., UK), fixed and dried more than 6 h on slide glasses for observation. Fluorescence microscope (Evos FL Auto 2 Imaging System, Thermo Fisher Scientific, USA) was used to visualize the penetration of peptides into the hair. The fluorescence images were captured with camera micro-scope, utilizing celleste image analysis software (Thermo Fisher Scientific, USA), the fluorescence intensity of hair fiber (N = 20) for each case was measured for further analysis.
3. Results and Discussion
3.1. Hair Strength
The tensile strength of hair was measured after shampoo-ing in order to explain the combination of peptide ligands, which can increase the affinity between peptide and carbox-ylate hair[19]. Table 1 exhibits the experimental tensile strengths of the hairs treated with various peptide sequences. Polylysine was generally used as the primary amine[20,22], and it (Mw ∼ 1,500) was employed for the internal modification in this study. RKKRR was used as the primary amine with extra amine groups in R groups of the comprising amino acids. The tensile strengths of vir-gin, damaged hair on which peptide only, peptide and PCI, peptide and EDC were treated, and were measured in order to compare the repair ratio of the peptides in different hair tresses. Due to the possibility that tensile strengths of virgin
hair from different Lots might be different, those of virgin and damaged hair were measured each time before the hair was damaged and treated with peptide.
In addition, before treatment with peptide, it was con-firmed that the tensile strengths of chemically damaged hair, KD decreased significantly from those of virgin hair for perm treatment. For the treatment of peptide solution only, KP values for DEEDD, LIILL, CMMCC, and RKKRR increased when compared to KD for each damaged hair. STTSS comprising amino acids with hydrophilic/ hy-droxyl groups and DEEDD consisting of acidic amino acids were easily washed off after shampooing, while LIILL con-sisting of hydrophobic amino acids, sulfur-containing CMMCC, and RKKRR comprising basic amino acids re-mained within hair and contributed to the increase of hair tensile strength.
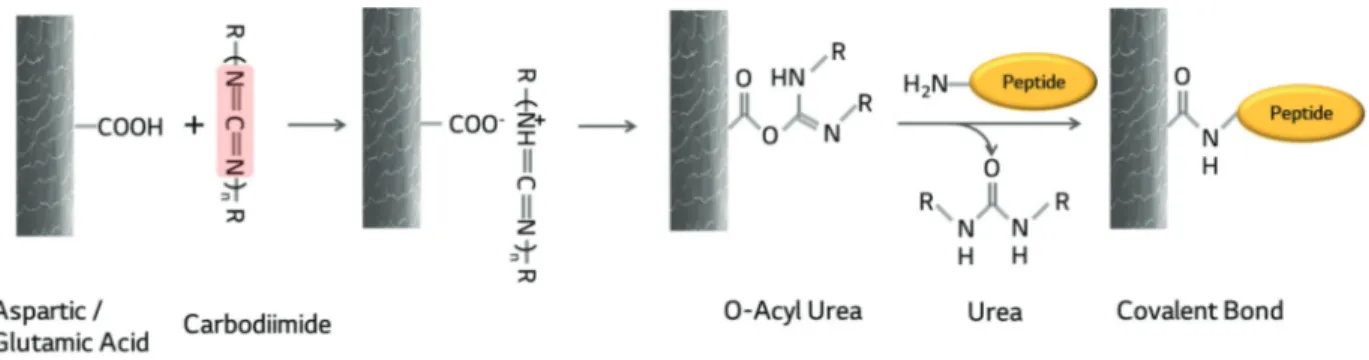
The two types of carbodiimide, PCI and EDC were used as the cross-linkers between peptide and hair following to carboiimide chemistry. The schematic diagram of the bind-ing mechanism for peptides to hair usbind-ing carbodiimide was depicted in Figure 1. The two significant differences
be-tween PCI and EDC are chemical structure and the molec-ular weight. The total number of N=C=N that functions as cross-linking agent for one molecule of PCI is more than one, while that of EDC is only one per one molecule. The expected molecular weight of PCI is approximately 1,500 Da, while that of EDC is 155.24 Da. From the previous study, it has been confirmed that when damaged hair was treated with PCI and hydrolyzed keratin (Seiwakasei, Japan) in a range of 400 – 100,000 Da, the hair treated resulted in higher tensile strengths. Thus, EDC was used in expectation that it can penetrate into the hair due to its small molecular weight and may easily enhance the hair strength.
The difference of binding affinity of peptides with the treatment of PCI and EDC can be found when comparing KPP and KPE values for Tab le 1. The KPP and KPE values for all peptides beside DEEDD, were very similar that there was no significant difference between them in spite of the differences of chemical structure and molecular weight of PCI and EDC. The difference between KPP and KPE for DEEDD may be have occurred from well bound peptide
Sequence Virgin,K V Damaged, KD Peptide, KP Peptide+PCI, KPP Peptide+EDC, KPE STTSS 362.18 318.73 316.34 319.13 327.70 DEEDD 359.04 304.27 318.36 325.38 342.67 LIILL 388.54 307.91 329.00 333.64 328.94 CMMCC 373.46 310.63 339.32 343.43 341.78 RKKRR 363.20 296.03 320.31 337.46 337.00
Table 1. Averaged Tensile Strength, K, for the Hairs Conjugated with Peptides and Polycarbodiimide/EDC after Shampoo (MPa)
in hair due to EDC coupling reaction of comparatively well penetrable peptide and EDC in the inner hair, while PCI or carbodiimide in polymer type remained more in the outer hair. The tensile strengths of KPP and KPE were compared to the KP, and they were increased in all peptides. Interestingly, the tensile strengths from KP to KPE were slightly increased at the STTSS, LIILL, and CMMCC due to no extra carboxyl or amine group in R group of the comprising amino acids. The representative primary amine, RKKRR showed high binding affinity with significantly in-creased KPP / KPE when compared to KP. Further we nu-merically analyze tensile strength of the hair formed by var-ious compositions of peptides in penetration of hair with carbodiimide chemistry.
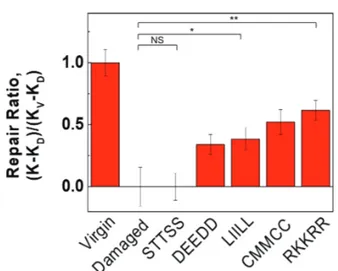
Statistical analysis was performed to analyze the hair binding affinity of each peptide with carbodiimide chem-istry, tensile strengths are normalized by the subtracted val-ue of virgin and damaged one using equation 1, and com-pared with the repair ratio of peptides as shown in Figure 2. The calculation of repair ratio for each peptide with re-spect to each damaged hair enabled more precise compar-ison for the binding affinity of peptides. There was no sig-nificant difference between the tensile strengths of hair
treated with STTSS and those of damaged hair, since hy-drophilic STTSS might easily be removed from hair after shampooing. Meanwhile, LIILL with hydrophobic amino acids, CMMCC with thiols, and RKKRR with primary amine groups showed significantly high repair ratio. As ex-pected, the maximum repair ratio was obtained at the hair of RKKRR with PCI, nearly 60% larger than that of dam-aged hair.
The tensile strengths in Figure 2 were done with one Lot of hair tress. All repair ratios of the experiment from the hair of different Lot number have shown pretty similar trend as a function of composition of peptides (data not shown). On the assumption that RKKRR supports the max-imum capability of repair regardless of hair sorts, further experiment was performed.
3.2. Fluorescence Signals
There was no significant difference between tensile strength of hair treated with PCI (KPP) and that with EDC (KPE) solutions when treated with STTSS, LIILL, and CMMCC, as shown in Figure 2. As mentioned above, the increasing pattern for KPE of DEEDD was different from the tensile strengths of other peptides as compared to the KP. The treatment with EDC solution showed a significant increase in hair tensile strength, while one with PCI sol-ution did not. Furthermore, RKKRR was effective in in-creasing hair tensile strength when treated with both PCI and EDC solution.
To further study for the behavior of treatment with EDC and PCI, fluorescence signals for TAMRA-STTSS, TAMRA-DEEDD, and TAMRA-RKKRR in hair were ob-served after treatment of TAMRA-peptide and EDC sol-ution in Figure 3. Since the treatment of STTSS on dam-aged hair exhibited no change in tensile strength in Figure 2, the damaged hair treated with TAMRA-STTSS was used as control. On the assumption that treatment of pep-tide-EDC penetrates into hair, further fluorescence study was performed in Figure 3. The red light from fluorescence microscope images in Figure 3A – C (400 X) and Figure 3D – F (200 X) reflects successful binding site of TAMRA-STTSS, TAMRA-DEEDD, and TAMRA-
Figure 2. Repair ratios on the normalized average tensile from Table 1. The error bar amplitude indicates SD. The lines above the bar graph denote a significant difference (N = 20) calculated using student’s t-test. NS: not significant, *p < 0.05, and **p <
RKKRR to hair after wash. While hair treated with TAMRA-STTSS showed almost no red light intensity in Figure 3A and Figure 3D, both hair treated with TAMRA- DEEDD and TAMRA-RKKRR showed red light intensity, however, resulted in different pattern of fluorescent signal in hair. TAMRA-DEEDD remained mostly in the inner-most of hair, medulla and cortex region. On the contrary, TAMRA-RKKRR was bound in a greater part of the outer-most layer, cuticle region.
Raman study and fluorescence signal upon the cross-sec-tioned hair elucidated a possibility of penetration for the peptide into hair even treated with polylysine and PCI[22]. The penetration of RKKRR enhances tensile strength of the perm-damaged hair towards that of virgin as shown in Figure 2. The carbodiimide reaction for hair inside has been proven by a prevention of lipid after wash[22]. However, EDC alone without peptide was not strong enough to in-crease tensile strength (data not shown). The best efficiency for increasing tensile strength has been found for the RKKRR with EDC coupling due to the carbodiimide re-action between the carboxylic acid of hair and the pep-tide[22].
RKKRR is protonated at the pH 5 in sample preparation and binds to anionic surface of hair, leading to the reaction
of the amine groups of RKKRR and O-acyl urea formed by EDC, and carboxylic group of hair. Polymerization of peptides within the hair may also occur since peptides con-tain both the carboxylate and amine groups. EDC may bind initially to the carboxyl groups of the peptides and form amine bonds with the amine groups of the peptides. The crosslinks within the peptides and between the peptides and hair enhanced the tensile strength of hair and resulted in high binding affinity.
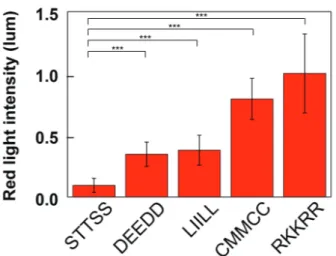
The averaged red light intensities from the fluorescent images of hair treated with TAMRA-STTSS, TAMRA- DEEDD, TAMRA-LIILL, TAMRA-CMMCC, or TAMRA- RKKRR, with EDC, were presented in Figure 4. The aver-age red light intensity of hair fibres for peptides corre-sponded to the hair repair ratio (data not shown), except for DEEDD. The average value of DEEDD was lower than that of LIILL, CMMCC, and RKKRR, although the highest repair ratio of hair for DEEDD was achieved among pep-tides when treated with EDC. The discordance of the aver-age red light intensity to hair repair ratio for DEEDD may be explained with the extraordinarily concentrated fluo-rescent area in small and middle portion, medulla or cortex of hair fib re, as shown in Figure 3C, resulting in lower light intensity. Nevertheless, regarding the average red light
Figure 3. Representative fluorescence microscope images (400 X, 200 X) of hair treated with TAMRA-STTSS (left column), hair treated with TAMRA-DEEDD (mid column), and hair treated with TAMRA-RKKRR (right column) followed by treatment of EDC solutions.
intensity for each peptide, it was concluded that DEEDD, LIILL, CMMCC, and RKKRR with EDC was significantly bound to hair when compared to STTSS. Among all pep-tides, RKKRR with primary amine group resulted in the highest binding affinity.
4. Conclusion
In this study, we observed hair repair capabilities for five types of pentapeptides, STTSS, DEEDD, LIILL, CMMCC and RKKRR, each consisting of a variety of amino acid sequences that represent hydrophilic, acidic, hydrophobic, thiol-friendly, and basic properties. Hair tensile strengths for treatment of peptides only, or with crosslinking agents, PCI or EDC to hair were measured, and fluorescent micro-scope images for each case were obtained to compare the hair repairing abilities in visual and in numerical fluo-rescent yields. It was investigated that LIILL, CMMCC, and RKKRR, peptides with hydrophobic amino acids, ami-no acids with thiol groups, or with extra amine groups con-tributed to the hair repair alone. Higher binding affinity of DEEDD and RKKRR to hair was attained when treated
with cross-linkers due to extra carboxyl or amine groups of the peptides. They enabled further strengthening of hair due to increased bonds within the hair after carbodiimide coupling reaction. The correspondance of hair repair ratio to the average fluorescent yield was found in all peptides except for DEEDD, which penetrated and bound mostly in the innermost region of hair. Polymerization within pep-tides and between peppep-tides and hair for DEEDD and RKKRR due to carbodiimide coupling reaction possibly contributed to the hair repair.
References
1. M. L. Tate, Y. K. Kamath, S. B. Ruetsch, and H. D. Weigmann, Quantification and prevention of hair damage, J. Soc. Cosmet. Chem., 44(6), 347 (1993). 2. Y. Yu, W. Yang, B. Wang, and M. A. Meyers,
Structure and mechanical behavior of human hair, Mater. Sci. Eng. C., 73, 152 (2017).
3. Y. Yu, W. Yang, and M. A. Meyers, Viscoelastic properties of α-keratin fibers in hair, Acta Biomaterialia, 64, 15 (2017).
4. M. V. R. Velasco, T. Dias, A. Z. Freitas, N. D. V. Júnior, C. A. S. Pinto, T. M. Kaneko, and A. R. Baby, Hair fiber characteristics and methods to evaluate hair physical and mechanical properties, Braz. J. Pharmac. Sci., 45(1), 153 (2009).
5. I. P. Seshadri, B. Bhushan, In situ tensile deformation characterization of human hair with atomic force microscopy, Acta Mater., 56(4), 774 (2008).
6. L. Kreplak, J. Doucet, and F. Briki, Unraveling double stranded α-helical coiled coils: an x-ray diffraction study on hard α-keratin fibers, Biopolymers, 58(5), 526 (2001).
7. C. R. Rob b ins, Chemical and physical b ehavior of human hair, 4, Springer-Verlag Berlin Heidelberg, New York (2012).
8. C. R. Robbins, R. J. Crawford, Cuticle damage and the tensile properties of human hair, J. Soc. Cosmet. Chem., 42(1), 49 (1991).
9. C. F. Cruz, M. Martins, J. Egipto, H. Osório, A.
Figure 4. Averaged red light intensity (luminescence) of hair fibres treated with TAMRA-STTSS, TAMRA-DEEDD, TAMRA-LIILL, TAMRA-CMMCC, and TAMRA-RKKRR followed by treatment of EDC solutions. The error bar amplitude indicates SD. The lines above the bar graph denote a significant difference (N = 20) calculated using student’s t-test. NS: not significant, *p < 0.05, and **p < 0.01.
Ribeiroa, and A. Cavaco-Paulo, Changing the shape of hair with keratin peptides, RSC Adv., 81, 7 (2017). 10. C. P. J. Bouillon, The science of hair care, ed. J.
Wilkinson, Taylor& Francis, (2017).
11. M. A. Rogers, L. Langbein, S. Praetzel-Wunder, H. Winter, and J. Schweizer, Human hair keratin- associated proteins (KAPs), Int. Rev. Cytol., 251, 209 (2006).
12. C. F. Cruz, N. G. Azoia, T. Matamá, and A. Cavaco-Paulo, Peptide-protein interactions within human hair keratins, Int. J. Biol. Macromol., 101, 805 (2017). 13. M. M. Fernandes, C. F. Lima, A. Loureiro, A. C. Gomes, and A. Cavaco-Paulo, Keratin-based peptide: biological evaluation and strengthening properties on relaxed hair, Int. J. Cosmet. Sci., 34(4), 1 (2012). 14. A. Tinoco, J. Goncalves, C. Silva, A. Loureiro, A. C.
Gomes, A. Cavaco-Paulo, and A. Ribeiro, Keratin- based particles for protection and restoration of hair properties, Int. J. Cosmet. Sci., 40(4), 408 (2018). 15. C. J. Silva, A. Vasconcelos, and A. Cavaco-Paulo,
Peptide structure: its effect on penetration into human hair, J Cosmet Sci, 58(4), 339 (2007).
16. A. Kelch, S. Wessel, T. Will, U. Hintze, R. Wepf, and R. Wiesendanger, Penetration pathways of fluorescent dyes in human hair fibers investigated by scanning near-field optical microscopy, J. Microscopy. 200(3), 179 (2000).
17. M. M. Fernandes, A. C. Gomes, A. Vasconcelos, F. D. Munteanu, T. Tzanov, M. S. T. Gonçalves, N. End, K. U. Schoening, G. Guebitz, and A. Cavaco-Paulo, Protein disulfide isomerase-assisted functionalization of keratin-based matrices, Appl. Microbiol. Biotech.,
90(4), 1311 (2011).
18. J. A. Swift, S. P. Chahal, N. I. Challoner, and J. E. Parfrey, Investigations on the penetration of hydrolyzed wheat proteins into human hair by confocal laser-scanning fluorescence microscopy, J Cosmet Sci, 51(3), 193 (2000).
19. S. K. Vahist, Comparison of 1-ethyl-3-(3- Dimethylaminopropyl) Carbodiimide based strategies to crosslink antibodies on amine-functionalized platforms for immunodiagnostic applications, Diagnostics (Basel), 2(3), 23 (2012).
20. S. H. Song, S. K. Son, and N. G. Kang, Dual case reports for lipid loss from human hair, Arch. Clinical Medical Case Rep., 3(6), 573 (2019).
21. C. Zviak, The Science of Hair Care, eds. C. Zviak, Marcel Dekker, New York (1986).
22. S. H. Song, J. H. Lim, S. K. Son, J. Choi, N. G. Kang, and S. M. Lee, Prevention of lipid loss from hair by surface and internal modification, Sci. Rep., 9, 9834 (2019).
23. H. G. Khorana, The chemistry of carbodiimides, Chem. Rev., 53(2), 145 (1953).
24. S. Son, W. Choi, B. T. Lim, S. H. Song, and N. G. Kang, Hair strengthening effect of silane coupling and carbodiimide chemistry, J. Soc. Cosmet. Sci. Korea, 44(2), 133 (2018).
25. S. H. Song, W. Choi, H. Park, B. T. Lim, K. R. Park, Y. Kim, S. Park, S. Son, S. M. Lee, and N. G. Kang, A new attempt to establish the extrinsic aging hair model to evaluate the response to aging in physical property, J. Soc. Cosmet. Sci. Korea., 45(2), 185 (2019).