저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Role of ICAM-1, a cell adhesion molecule, in
morphological changes of BV2 microglial cell.
By
Sumit Barua
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Role of ICAM-1, a cell adhesion molecule, in
morphological changes of BV2 microglial cell.
By
Sumit Barua
A dissertation submitted to the graduate school of
medicine, Ajou University in partial fulfillment of the
requirement for the degree of
Master of Neuroscience
Supervised by
Eun Joo BAIK, M.D., Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Sumit Barua is approved.
SUPERVISORY COMMITTEE
Soo Hwan Lee
Yi-Sook Jung
Eun Joo Baik
The Graduate School, Ajou University
December 23
rd2010
i
-ABSTRACT-
Role of ICAM-1, a cell adhesion molecule, in morphological
changes of BV2 microglial cell.
Microglial cells, the major immune effector cells in the central nervous system, are activated in various brain injuries. Lipopolysaccharide (LPS), found in the outer membrane of Gram-negative bacteria, one of the major activator of microglia induces morphological changes and activation of the microglial cells. There are a series of cell adhesion molecules (CAM) related with these changes. In our present study, we studied the role of adhesion molecules including Intracellular adhesion molecule-1 (ICAM-1), which are responsible for the microglial activation using immortalized BV2 microglial cell line. After seeding in the plastic, BV2 cells showed their morphological changes, from the round shape to bipolar or multipolar (spread) shape, according to time course. The treatment of LPS (1 μg/ml) influenced their morphological change at earlier time, no longer than 6 hrs. LPS treatment increased the numbers of cells into spread form with short and large proximal processes and their distance ramification reduced, which were coincided with the mRNA and protein expression of ICAM-1. The monoclonal neutralizing antibody to ICAM-1 significantly reduced the numbers of the multipolar cells. However, there was no change in other cell adhesion molecules like LFA-1, MAC-1 and β1 integrin a subunit of fibronectin
ii
receptor α5β1 even in LPS treated group. LPS also increase the trace number of ramified cells. Therefore; ICAM-1 might play an important role for the LPS-induced morphological changes.
iii
TABLE OF CONTENTS
ABSTRACT... i LIST OF FIGURES...v LIST OF TABLES ... vi ABBREVIATION... vii I. INTRODUCTION... 1II. MATERIAL AND METHODS... 6
A. Cell Culture ... 6
B. Morphological observations ... 7
C. Immunofluorescence assay for actin... 7
D. RT-PCR (Reverse transcription-Polymerase Chain Reaction)... 8
E. Western blotting...11
F. Inhibition of ICAM-1 by neutralizing antibody for ICAM1...11
G. Inhibition of ICAM1 downstream Rho/ROCK kinase...12
III. RESULTS ...13
iv
B. Immunofluorescence assay of Actin to check BV2 cell spread...16
C. Role of cytoskeleton in cell shape change...18
D. LPS-induced cell adhesion molecule mRNA Expression By BV2 cell ...21
E. ICAM-1 protein Expression by BV2 cell ...21
F. Cytokine expression By the BV2 cell...24
G
.
Spread inhibition by monoclonal neutralizing ICAM1antibody in BV2 cells27
H. Immunoflruorecense Assay to check the effect of monoclonal
antibody to ICAM1.
30
I. Inhibition of ICAM1 downstream molecule Rho/ROCK.
32
IV. DISCUSSION ...33V. CONCLUSION ...37
VI. REFERENCES...38
v
LIST OF FIGURES
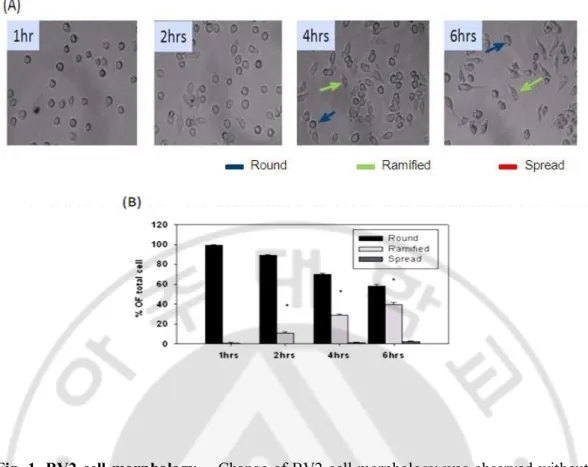
Fig. 1. BV2 cell morphology. ...14
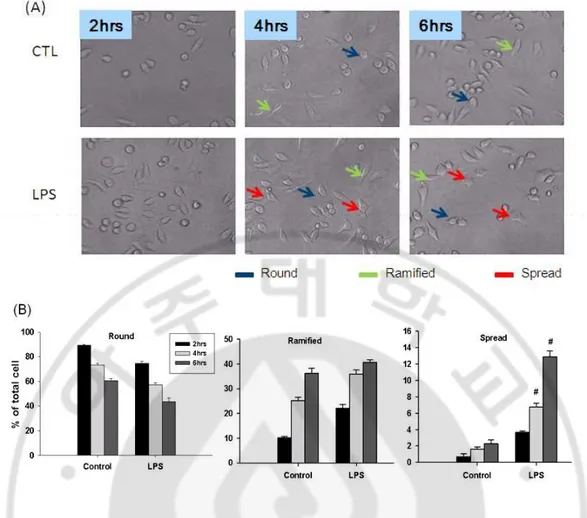
Fig. 2. Earlier change of LPS treated BV2 cell morphology. ...15
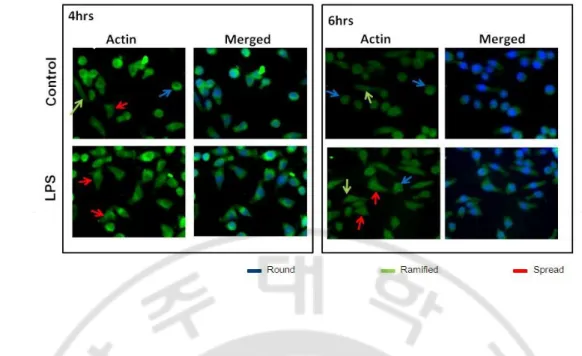
Fig. 3. Immunofluorescence assay of BV2 cell morphology. ...17
Fig. 4. Immunofluorescence assay cytoskeleton of BV2 cell. ...20
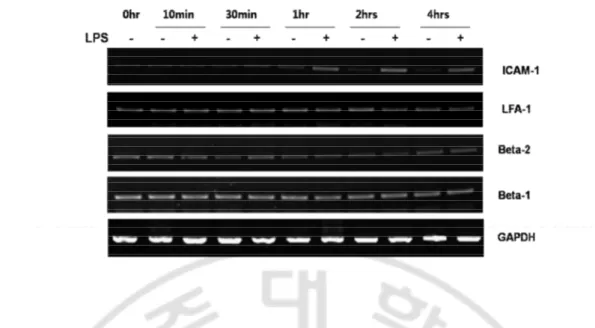
Fig. 5. Cell adhesion molecules express in BV2 cell. ...22
Fig. 6. ICAM-1 protein expression with or without LPS...23
Fig. 7. Cytokine expression by BV2 cell without treatment. ...25
Fig. 8. Cytokine expression by LPS treated BV2 cell. ...26
Fig. 9. Inhibition of ICAM1 by monoclonal neutralizing antibody. ...29
Fig. 10. Expression of actin in LPS and monoclonal antibody treated BV2 cell. ...31
vi
LIST OF TABLES
vii
ABBREVIATION
LPS : Lipopolysaccharide CAM : Cell adhesion molecules
ICAM-1: Intracellular adhesion molecules 1 LFA-1 : Lymphocyte function-associated antigen 1 MAC-1 : Macrophage Integrin 1
TNF-α : Tumor necrosis factor-alpha IL-1β : Interleukin-1 beta
IFN-γ : Interferon-gamma
YN1/1.7.4 : Rat monoclonal to ICAM 1 Y27632 : Inhibitor of Rho/ROCK MEM : Minimum essential medium
1
I. INTRODUCTION
Microglial cells, members of brain innate immune system (Hussain et al, 2006), are the resident professional macrophages in the CNS, which are responsible for the principal immune effector cells of the CNS in any pathological environment. Pathogenesis of a variety of neurodegenerative diseases, including Alzheimer disease, Parkinson disease, Creutzfeld-Jacob disease, HIV-associated dementia, stroke, and multiple sclerosis (Gonzalez-Scarano et al, 1999) is closely related to microglial activation. Activated microglial cells accumulated at the injury sites or plaques in the neurodegenerative CNS (Barcia et al, 2004; Dauer et al, 2003; Wu et al, 2002). Even though activated microglial cells scavenge dead cells from the CNS and secrete a different types of neurotrophic factors for neuronal survival (Gonzalez-Scarano et al, 1999; Carson et al, 2002), it was found that various autoimmune responses due to over activation lead to neuronal death and brain injury (Gonzalez-Scarano et al, 1999; Carson et al, 2002 Dauer et al, 2003; Wu et al 2002). Morphological changes in microglial cells and bone marrow derived macrophages in the brain are associated with dramatic changes in cell behavior. For example, amoeboid microglial cells are responsible for removing apoptotic cells during development, mounting an innate immune response against pathogenic invaders, and destroying neurons during neurodegenerative diseases (Simone et al, 2004; Streit et al, 2001; Streit et al, 2004). In contrast, ramified microglial cells are characterized by a resting state. Therefore, factors inducing morphological changes in microglial cells have important physiological consequences.
2
After long debate on the origin of microglial cells (Ling et al, 1993; Theele et al, 1993) it is now generally accepted that they originate in mesodermal ameboid cells that enter brain parenchyma during embryonic development. Microglial cells, when differentiating from amoeboid cells into ramified ones, lose macrophage like-properties such as phagocytotic activity and expression of macrophage markers (Chugani et al, 1991; Streit et al, 1988). On the other hand, amoeboid microglial cells may be derived from local ramified cells in mature brain when the brain is damaged. The activated microglial cells with phagocytotic activity express macrophage-like markers such as ED1 and major histocompatibility complex antigens (Andersson et al, 1991; Flaris et al, 1993; Ling et al, 1992; Streit et al, 1988). Thus, the morphological change between amoeboid and ramified cells takes place during development and neuropathological events. There are several candidates involved in the mechanisms underlying the ramification, such as soluble factors including astrocyte conditioned medium and cytokines such as TGF h1, M-CSF, GM-CSF (Schilling et al, 2001), as well as membrane bound factors on astrocytes and epithelial cells, have been reported (Wilms et al, 1997), intracellular factors like different ion channels are involved in induction of microglial ramification. However, little attention was given in the cytoskeleton remodeling in BV2 microglial cells. As microglia are highly dynamic cells that undergo rapid cellular remodeling during membrane extension, migration, and phagocytosis. These processes are orchestrated by changes in the organization of the actin cytoskeleton and the assembly and disassembly of focal adhesions (Lynda et al, 2007).
Microglial activation through inflammation is reflected by changing morphology, migration, proliferation, phagocytosis, expression and release of different molecules (Kreutzberg et al, 1996; Zielasek et al, 1996; Raivich et al, 1999). Previous Studies
3
explain that any manipulation or insult to the brain that disturbs homeostasis leads to microglial activation, which manifests as a change in morphology and the up regulation or de novo synthesis of cell-surface receptors, CAMs like ICAM-1, LFA-1 and α5β1 (Perry et al, 2010; Chang et al, 2006). However, there is huge controversy about the morphological change according to the function, which has not been solved yet. According to those studies, it is summarized that when microglial cells are activated by inflammatory response they go changes in morphology, expression of cell adhesion molecules (CAMs) which increases their adhesive property that helps in migration of the cells to the injury site, and microglial cells exert their function, lastly MAPK/NF-kB pathways plays the key role in CAMs expression.
The idea about adhesion molecules was first presented on leukocytes and endothelial cells, and functionally characterized as cell surface receptors enabling cell– matrix and cell–cell interactions (Springer et al, 1990). Among all CAMs three families are of major importance: the monomeric selectins (e.g., E-selectin), the membrane proteins belonging to the immunoglobulin-supergene family (e.g., ICAM-1), and the heterodimeric integrins (e.g., leukocyte function antigen-1[LFA-1] (Shimizu et al, 1992). Microglial activation in neurodegenerative diseases (McGeer et al, 1988; Hall et al, 1997; Reichertet al, 2003; Miklossy et al, 2006; Mount et al, 2007) increases the expression of membrane molecules like ICAM-1 (CD54) (Richard Milner et al, 2003). As described previously, ICAM-1 is a trans-membrane glycoprotein molecule of the immunoglobulin super family and ICAM-1 associates with receptors of the integrin (LFA-1) family, thereby mediating cell-cell interactions and allowing for signal transduction. ICAM-1 is barely detectable in normal brain cells, but its expression is enhanced on the endothelial and glial cells during inflammatory situations such as multiple sclerosis and experimental
4
allergic encephalomyelitis (Sobel et al, 1990; Cannella et al, 1991; Bo et al, 1996). In vitro, ICAM-1 expression can be up-regulated in response to proinflammatory cytokines, such as TNF-α, IL-1β, or IFN-γ (Fabry et al, 1992). Every CAMs are distinct due to their wide range of role in function. ICAM-1 plays an important role in immune-mediated cell-cell adhesive interactions (Wager et al, 2005). In case of LFA-1, ICAM1 is a major counter-receptor for LFA-1 (Takeo et al, 1999) and α5β1 integrin is a major receptor for extracellular matrix glycoprotein fibronectin which is involved in cell adhesion, growth, migration and differentiation (Ruoslahti et al, 1988). It was reported that leukocyte ligand engagement may induce ICAM-1 oligomerization, contributing to ICAM-1-mediated signaling by clustering endothelial ICAM-1 molecules. However, it is possible that at sufficiently high levels of expression, ICAM-1 may spontaneously oligomerize on the endothelial cells and signal without engagement of other cell, and it includes cytoskeleton remodeling by activating Rho kinase pathway.
Lipopolysaccharide (LPS, a bacterial endotoxin), a major component of the cell wall of the gram-negative bacteria, is a potent stimulus for microglial activation. LPS is capable of transforming microglia into amoeboid, phagocytotic cells both in vitro (Giulian et al, 1995; Suzumura et al, 1991) and in vivo (Buttini et al, 1996; Kitamura et al, 1996). In the innate immune system, the lipopolysaccharide (LPS) receptor complex are Toll-like receptor (TLR), especially TLR4 (Buttini et al, 1996), predominately expressed on microglial cells but not neurons or oligodendrocytes (Rivest, 2003), and MAPKs are the downstream signals of TLRs (Chen et al, 2002) which is responsible for CAMs expression. Recent evidence indicates microglial cells, like their peripheral innate immune cell counterparts, express a wide array of TLRs, including mRNA for TLRs 1–9
5
in mice (Olson et al, 2004). Several observations indicate that TLR2 also involved in LPS signaling (Yang et al, 1998; Kirschning et al, 1998). It is also evident by different studies
that both MAPK and NF-kB are involved in CAMs expression (Arancibia et al, 2007; Liu et al, 2008; Renshaw et al, 1997; Andrew et al, 1999). According to previous studies it was concluded that inflammatory stimuli cause the activation of the microglial cells, which is characterized by early changes in morphology, expression of different CAMS like ICAM-1, LFA-1, α5β1 etc, migration and phagocytosis. Especially, individual function of CAMS in microglial cell spread needs to be revealed.
6
II. MATERIAL AND METHODS
Dulbecco's Modified Eagle Medium was purchased from WELGENE Bioscience (Seoul, S. Korea), Fetal Bovine Serum (FBS), L-glutamine were obtained from Gibco-BRL (Gaithersburg, MD). I purchased 100U/ml Penicillin/Streptomycin Invitrogen (Carlsbad, CA, USA), Total RNA extraction solution Easy BLUE TM iNtRON (Seoul,
Korea), RT-PCR kit AMV reverse transcriptase purchased from (Roche, Germany), Oligo primer for different molecule purchased from from GenoTech Corp. (Daejon, Korea). Cell was incubated in MCO-17 AI CO2 incubator (SANYO, Japan), to measure total
RNA after extraction we use Nano drop-1000 spectrophotometer (Thermo Scientific, Delaware, USA) for RT-PCR My Cycler TM (Biorad, Hercles , CA USA) was used, to measure the amount of protein in lysate VersaMax ® Microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used. PVDF membrane (Millipore, Bedford, MA, USA) was used in the step of protein transfer of western blotting. The picture of the membrane was taken by LAS-1000 (Fuji, Honshu, Japan) in western blotting.
A. Cell Culture
The BV2 cell line, established as previously described (13), was routinely maintained in medium consisting of equal volumes of minimum essential medium (MEM) and Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat
7
inactivated fetal bovine serum (FBS), 100 IU/ml penicillin/streptomycin in a humidified 5% CO2 atmosphere at 37°C. The cells were harvested and reseeded when confluent. For morphological and functional observation the cells were seeded in the 24 well plastic plates with a concentration of 2x105 cells/well and maintained in the incubator for 1, 2, 4
and 6 hrs.
B. Morphological observations
BV2 cells were cultured in DMEM supplemented with 1% FBS in a concentration of 2x105 cells/ well in the 24 well plastic plates and incubated in the
humidified 5% CO2 atmosphere at 37°C for 1, 2, 4 and 6 hrs then the picture was taken by Coolpix 995 (Nikon, Japan). Afterward the cell number was counted according to their morphology as round or non- spread (N/S) cells, Ramified cells and Spread cells. To check the effect of LPS on BV2 cell morphology cells were treated with or without LPS (1ug/ml) and the picture was taken and the number of the cells was counted in the same procedure.
C. Immunofluorescence assay for actin
BV2 cell were treated with or without LPS (1ug/ml) for 4 and 6hrs. After the expected time the cells were fixed with 4% Para formaldehyde (PFA) for 30 mins or overnight and then after washing with PBST ( PBS with 0.1% trition-100) block with 3%
8
Bovine serum Albumin (BSA) in Phosphate buffer saline (PBS) for 1 hour. The cells were then incubated with Actin antibody sc-10731 purchased from Santa Cruz Biotechnology, INC (CA, USA) for overnight in 4o C. then after sequentially
permeabilization with PBST, the cells were incubated with secondary antibody Alexa Fluor 488 purchased from Invitrogen (Oregon, USA) for 2 hrs and then after washing 2 times with PBST and the incubated with Hoechst 33258, catalogue number B-2883, was purchased from Sigma Chemical Co. (St. Louis, Missouri), for 30 minutes. Image was then taken by ZEISS fluorescence microscope (Germany). In the same way immunoassay for actin stress fiber and focal adhesion of BV2 cell by LPS on plastic was detected by Alexa Fluro Phalliodin (A12379) from Invitrogen and mouse monoclonal antibody to Vinculin (ab11194) respectively.
D. RT-PCR (Reverse transcription-Polymerase Chain Reaction)
BV2 cell was seeded in the 6 well plates same as before and incubated for the expected time course. Then the cells were washed twice with PBS and later total RNA was extracted by using easy blue (1 ml) according to the recommended by the manufacturer. Briefly, Cell lysed by easy blue taken into 1.5ml tube and 500 ul of Chloroform was added and vortex well then centrifuged for 15 minutes. Then the 400 ul clear supernatant was carefully taken in a newly prepared 1.5 ml tube and Ice cold isopropanol (400ul) was added there and mixed well by inverting the tube for few times and keep for 10 mins (or overnight). Then centrifuged for 15 mins and thereafter the supernatant was thrown out carefully and ice cold 70% EtOH was added and mixed well
9
then again centrifuged for 10 mins. Later the upper layer was discarded and the pellet was let it to dry to get rid of moisture. Dried RNA was dissolved in 20-25ul of DEPC. After measuring the RNA, by Nano drop spectrophotometer (Thermo Scientific, Delaware, USA), cDNA synthesis was performed by using 1ug of total RNA and RT-PCR kit AMV reverse transcriptase (Roche, Germany). Single strand cDNAs were subsequently used for PCR analysis by perfectShotTM Ex Tag Kit (Takara, Japan) with the following primers:
10 Table 1. Primer Sequences for RT-PCR
Name Sense Anti-sense
GAPDH CGGCTTTGATCTCTGCTTAA ACAGGTCTCCTCCGTCTT GA
IL-1β GCAACTGTTCCTGAACTC CTCGGAGCCTGTGT GCA TNF-a CCATGACCTATACTCAGGCTT CAGG GAAGCTCCATATCCCTGGGTGG AAAG ICAM1 GCAAGTAGGCAAGGACCTCA C CGACGCCGCTCAGAAGAA CCA C LFA-1 TGCTGACCAATACCTTTC GTG C TGAGGCAAATATGTGGAG CGT C
Integrin β1 AATGTTTCAGTGCAGAGC C TTGGGATGATGTCGGGAC Integrin β2 AATGAAGCAAGAGGGCAA
TGCGAC
ACAGTCGCAGAAGGTGCCATA GAT
11
E. Western blotting
BV2 cells were seeded in the 6 well plates as before and incubated for 4 and 6hrs. After the incubation time cells were washed with Phosphate Buffer Saline (PBS) twice and harvested by RIPA buffer (150 mM NaCl, 10 mM Na2HPO4 [pH 7.2] , 0.5% sodium
deoxycholate and 1% Nonident P-40) containing 5 mM EDTA, 10 µg/ml Leupeptin, 0.5 mM Phenylmethylsulfonyl Fluride (PMSF), 1 µg/ml of pepstatin and 10 µg/ml Aptotinin. Cell Lysate were further homogenized by brif sonication and later on centrifuged at 14,000 g for 20 minutes at 4° C and the resulting supernatant collected. Protein concentration was determined by using BCA reagent and sample was prepared with adding 1x Laemmli buffer (250 mM Tris-Hcl[pH 6.8], 40% Glycerol, 10% SDS, 20% 2-Mercaptoethanol, 0.032% Bromophenol Bule) . The protein sample was separated in the 10% SDS-PAGE and transfer to the PVDF/ millipore membrane. Then the membrane was blocked with 5% skim milk for 1 hour followed by primary antibody binding for expected protein and was kept in the 4°C for overnight. Secondary antibody was bound with the membrane for 2 hours the membrane was scanned by using chemiluminescence WB kit (ECL, intron, Korea) in the LAS-1000 (Fuji, Japan).
F. Inhibition of ICAM-1 by neutralizing antibody for ICAM1
After subculture BV2 cell were separated by two groups in 2x105 cells/well. One of them was for control and the other was treated with monoclonal neutralizing antibody to ICAM-1 (YN1/1.7.4 2.5 ug/ml) and incubated in the 37°C and 5% CO2 chamber for 30
12
mins and then treated with LPS (1 µg/ml) and incubated for 4 and 6hrs. Then the picture was taken by using Coolpix 995 (Nikon, Japan) and the cell was counted. The effect of YN1/1.74 was also observed on the rearrangement of actin filament the phalloidin staining..
G. Inhibition of ICAM1 downstream Rho/ROCK kinase
BV2 cell was first incubated with Y27632 (10 μM/ml) (Sigma-Aldrich, USA), a well known inhibitor Rho kinase, of for 30 minutes then LPS (1 μg/ml) was added and cell was seeded. The concentration of the cell was 2x105 in each group. The cell was
13
III. RESULTS
A. BV2 cell morphology
BV2 microglial cells were subcultured when the cells was confluent and plated in 24 well plate. After seeding BV2 cells showed round morphology. After 2 hrs they gradually started to get their ramified form which showed slender cell body with smooth membrane and short proximal process (Fig.1A). However, at 4 and 6 hrs more than 30- 40% of total cells became ramified with small body, usually bipolar short thickened proximal process and long distal ramification, and negligible amount of spread cells. The spread cells showed little short proximal process and distal attachment (Fig.1B). When the BV2 cells were treated with LPS (1 µg/ml), they showed the change of morphology but similar as untreated group until 2hrs (Fig.2A and B). After 4 and 6 hrs there was significant increase in the number of spread cells, with distorted uneven membrane, short contracted process and large in size (Fig.2A).
14
Fig. 1. BV2 cell morphology. Change of BV2 cell morphology was observed without any treatment in different time course. (A) BV2 cells plated on plastic started to change morphology as early as 2 hr and the number of cells changing morphology increased after then respectively. The increase of spread cells was not significant. (B) Bar chart showed the ramified cells increased significantly whereas the change of round cells and spread cells showed no significant difference Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells.(*p < 0.05, compared with control). Phase contrast photo micrograph of the corresponding fields X100.
15
Fig. 2. Earlier changes of LPS-treated BV2 cell morphology. Microglial cell line BV2 cells were treated with or without LPS (1 μg/ml) for 2hrs, 4hrs and 6hrs. (A) Showed the increases of ramified cells with long distal processes and spread cells with shortening the long processes and ruffling of the cell body in LPS-treated group. (B) Bar chart showed the changes of three types of cells in time. Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells. (#p < 0.05, compared with control of different time point). Phase contrast photo micrograph of the corresponding fields X100.
16
B. Immunofluorescence assay of actin to check BV2 cell spreading
In BV2 cells the immunofluorescence assay for actin was done 4 and 6 hrs after treated with or without LPS (1 µg/ml). Without LPS, that is control condition, the cell showed round shape cell body sign of having process like short proximal process and long distal ramification and cell membrane looks even in both 4 and 6 hrs (Fig.3). In the other hand, actin expression in the LPS-treated group showed changes in the cell shape and sign of having more than two pole, possible retraction of process, their uneven membrane (Fig.3).
17
Fig. 3. Immunofluorescence assay of BV2 cell morphology. Microglial cell line BV2 cell were treated with or without LPS (1 μg/ml) for 4hrs and 6hrs and labeled with antibody for actin. Treatment of LPS increased the number of cells containing uneven membrane, thick proximal process with short or no distal ramification, which indicated the spread BV2 cells. Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells. Immunefluorescence photomicrograph of the corresponding fields is X400.
18 C. Role of cytoskeleton in cell shape change
Cytoskeleton is one of the main factors that are responsible for the maintenance of the cell shape. Therefore, I performed immunoassay for the stress fibers, components of cytoskeleton, in BV2 cells. After treatment with LPS (1 μg/ml) for 4hrs and 6hrs, the cells were fixed and labeled with antibody against a focal adhesion molecule vinculin and phalloidin specific for F-actin. It showed that LPS increased the focal adhesion area and changed the arrangement of stress fibers (Fig.4A). In LPS-treated group, the numbers of spread cells increased while the numbers of round cells decreased than the control group, which was same as previous data in phase contrast picture. In cytoskeleton-stained pictures, the cell shape change is suggested as due to rearrangement of the actin filaments.
20
Fig. 4. Immunofluorescence assay cytoskeleton of BV2 cell. Microglial cell line BV2 cell were treated with or without LPS (1 μg/ml) for 4hrs and 6hrs and labeled with antibody for vinculin (red) stress fiber (green). LPS increased the focal adhesion area of the cells and changed the rearrangement of F-actin (A). Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells. According to the cell shape the cell numbers also counted into three different groups as previously mentioned (B). Immunefluorescence photomicrograph of the corresponding fields is X200.
21
D. LPS-induced cell adhesion molecule mRNA expression in BV2 cells
Cell adhesion molecules expressed by LPS (1 ug/ml) in BV2 cells were evaluated by RT-PCR. The BV2 cells were treated with or without LPS and incubated for 1, 10 and 30 mins, 1hr, 2hr and 4 hrs. The mRNA for ICAM-1, LFA-1 and subunits of different integrin (β1 & β2) were done. Results showed that there was no change of ICAM-1 mRNA expression in the control group in any time course, whereas LPS induced ICAM-1 mRNA expression as early as 1 hr and maintained in 2 and 4 hrs (Fig. 5), which coincided with the time of BV2 cell spread. However, the expression of LFA-1 and integrin subunits (β1 & β2) mRNA was not much changed in time or by LPS treatment.
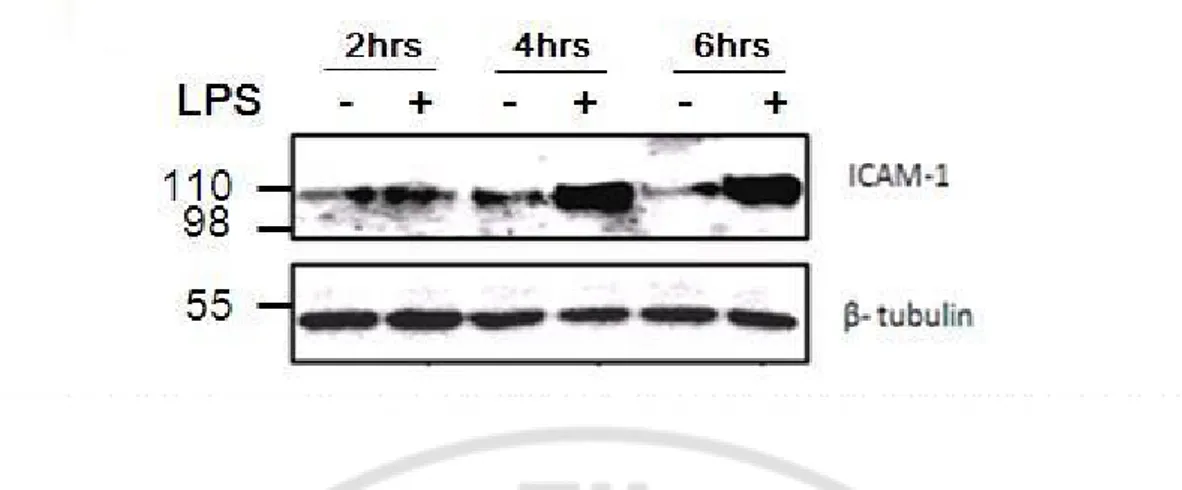
E. ICAM-1 protein expression in BV2 cells
LPS induced ICAM-1 mRNA expression in BV2 cell as early as 1 hr. The ICAM-1 protein expression was observed at 2, 4 and 6 hrs with western blotting. There was no change in ICAM-1 protein expression in the control group in any time course (Fig. 6), while LPS began to induce ICAM-1 protein expression at 2hrs and increased the expression significantly at 4 and 6 hrs (Fig. 6).
22
Fig. 5. Cell adhesion molecule express in BV2 cells The cells were treated with or without LPS for 0, 10 and 30 min, 1hr, 2 hrs and 4hrs to check mRNA expression of different CAMs. The expression of ICAM-1 mRNA increased in BV2 cells treated with LPS at 1 hr and continued in 2 hrs and 4 hrs, which also correlated with change of morphology. In case of CAMs from integrin family there were no significant changes in their mRNA expression by LPS.
23
Fig. 6. ICAM-1 protein expression with or without LPS. Western blotting was done to measure ICAM-1 protein expression in BV2 cells treated with or without LPS for 2, 4 and 6 hrs. It showed that the ICAM-1 protein expression increased at 2hr and more increased at 4 and 6hrs. This result coincided with the ICAM-1 mRNA expression and the increase in number of spread cells.
24 F. Cytokine expression By the BV2 cell
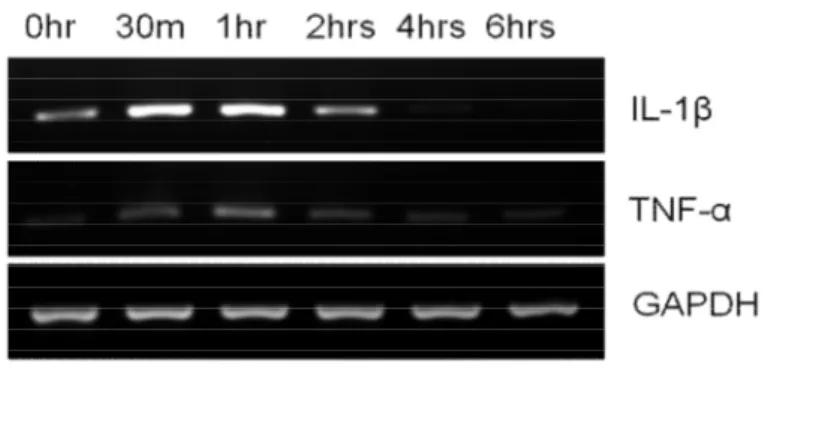
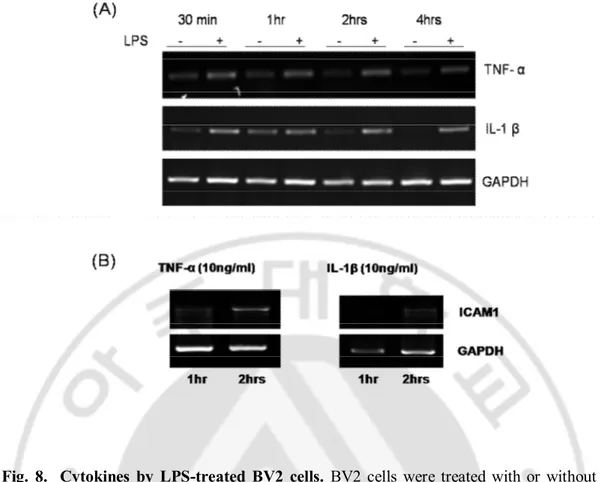
After seeding BV2 cells, the expression of cytokines IL-β and TNF-α was observed at the time of seeding, 30 min, 1hr, 2hr, 4hr and 6hr (Fig. 7). Both of the cytokines gradually increased until 1 hr, and gradually decreased after 2 hrs and later at 6hr become hardly traceable. The treatment of LPS (1ug/ml) increased the expression of both cytokines as early as 30 mins and the expression remained same until even 4hrs. Therefore, the LPS-treated cells started spread after 2 hrs but the cytokines expressed far earlier than the cell spread (Fig. 8). As cytokines expressed ICAM1 within 1-4 hrs, they may have some roles in cell spread in the earlier spread of BV2 cell.
25
Fig. 7. Cytokines Expression by BV2 cells without treatment. Cytokines IL-1β and TNF-α mRNA expression was measured in the BV2 cells without LPS treatment. Both of the cytokine expression increased as early as 30 min but gradually decreased after 2 hrs and become hardly traceable in 6 hrs. This suggested that the cytokines had little roles in BV2 cell morphology without LPS treatment.
26
Fig. 8. Cytokines by LPS-treated BV2 cells. BV2 cells were treated with or without LPS for 30 min, 1 hr, 2hrs and 4hrs to check the effect of LPS on the expression of cytokines IL-1β and TNF-α mRNA. LPS increased the expression of both cytokines at 30 min and remained same until 4 hrs (A). In case of control group the expression of mRNA showed as previously mentioned. Both cytokines increased the ICAM1 expression at 2 hrs but the expression was not much significant in the earlier time (B).
27
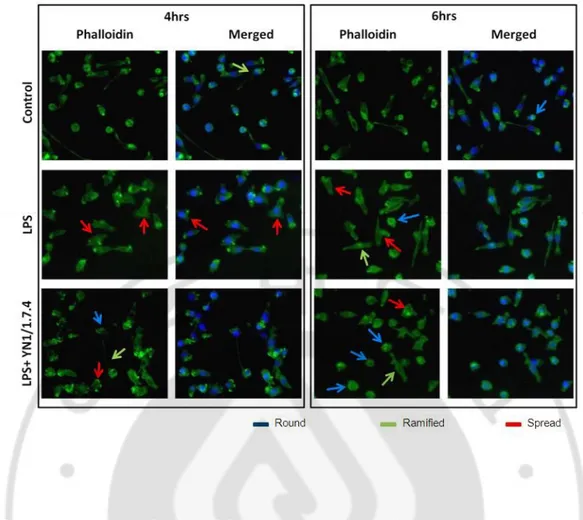
G. Spread inhibition by monoclonal neutralizing ICAM1antibody in BV2 cells
As BV2 cells showed spreading at 4 and 6 hrs, the cellular shape changes were observed at those time course after treatment with or without LPS and a monoclonal neutralizing ICAM1antibody YN1/1.7.4 (2.5 ). In Fig. 9A phase contrast pictures showed that the treatment of neutralizing antibody to ICAM1 attenuated the number of spread of BV2 cells and although the numbers of ramified cells was reduced, there was no significant change (Fig. 9). However, the portion of spread cells showed a significant reduction in LPS and YN1/1.7.4 treated group compared with the only LPS-treated group (Fig. 9A and 9B). This result indicates that ICAM-1 plays roles in LPS –induced morphologic changes of BV2 cells.
29
Fig. 9. Inhibition of ICAM1 by monoclonal neutralizing antibody. BV2 cell were treated with or without LPS (1ug/ml) and ICAM1 monoclonal antibody YN1/1.7.4 (2.5uM/ml) for 2, 4 and 6hrs. In (A). Phase contrast picture shows that monoclonal neutralizing antibody to ICAM1 reduce the number of spread of BV2 cell. Pai chart shows that Neutralizing antibody for ICAM-1 reduce the number of spread cells in LPS treated groups in both 4 and 6 hrs (B). But there is no significant difference in other type of cells. Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells. Phase contrast photo micrograph of the corresponding fields X100.
30
H. Immunoflruorecense Assay to check the effect of monoclonal antibody to ICAM1.
To confirm the effect of the YN 1/1.7.4 the monoclonal antibody to ICAM1 on the morphologic changes by LPS in BV2 cells, I examined the effect of ICAM1 mAb on actin remodeling, LPS-induced spread, which was evident in the phase contrast. The actin remodeling was observed 4 and 6hrs after LPS treatment. After fixation with 4%
paraformaldehyde the BV2 cells were stained with Phalloidin. LPS treatment increased the actin rearrangements and those were attenuated significantly by treatment of mAb to ICAM1 (Fig.10). It gives that idea that monoclonal antibody to ICAM1 blocks the effect of LPS-induced ICAM1 which causes the actin rearrangement.
31
Fig. 10. Expression of actin in LPS and monoclonal antibody treated BV2 cell LPS (1μg/ml) and ICAM1 monoclonal antibody YN1/1.7.4 (2.5 μg/ml) were treated in BV2 cell for 4 and 6hrs. The cells were fixed with 4% PFA and later was stained with phalloidin. It showed that mAb for ICAM1 attenuated the spread of BV2 cells by reducing the rearrangement of actin fiber. Blue arrow shows the round cell, green arrow shows the ramified cell and red arrow shows the spread cell. Immunefluorescence photomicrograph of the corresponding fields is X200.
32
I. Inhibition of ICAM1 downstream molecule Rho/ROCK.
Rho/ROCK, serine-threonine kinases are the downstream molecule of ICAM1 signaling pathway. The effect of Rho/ROCK kinase inhibitor Y27632 (10 μM/ml) on BV2 cells with or without LPS was observed. It showed that Y27632 developed long process in most cells which resembled the ramified cells (Fig.10). When the cells were treated with LPS for 4 and 6 hrs, there was little changing in the cell shape or process. However, treatment of LPS without Y27632 showed the similar result as previously shown.
33
Fig. 11. Inhibition of ICAM1 downstream pathway by Rho/ROK inhibitor. BV2 cells were first incubated with or without Rho/ROK inhibitor Y27632 (10 μM/ml) and later with or without LPS (1μg/ml). The time course was chosen as 4 and 6hrs. Then the pictures was taken in the contrast phase. Blue arrow shows the round cell, green arrow shows the ramified cells and red arrow shows the spread cells. Phase contrast photomicrograph of the corresponding fields is X100.
34
In this study it is characterized that BV2 microglial cells change in morphology, which is time dependent, and the expression of ICAM-1 is parallel for the time course change of microglial shape especially for spreading. When the BV2 cells was pre-incubated with ICAM-1 neutralizing antibody YN1/1.7.4 prior to LPS, the number of spreading cells was significantly reduced.
Microglial cells, the resident macrophages of the CNS, are exquisitely sensitive to brain injury and disease, altering their morphology and phenotype to adopt a so-called activated state in response to pathophysiological brain insults. Morphologically activated microglial cells like other tissue macrophages, exist as many different phenotypes, depending on the nature of tissue injury (Perry et al, 2010). Lipopolysaccharides (LPS), also known as lipoglycans, are large molecules consisting of a lipid and a polysaccharide joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria, act as endotoxins and elicit strong immune responses in animals. LPS treatment has been used extensively in inflammatory studies (Carter et al, 2003; Maekawa et al, 2002). It has also been shown that LPS activates microglia and exerts neurocytotoxic effects in both in vitro and in vivo systems (Hughes et al, 2004; Nakamura et al, 1999). Microglia shows diverse morphological changes which also indicate activation after treated with LPS. Morphologically, this activation was characterized by the swelling of the microglial cell body, a thickening of the proximal processes, and a reduction in distal ramification (Christian et al, 2000). In this study the types of BV cells were classified into three groups round, ramified and spread. The classification was done according to their cell size, condition of the membrane,
35
proximal process and distal ramification. Round cells with smooth cell surface membrane resembles as either myelomonocytic cells or activated BV2 cell (amoeboid activated cells), ramified are bipolar with small cell body and have distal ramification and proximal process, lastly the spread cells tri- or multipolar with short or no distal ramification, thickened proximal process and uneven cell surface membrane. In primary microglial cells, treatment of LPS has a more extended morphology than untreated cells on glass (Summers et al, 2009). In this study BV2 microglial cells treated with LPS (1 µg/ml) showed different time duration but the result supported the previous study. In present study BV2 cells started to take ramified morphology after 2 hours in both LPS treated and untreated group. However, LPS increased the number of spread cells at 4 and 6 hrs in Fig. 1 and 2, which also could be manifested as pro-amoeboid cells (Fig. 2). I also confirmed the changes of the cell shape with immunofluorescence assay, by using antibody binds to actin, in the control and LPS-treated group at 4 and 6 hours. It showed that BV2 cells increased in size and got spread shape in LPS-treated group in both 4 and 6 hrs. Thus, it is certain that LPS induce the spreading of BV2 cells and it is time dependent.
Cell adhesion molecules (CAMs) are located on the cell surface, which are involved with the binding with other cells or with the extracellular matrix (ECM) in the process called cell adhesion. Microglial cells express a number of CAMs like ICAM-1 (CD54), lymphocyte function associated antigen-1 (LFA-1) (CD11a) and α5β1 integrin in neurodegenerative diseases (Milner et al, 2003). Previous study reveals that cell adhesion molecules like ICAM-1, LFA-1 has a strong role in change of size in human dermal microvessel endothelial cells (HDMEC), human monocyte cell line THP-1, T-cell and other cells (Ronald et al, 2001; Mueller et al, 2004). In present study, cell adhesion
36
molecule expression with or without LPS (1 µg/ml) in BV2 cells was measured. The most of the CAMs or their subunits expressed by microglia such as LFA-1, β2 and β1 subunits of MAC-1 and α5β1 were present after seeding in the plastic (Milner et al, 2003; Färber et al, 2008). After seeding, mRNA of these integrins expressed at earlier time and there are no significant changes of their expression in the course of time and also irrespective of the control and the LPS-treated group (Fig. 5). In contrast, the expression of ICAM-1, a cell adhesion molecule related to Immunoglobulin super family, increased significantly after 1 hr and it continued even until 4 hrs (Fig. 5). In morphology, BV2 cells showed spreading after 2 hrs and at 4 hrs and it was suggested that the increase of ICAM-1 mRNA expression might have somewhat relationship with the spreading. Further the expression of ICAM-1 protein in BV2 cells was measured at 2, 4 and 6hrs. LPS increased ICAM-1 protein expression at 2 hrs, more significantly increased at 4 hrs and continued at 6hrs (Fig. 6). ICAM-1 protein expression fully coincided with the BV2 cell spreading, which was observed previously.
LPS induces proinflammatory cytokines including Tumor necrosis factor (TNF)-α and Interleukin beta (IL- β) in microglial cells (Ye et al, 2001; Astrid et al, 2010). The release of these factors was time-dependent (Wang et al, 2005). In the present study, the expression of TNF-α and IL- β in microglial cells increased after seeding cells on the plastic in 1st hr but after 2nd hr gradually decreased and the mRNA expression of both
cytokines in 6 hr diminished. When LPS was treated, the expression of both cytokines increased as early as 30 mins after seeding and remained same until 4 hrs in Fig. 4. Also, the treatment of TNF-α and IL- 1β did not significantly enhance ICAM-1 mRNA levels in microglial cells, and change little in the morphology. Therefore, cytokines such as TNF-α and IL- 1β had little role in spreading.
37
Later to ensure the role of ICAM-1 in cell spreading, ICAM-1 was blocked by YN1/1.7.4 a neutralizing antibody specific for ICAM-1. The YN1/1.7.4 monoclonal antibody efficiently blocks the binding of ICAM-1 to LFA-1 and Mac-1 and inhibits ICAM-1-mediated functions including cell-cell adhesion, antigen presentation to T-cells and leukocyte migration to inflammatory tissues (Johnson LA, 2006; Kooyk YV, 1994). The YN1/1.7.4 monoclonal antibody was examined whether the ICAM-1 is responsible for LPS-induced cell spreading in BV2 cells. When the BV2 cells were pre-incubated with YN1/1.7.4 for 30 mins, the number of spreading cell in LPS-treated BV2 cell was reduced. It was also observed when the cells were stained with phalloidin. Blocking of ICAM-1 attenuated the LPS-induced rearrangement of the actin filament, however there was no significant change in the number of ramified cells, which indicated that the ICAM-1 exerted on the spreading rather than ramification. However, when Rho kinase ICAM1 downstream molecule was inhibited with Y27632, BV2 cells became ramified with long process in the control group while LPS-induced spreading was not affected by Rho kinase inhibitor. It suggested that LPS-induced Rho activation is closely related with retraction of the process.
38
ICAM-1 is a well known cell adhesion molecule from the Immunoglobulin super family, and associates with receptors of the integrin (LFA-1) family, thereby mediating cell-cell interactions and allowing for signal transduction. In this study LPS significantly increased a high level of ICAM1 mRNA and protein expression using RT-PCR and western blotting and concomitantly induced the morphologic changes, which increased spreading as early as 4 and 6 hrs with actin rearrangement. The monoclonal neutralizing antibody for ICAM-1 (YN1/1.7.4) attenuated the number of the spreading cells. The LPS-induced spreading was related with retraction of ramification, which might be closely involved with ICAM-1 downstream signaling ROCK/Rho activation. Thus ICAM-1 plays an important role in the cell shape or morphological change.
39
1. Andersson PB, Perry VH, and Gordon S: The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience (42):201-214, 1991
2. Andrew E Aplin, Alan K Howe and Juliano RL: Cell adhesion molecules, signal transduction and cell growth. Current Opinion in Cell Biology (11):737–744, 1999
3. Arancibia SA, Beltran CJ, Aguirre IM, Silva P, Peralta AL, et al: Toll-like receptors are key participants in innate immune responses. Biol. Res (40):97–112, 2007
4. Astrid VF, Mark C, Hajo H, Markus K, Yu-Mi R, Rolf R, Cordian B, Xenon: Enhances LPS-Induced IL-1β Expression in Microglia via the Extracellular Signal-Regulated Kinase 1/2 Pathway. J Mol Neurosci 2010.
5. Barcia C, Sanchez Bahillo A, Fernandez-Villalba E, Bautista V, Poza Y, Poza M, Fernandez-BarreiroA, Hirsch EC, Herrero MT: Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia (46):402–409, 2004
40
6. Bo¨ L, Peterson JW, Mork S, Hoffman PA, Gallatin WM., Ransohoff RM, and Trapp BD: Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the b2 integrin LFA-1 in multiple sclerosis lesions. J Neuropathol Exp Neurol (55):1060-1072, 1996
7. Buttini M, Limonta S, and Boddek HW: Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem. Int (29): 25–35,1996
8. Carson MJ: Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia (40):218–231, 2002
9. Carter DA, Dick AD: Lipopolysaccharide/interferon-gamma and not transforming growth factor beta inhibit retinal microglial migration from retinal explant. Br. J. Ophthalmol (87): 481–487, 2003
10. Cannella B, Cross AH, and Raine CS: Adhesion-related molecules in the central nervous system. Lab Invest ( 65):23, 1991
11. Chang YP, Fang KM, Lee TI, Tzeng SF: Regulation of Microglial Activities by Glial Cell Line Derived Neurotropic Factor. jcb (97):501–511, 2006
12. Chen LY, Zuraw BL, Liu FT, Huang S, and Pan ZK: IL-1 receptor associated kinase and low molecular weight GTPase RhoA signal moleculesare required for
41
bacterial lipopolysaccharide-induced cytokine genetranscription. J Immunol (169): 3934–3939, 2002.
13. Christian UAK, Marion B, Georg WK, Gennadij R: Effect of Lipopolysaccharide on the Morphology and Integrin Immunoreactivity of Ramified Microglia in the Mouse Brainand in Cell Culture mechanism. Exp. Eye Res (78): 1077–1084, 2001
14. Chugani DC, Kedersha NL: and Rome LH. Vaultimmunofluorescence in the brain: New insights regarding the origin of microglia. J. Neurosci (11):256-268, 1991
15. Dauer W, Przedborski S: Parkinson's disease: mechanisms and models
.
Neuron (39):889–909, 200316. Fabry Z, Waldschmidt MM, Hendrickson D, Keiner J, Love-Homan L, Takei F, Hart MN: Adhesion molecules on murine brain microvascular endothelial cells: expression and regulation of ICAM-1 and Lgp 55. J. Neuroimmunol (36):1-11, 1992
17. Farber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N,
Kettenmann H: An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci (39):579–585, 2008
42
18. Flaris NA., Densmore TL., Molleston MC., and Hickey WF: Characterization of microglia and macrophages in the central nervous system of rats: Definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia ( 7):34-40, 1993
19. Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB: Cell surface
morphology identifies microglia as a distinct class of mononuclear phagocyte. J. Neurosci (15): 7712–7726, 1995
20. Gonzalez-Scarano F, Baltuch G: Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci (22):219–240, 1999
21. Hailer NP, Bechmann I, Heizmann S, Nitsch R: Adhesion molecule expression on phagocytic microglial cells following anterograde degeneration of perforant path axons. Hippocampus 7(3):341-9, 1997
22. Hall GL, Wing MG, Compston DA, Scolding NJ: Beta-interferon regulates the immunomodulatory activity f neonatal rodent microglia. J Neuroimmunol (72): 11–19, 1997
43
23. Hughes EH, Schlichtenbrede FC, Murphy CC, Broderick C, van Rooijen N, Ali RR, Dick AD: Minocycline delays photoreceptor death in the rds mouse through a microglia-independent. Exp Eye Res 78(6):1077-84, 2004
24. Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB: Innate immune functions of microglia isolated from human glioma patients. Journal of Translational Medicine (30):4-15, 2006
25. Kitamura Y, Takahashi H, Matsuoka Y, Tooyama I, Kimura H, Nomura Y, Taniguchi T: In vivo induction of inducible nitric oxide synthase by microinjection with interferon-gamma and lipopolysaccharide in rat hippocampus. Glia 18(3):233-43, 1996
26. Kirschning CJ, Wesche H, Merrill AT, Rothe M: Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 188(11):2091-7, 1998
27. Kosslyn SM, Rose RM,Cohen JD: Placebo-induced changes in fMRI in the anticipation and experience of pain. Science (303)1162–1167, 2004
28. Kreutzberg GW: Microglia/macrophages: a sensor for pathologicalevents in the CNS. Trends Neurosci (19):312–3180, 1996
44
29. Ling EA, Wong WC: The origin and nature of ramified and amoeboid microglia: A historical review and current concepts. Glia 7(1):9-18, 1993
30. Liu Y, Kimura K, Yanai R, Chikama T, Nishida T: Cytokine, chemokine, and adhesion molecule expression mediated by MAPKs in human corneal fibroblasts exposed to `poly(I:C). Invest Ophthalmol Vis Sci 49(8):3336-44, 2008
31. LyndaM S, Susan AB, Cameron RS,, JessicaM S, James R,Julie G,Anita A T, Ailiang Z, Joseph BE, Kathryn JM: CD36 Signals to the Actin Cytoskeleton and Regulates Microglial Migration via a p130Cas Complex. J Biol Chem 282(37): 27392-401, 2007
32. Maekawa S, Aibiki M, Si QS, Nakamura Y, Shirakawa Y, Kataoka K: Differential effects of lowering culture temperature on mediator release from lipopolysaccharide-stimulated neonatal rat microglia. Crit. Care. Med (30): 2700–2704, 2002
33. McGeer PL, Itagaki S, Boyes BE, McGeer EG: Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology (38), 1285–1291, 1988
34. Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL: Role of ICAM-1 in persisting inflammation in Parkinson’s disease and MPTP monkeys. Exp Neurol 197(2):275-83, 2006
45
35. Milner R, Campbell IL: The extracellular matrix and cytokine regulate microglial
integrin expression and activation. J. Immunol (170):3850–3858, 2003
36. Mueller KL, Daniels MA, Felthauser A, Kao C, Jameson SC, Shimizu Y: Cutting Edge: LFA-1 Integrin-Dependent T Cell Adhesion is Regulated by Both Ag Specificity and Sensitivity. The Journal of Immunology (173): 2222-2226, 2004
37. Nakamura Y, Si QS, Kataoka K: Lipopolysaccharide-inducedmicroglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35(2):95-100,1999
38. Olson JK, Miller SD: Microglia initiate central nervous system innate and adaptive immune responses through multipleTLRs. J Immunol 173(6):3916-24, 2004
39. Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol (4):193-201, 2010
40. Raivich G, Bohatschek M, Kloss CUA, Werner A, Jones LL, Kreutzberg GW: Neuroglial activation repertoire in the injured brain: graded response molecular mechanism and cues to physiological function. Brain Res Rev (30):77–105, 1999
46
41. Reichert F, Rotshenker S: Complement-receptor-3 nd scavenger-receptor-A I=II mediated myelin phagocytosis n microglia and macrophages. Neurobiol Dis (12): 65–72, 2003.
42. Renshaw MW, Ren XD, Schwartz MA: Growth factor activation ofMAP kinase requires cell adhesion. EMBO J (16):5592-5599, 1997
43. Richard M, Iain L C: The Extracellular Matrix and Cytokines Regulate Microglial Integrin Expression and Activation. J. Immunol (170):3850-3858, 2003
44. Rivest S: Molecular insights on the cerebral innate immune system. Brain Behav. Immun (17): 13–19, 2003
45. Ronald JA, Ionescu CV, Rogers KA, Sandig M: Differential regulation of transendothelial migration of THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4. J Leukoc Biol 70(4):601-9, 2001
46. Ruoslahti E: Structure and biology of proteoglycans. Annu. Rev. Biochem (57), 375-413, 1988
47
47. Schilling T, Nitsch R, Heinemann U, Haas D, Eder C: Astrocytereleased cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur. J. Neurosci (14) 463–473, 2001
48. Shimizu Y, Newman W, Tanaka Y, Shaw S: Lymphocyte interactions with endothelial cells. Immunol Today (13):106–112, 1992
49. Simone R D, Ajmone-Cat MA, Minghetti L: Atypical antiinflammatoryactivation of microglia induced by apoptotic neurons: possible role of phosphatidylserine– phosphatidylserine receptor interaction,Mol. Mol Neurobiol 29(2):197-212, 2004
50. Sobel RA, Mitchell ME, Fondren G: Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human central nervous system. Am. J. Pathol 136(6):1309. 1990
51. Springer TA: Adhesion receptors of the immune system. Nature (346):425–434, 1990
52. Streit WJ, Graeber MB, Kreutzberg GW: Functional plasticity of microglia: A review. Glia ( 1):301-307, 1988
53. Streit WJ: Microglia and macrophages in the developing CNS. Neurotoxicology (22):619– 624. 2001
48
54. Streit WJ: Microglia and Alzheimer’s disease pathogenesis. J. Neurosci.Res (77):1 – 8, 2004
55. Summers L, Kielty C, Pinteaux E: Adhesion to fibronectin regulates interleukin-1 beta expression in microglial cells. Molecular and Cellular Neuroscience (41):148–155, 2009
56. Suzumura A, Marunouchi T, Yamamoto H: Morphological transformation of microglia in vitro. Brain Res (545):301–306, 1991
57. Takeo M, Yoshihiro Y, Hiroyuki K , Keiko O,Yoshinori I, Shinichi K , Kensaku M: Neuronal adhesion molecule telencephalin induces rapid cell spread of microglia. Brain Research (849).58–66, 1999
58. Theele DP, Streit, WJ:A chronicle of microglial ontogeny. Glia 7(1):5-8. 1993
59. Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD: Placebo-induced changes in fMRI in the anticipation and experience of pain. Science (303): 1162–1167, 2004
60. Wang L, Yu A CH, Lau L T, Lee C, Wu L M, Zhu X, M. Tso O M: Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int (4):152-158, 2005
49
61. Wilms H, Hartmann D, Sievers J: Ramification of microglia,monocytes and macrophages in vitro: influences of various epithelialand mesenchymal cells and their conditioned media. Cell Tissue Res (287):447– 458, 1997
62. Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H: NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad
Sci USA 100(10): 6145–6150. 2003
63. Ye SM, Johnson RW: Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol 117(1-2):87-96, 2001
64. Zielasek J, Hartung HP: Molecular mechanisms of microglial/macrophage activation. Adv Neuroimmuno (6):191–122, 1996
- 국문요약 -
BV2 미세아교세포에서 형태적 변화에 대한 세포부착인자
ICAM-1의 역할에 관한 연구
50 아주대학교 대학원 의생명과학과 Sumit Barua (지도교수: 백 은 주) 중추신경계에 존재하는 미세아교세포는 주요한 면역세포로 여러 형태의 뇌손상상태에서 활성화된다. 그람음성세균의 외막에 존재하는 lipopolysaccharide (LPS)는 미세아교세포를 활성화시키는 것으로 잘 알려져 있으며 또한 미세아교 세포의 형태적 변화를 일으킨다. 이러한 형태적 변화는 활성화와 긴밀한 관계를 가지고 있으나 이에 대한 자세한 설명은 잘 되어 있지 않으나 여러 세포부착물질들이 관여할 것으로 추측된다. 본 연구에서는 BV2 미세아교세포를 이용하여 형태적 변화에서의 intracellular adhesion molecule ICAM-1를 비롯한 세포부착물질의 역할을 연구하고자 하였다. 미세아교세포들을 플라스틱에 배양할 때 둥근모양에서 시간이 지남에 따라 이극화(biopolar 또는 ramified) 또는 다극화(multipolar 또는 spread) 모양의 세포로 변화한다. LPS(1 µg/ml) 처치후 6 시간안에 이러한 변화들이 더 이르게 나타난다. LPS는 미세아교세포의 가지 기시부 (proximal process)가 짧고 크게 보이는 다극화세포의 수를 늘이고 이극화 세포의 가지 길이를 짧게 하는 동시에 ICAM-1의 mRNA와 단백질이 모두 증가하게 된다. 이때에 ICAM-1를 차단하는 neutralizing antibody는 이러한 다극화세포를 현저히 감소시켰다. 그러나 LFA-1, MAC-1와 fibronectin receptor α5β1 의 subunit인
51 β1 integrin의 변화는 LPS투여로도 관찰할 수 없었다. 또한 LPS는 대조군에 비해 이극화세포의 수를 증가시켰으나 ICAM-1 antibody로는 이것의 변화를 유도하진 못하였다. 따라서 ICAM-1은 LPS로 유도되는 활성화에 관여된 형태적 변화 특히 다극화세포로 변화하는 데에 주요하게 작용하는 것을 확인할 수 있었다. 핵심어 :