관련 문서
interaction with anti-apoptotic Bcl-2 family members, we investigated whether Mcl-1 can inhibit the Noxa-induced cell death.. As expected, Mcl-1 significantly inhibits
– Embryonic stem cell : Pluripotent cells that can give rise to all tissue types. – Adult stem cell : Multipotent cells
Therefore, in this paper, the solar array of HST is modeled as a simple model with beams and membranes, and the phenomenon of thermal induced vibration is verified and
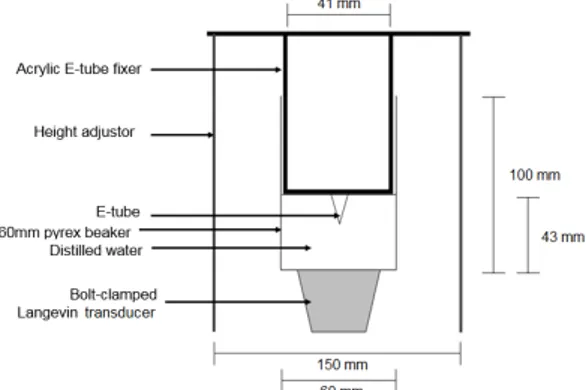
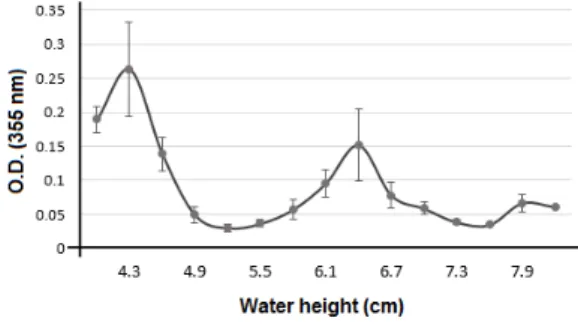
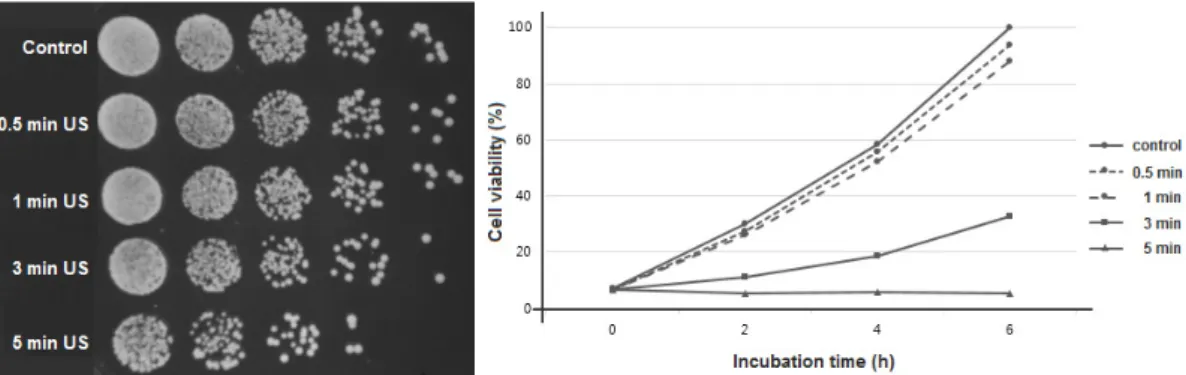
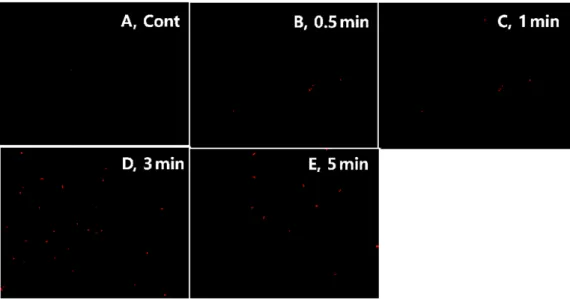
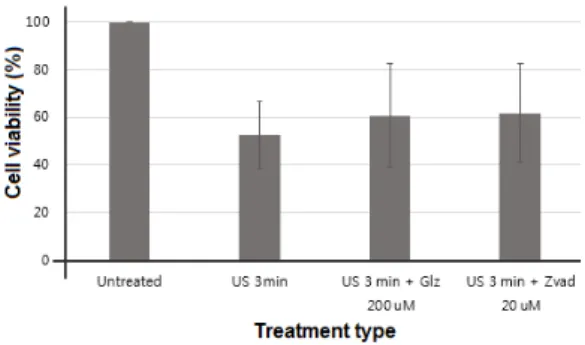
Low intensity pulsed ultrasound used in this
Objective: The purpose of this study was to analyze recent trend in incidence of basal cell carcinoma and squamous cell carcinoma in patients from the Gwangju City
Glutamate excitotoxicity induced by excessive activation of NMDA receptor causes various damage to cells, which leads to cell death.. In previous studies, increased ROS
These results suggest that the bilobalide inhibits the cell proliferation and induces the apoptotic cell death in FaDu human pharyngeal squamous cell carcinoma via both
Taken together, these results suggested that latex containing the ficin inhibited the cell growth and induced apoptosis by caspase and Bcl-2 family signaling pathway in