Micro-morphological Features of Liquid Urea-Formaldehyde Resins during Curing Process at Different Levels of Hardener and Curing Time Assessed by Transmission Electron Microscopy
전체 글
수치
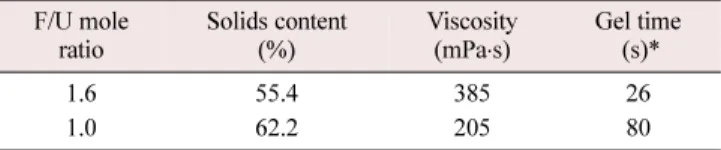

관련 문서
Specifically, Mises showed that the effect of inflation was not the same in the different periods since German people at that time had changed the expectation of
So, in order to clarify, the researchers tried to identify the formation process, structure and meaning, and stylistic features of the novel.. However, the
웹 표준을 지원하는 플랫폼에서 큰 수정없이 실행 가능함 패키징을 통해 다양한 기기를 위한 앱을 작성할 수 있음 네이티브 앱과
_____ culture appears to be attractive (도시의) to the
각 브랜드의 강점과 약점이 서로 상충되는 경우, 각 브랜드에 있어서 어떤 속 성의 약점을 다른 속성의 강점에 의해 보완하여 전반적인 평가를 내리는 것.
The index is calculated with the latest 5-year auction data of 400 selected Classic, Modern, and Contemporary Chinese painting artists from major auction houses..
1 John Owen, Justification by Faith Alone, in The Works of John Owen, ed. John Bolt, trans. Scott Clark, "Do This and Live: Christ's Active Obedience as the
That is, the expansion of material by high temperature and distortion by cooling during welding process is caused by tensile and compressive residual stresses in welding